Abstract
Introduction
To investigate the efficacy and safety of proton-beam irradiation (PBI) combined with intravitreal conbercept (IVC) injection for refractory or recurrent polypoidal choroidal vasculopathy (PCV).
Methods
A prospective interventional clinical trial included 12 patients with refractory PCV (defined as persistent exudation or fluid after six consecutive injections at monthly intervals and/or photodynamic therapy) or recurrent PCV (defined as new exudative signs after six monthly injections and/or photodynamic therapy) treated between January 2019 and September 2020. Every patient underwent single PBI (14 GyE) with concomitant IVC (0.5 mg) within 1 week and further doses of IVC were administered pro re nata.
Results
By the 12-month follow-up, the subretinal fluid was completely absorbed in 9 eyes (81.8%). The angiographic regression and closure rates of the polyps were 60% (12/20) and 90% (18/20), respectively. The mean number of IVC injections was 3.1 ± 1.37. The mean BCVA improved by 20 letters (P = 0.006). The mean central macular thickness (CMT) decreased from 476.50 ± 123.63 μm to 317.70 ± 89.34 μm (P = 0.004). The areas of branching vascular networks and polyps decreased by 37.2% and 72.3%, respectively. Radiation retinopathy was observed in five eyes, but no systemic adverse events were observed.
Conclusion
PBI combined with IVC appears to promote polyp regression and closure, reduce CMT, and improve BCVA, with a favorable safety profile, after 12 months. Therefore, PBI may be a useful adjuvant therapy for patients with refractory or recurrent PCV.
Trial Registration
Proton-Beam Irradiation Combined with Intravitreal Conbercept for Refractory or Recurrent Polypoidal Choroidal Vasculopathy: Prospective Phase II Clinical Study (ChiCTR2000038987).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Polypoidal choroidal vasculopathy (PCV) was estimated to affect 50% of neovascular age-related macular degeneration (nAMD) cases in Asia. |
The treatment for refractory or recurrent PCV has remained challenging. |
Proton-beam irradiation has been studied as an alternative treatment for the management of nAMD. |
Proton-beam irradiation combined with intravitreal conbercept injection can achieve a high polypoidal lesion regression rate and good visual outcomes at 12 months. |
Our research could provide a new approach for the therapy of refractory or recurrent PCV. |
Introduction
Polypoidal choroidal vasculopathy (PCV), first termed by Yannuzzi et al. in the 1990s, is characterized by polypoidal lesions and abnormal branching vascular networks (BVNs) of choroidal vessels [1]. PCV is predominantly reported among Asian patients with age-related macular degeneration (AMD), with a prevalence of 20–60% in these patients [2]. Compared with the management of neovascular AMD, the management of PCV remains a conundrum because the disease follows a natural relapsing course without proactive treatment, and PCV is more likely than AMD to affect long-term visual acuity [3, 4].
Intravitreal anti-vascular endothelial growth factor (VEGF) has become the mainstay therapy for typical patients with neovascular AMD. However, the effective therapy for PCV is still contentious. A combination of an anti-VEGF agent with photodynamic therapy (PDT) is superior to anti-VEGF monotherapy not only in terms of the improvement in best corrected visual acuity (BCVA) and the closure of polyps but also in terms of the number of anti-VEGF agent injections required in the first year of treatment [3]. Refractory or recurrent PCV is defined as PCV in which the fluid persists or the development of new active signs after six monthly injections or/and PDT, respectively [5, 6]. Refractory and recurrent PCV are not uncommon, and the recurrence rates are reported to be as high as 40–78.6% after at least 3 years of follow-up [7, 8]. Until now, the treatment for refractory or recurrent PCV has remained challenging.
Therefore, alternative approaches to manage PCV must be explored. Introini et al. recently evaluated the efficacy of stereotactic radiation therapy (SRT) combined with ranibizumab in the treatment of 12 patients with PCV [9]. A high polypoidal lesion regression rate (83.3%) and good visual outcomes were achieved after 12 months. Unlike SRT, proton-beam irradiation (PBI) delivers more than 90% of the radiation dose to the target tissue, thus minimizing damage to the adjacent tissue [10]. This advantage allows fixed-beam irradiation to target the subfoveal neovascular membranes, while delivering a minimal dose to nontarget tissues. PBI has been studied as an alternative treatment for the management of wet AMD [10,11,12,13,14], and is thought to inhibit inflammation and fibrosis and to induce the regression of choroidal neovascularization (CNV) [15, 16].
On the basis of previous encouraging studies, we evaluated the efficacy and safety of PBI in conjunction with intravitreal conbercept (IVC) injections in patients with either refractory or recurrent PCV.
Methods
The study protocol was approved by the institutional review board. All procedures were performed in compliance with the principles of the Declaration of Helsinki. Written informed consent was obtained from every patient.
Study Design
This was a prospective, noncomparative, interventional trial of patients with refractory or recurrent PCV treated between January 2019 and September 2020. All patients received a single dose of 14 cobalt Gray equivalent (GyE) PBI combined with an IVC injection (0.5 mg) (Lumitin, Chengdu Kanghong Biotech Co., Ltd, P. R. China) at baseline. All the patients received PBI therapy at baseline and were then treated with IVC within 48 h. After PBI, all patients were followed up monthly, and received further doses of IVC at each visit if the retreatment criterion was met.
Patient Selection
Patients (age 50 years or older) with refractory PCV (defined as persistent fluid after six consecutive injections with anti-VEGF agent at monthly intervals and/or PDT) or recurrent PCV (defined as new active polyps signs after six monthly injections and/or PDT) with a BCVA of 20/40–20/320 (Snellen equivalent) and of 73–24 letters (Early Treatment Diabetic Retinopathy Study, ETDRS) in the study eye were eligible for the study. Macular PCV was diagnosed using spectral-domain optical coherence tomography (SD-OCT), fluorescein angiography (FFA), and indocyanine green angiography (ICGA). Both branching vascular networks (BVNs) and polyps were located within a circle (6 mm diameter) centered on the fovea. The activity of polyps was based on presence of leakages on FFA and ICGA, and subretinal and/or intraretinal fluid OCT imaging at final visit. The ability to give informed consent and to return for monthly follow-up visits were also criteria for enrollment. The main exclusion criteria were subretinal hemorrhage (at least 6PD), RPE tear, subretinal fibrosis, a vision-compromising disease other than PCV, a globe axial length of less than 20 mm or greater than 26 mm in the study eye, or type 1 or type 2 diabetes mellitus (see Fig. 5).
PBI Therapy
To immobilize the patient for PBI, a customized bite block and thermoplastic head mask were manufactured 1 day before PBI. During the treatment, the patient was fixed on a six-dimensional treatment chair with the bite block and the head mask (Fig. 1). The isocenter was first aligned to the center of the pupil of the affected eye in a left–right and head–feet direction, and then aligned to the canthus in an anterior–posterior direction. A retractor was placed on the eye to move the eyelids away from the proton beam (Fig. 2). To avoid the effect of high-dose irradiation on the lens, the patients were asked to stare at a red blinking fixation light mounted to the side of the beamline [11]. The chair was then translocated laterally to align the macula at the center of the beam and verified with a light field projected through the treatment aperture.
A beam applicator with a ridge filter, a collimator, and a treatment aperture of 10 mm diameter was used to deliver a uniform dose (14 GyE) of 8 mm diameter, centered on the macula, in one fraction (Fig. 5). The beam-on time was about 1 min.
Study Procedures
All patients underwent a detailed ophthalmological examination, including the measurement of BCVA, fundus photography (FP, Topcon TRC50LX; Topcon, Tokyo, Japan), fundus autofluorescence (FAF, Heidelberg Engineering, Heidelberg, Germany), SD-OCT (Heidelberg Engineering), FFA & ICGA (Heidelberg Engineering, Heidelberg, Germany), ultra-wide-field scanning laser ophthalmoscopy FFA (UWF-FFA, Optos, PLC, Dunfermine, Scotland), full-field electroretinography (ff-ERG), and swept source OCT angiography (SS-OCTA, PLEX Elite 9000; Carl Zeiss Meditec, Dublin, CA) at baseline. The central macular thickness (CMT) in the 1-mm-diameter central region of the macula, according to the Early Treatment of Diabetic Retinopathy Study thickness map, was measured using Spectralis software. The sizes of polyps and BVNs were measured with the on-board software of ICGA (Heidelberg Retina Angio-graph 2). Eligible patients underwent PBI combined with IVC (0.5 mg/0.5 ml) at baseline, and further doses of IVC were given pro re nata (PRN), according to the retreatment criterion (presence of either subretinal or intraretinal fluid on OCT). Patients returned for clinical assessment (BCVA, FP, SD-OCT, FAF, and SS-OCTA) every month and for safety evaluation (FFA, ICGA, and UWF-FFA) every 3 months. At the final visit, ff-ERG was used by a certified examiner to evaluate the retinal functions. OCTA was an advanced technology to detect the abnormal microvascular at different layers of retina. All intravitreal injections were performed by a retinal specialist and PBI was performed by an expert.
Primary and Secondary Outcomes
The primary objectives of our study were to evaluate the rates of polyp regression (defined as the absence of polyps on ICGA) and polyps closure (defined as the presence of polyps on ICGA but no new subretinal or intraretinal fluid on OCT), and the mean number of IVC injections given within 1 year. The secondary outcome measures were the changes in BCVA, central macular thickness (CMT), and the areas of polyps and BVNs from baseline.
Vision Testing
A certified examiner tested both eyes of each patient at every visit. BCVA was recorded as described by the ETDRS protocol. If the patient could read fewer than 20 letters at 2 m, visual acuity was measured at 1 m.
Radiation Vasculopathy Assessment
Radiation retinopathy and radiation papillopathy were assessed retrospectively at each visit from color fundus photographs and every 3 months with fluorescein angiograms by two specialists. Furthermore, ff-ERG was used to detect any potential radiation retinopathy.
Data Analysis
CMT and SFCT were determined by SD-OCT. The polyps numbers and the areas of BVNs and polyps were evaluated by ICGA. The changes in BCVA, CMT, subfoveal choroidal thickness (SFCT), polyps numbers, and the areas of BVNs and polyps were compared between baseline and the final visit with paired-samples t tests. Significance was defined as P < 0.05. All statistical analyses were performed with IBM SPSS Statistics v19 (SPSS, Chicago, IL, USA).
Results
Patients
Twelve eyes of 12 consecutive patients (five women and seven men; mean age 62.58 ± 7.09 years, range 52–73 years) with refractory or recurrent PCV were enrolled. One of the 12 patients discontinued during the follow-up period because of newly diagnosed pancreatic carcinoma, and was excluded from efficacy assessments. The remaining 11 patients were followed up for 12 months. We have evaluated the macular atrophy by autofluorescence shown in Fig. 3. In our cohort, no patients showed macular atrophy detected by autofluorescence at baseline and final visit. The demographic and clinical data of all the patients are presented in Table 1.
Treatments
During the study period, the mean number of conbercept injections administered was 3.1 ± 1.37, and six of the 11 eyes (54.5%) required three injections or fewer during the 12-month follow-up. The presence of recurrent polypoidal choroidal vasculopathy (Fig. 4) and refractory polypoidal choroidal vasculopathy (Fig. 5) before and after therapy.
Multimodal imaging findings of recurrent polypoidal choroidal vasculopathy before and after therapy in case 2. At baseline, optical coherence tomography (OCT) showed intraretinal fluid with hyperreflective materials between the retinal pigment epithelium and Bruch’s membrane (a), corresponding to early hyperfluorescence on fluorescein angiography (FFA) (b) and the branching vascular network (BVN) and polyps on early indocyanine green angiography (ICGA) (c). OCT angiography showed the morphology of the vasculature (d). After a single round of proton-beam irradiation and two intravitreal injections of conbercept, the fluid was completely absorbed (e) and no active polyps were detected with FFA or ICGA (f, g). The area of the BVNs was clearly reduced on OCT angiography (h)
Multimodal imaging of an eye with refractory polypoidal choroidal vasculopathy (PCV) before and after therapy. This 57-year-old man was diagnosed with refractory PCV in his right eye. He had previously received 12 intravitreal conbercept (IVC) injections in his right eye. The best-corrected visual acuity was 20/200 in his right eye. Optical coherence tomography (OCT) showed subretinal fluid, pigment epithelial detachment, and hyperreflective materials between the retinal pigment epithelium and Bruch’s membrane (a). Fluorescein angiography demonstrated early focal hyperfluorescence (b), while indocyanine green angiography (ICGA) revealed late leakage corresponding to the branching vascular networks (BVN) and polyps (c). After proton-beam irradiation and four monthly injections of IVC, reduced BVN and polyp areas were detected with ICGA (c, g) and OCT angiography (d, h); the fluid was completely absorbed (e)
Polypoidal Lesion Changes on ICGA
At 3 months, the subretinal fluid was completely absorbed in 6 eyes (54.5%); the angiographic regression and closure rates of the polyps were 30% (6/20) and 60% (12/20), respectively (Table 3). At the final visit, the subretinal fluid was fully absorbed in 81.8% (9/11) of the patients, the polyps closure rate was 90% (18/20), and the complete polyp regression rate was 60% (12/20). The areas of BVNs and polyps decreased by 37.2% and 72.3%, respectively. There were 20 active polyps of 11 participants at baseline and two active polyps of two patients at final visit (shown in table 3 and table 4).
Visual Acuity Outcomes
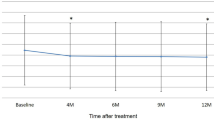
The mean BCVA was significantly improved at the final visit (61.55 ± 15.17 letters) compared with that at baseline (41.09 ± 10.94 letters) on the ETDRS chart (P = 0.006). The mean improvement in BCVA from baseline was 20 letters.
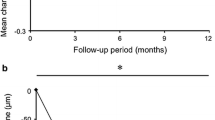
The mean CMT decreased significantly from 476.50 ± 123.63 μm to 317.70 ± 89.34 μm by 12 months (P = 0.004). The mean SFCT decreased from 211.90 ± 101.78 μm at baseline to 176.20 ± 86.89 μm at the final visit (P = 0.041). OCT revealed that new fluid appeared in two of the 11 patients (case 3 and case 9) at 12 months. Clinical features of eyes with PCV detected by OCT and OCTA before and after therapy are shown in Table 2.
Radiation-Related Changes
In total, five of the 11 eyes (45.5%) showed radiation-related changes during the 1-year period after their treatment. Radiation retinopathy occurred in five eyes, with microvascular telangiectasia. No other radiation papillopathy was observed in this study. Ff-ERG detected no obvious radiation-related retinal abnormalities.
Discussion
To our knowledge, this is the first prospective study of PBI combined with IVC for the treatment of refractory or recurrent PCV. Our results suggest that PBI promoted polyp regression or closure, reductions in CMT and the size of BVNs, and an improvement in BCVA, with a favorable safety profile, at the 12-month follow-up (Table 3).
Previous studies have shown that anti-VEGF monotherapy and a combination therapy with PDT are the optimal treatment options for patients with symptomatic PCV [3, 17]. However, refractory or recurrent PCV is not uncommon. In the EVEREST-II and PLANET studies, despite regular treatment, fluid persisted for over 12 months in 8.8% and 15% of patients, respectively [18, 19]. Patients with refractory or recurrent PCV require multiple injections, with the attendant risks entailed by the intravitreal injection procedure and exposure to anti-VEGF agents. Patients with persistent fluid may also develop resistance to the anti-VEGF therapy, including drug tolerance, tachyphylaxis, and changes in the neovascular architecture, which can diminish the therapeutic effects of the drug [3, 4, 20]. In patients with recurrent PCV, the defects associated with repeated sessions of PDT, including less favorable long-term vision, subretinal hemorrhage, choroidal ischemia, and retinal pigment epithelium (RPE) tear, cannot be ignored [21]. The lack of effective therapies and the heavy burden of treatment on these patients highlight the need for new therapeutic modalities (Table 4).
Radiation is a potentially beneficial modality for the treatment of macular CNV. SRT had been investigated as an optional treatment for exudative AMD [22, 23], and the SRT device used in the INTREPID study reduced the frequency of ranibizumab injections required and preserved good vision at 12 months [22]. Introini et al. subsequently used SRT in conjunction with ranibizumab to treat patients with PCV, with encouraging results [9]. The characteristics of the Bragg peak mean that the proton beam has the unique advantage of concentrating the maximum radiation dose within a designated target area, while minimizing the radiation to the surrounding tissues [24]. Preliminary evaluations of the treatment of macular CNV with PBI have demonstrated its beneficial effects and safety [10,11,12,25,26,27]. Here, we investigated, for the first time, the effects of PBI in patients with recurrent or refractory PCV. Because the therapeutic effects of PBI are not immediate [28], the concomitant use of IVC was expected to promote the rapid absorption of SRF and improve the visual outcomes in this study. Our encouraging results showed a mean improvement in BCVA of 20 letters from baseline, with the complete absorption of SRF in 81.8% of the patients, and 54.5% of the patients had required three or fewer injections at the 12-month follow-up.
Polyps closure or regression, assessed with ICGA, is a vital prognostic indicator. Limited complete polyp regression was an important cause of persistent fluid despite the current therapy, and also might be associated with the high recurrence rate during the long-term follow-up. In the present study, complete polyp regression rate of 60% (12/20) was achieved and 90% (18/20) of participants showed no active polyps on FFA and ICGA, and OCT imaging at the final visit. Our results suggest that PBI not only affects the polyps itself but also enhances the impact of the anti-VEGF agent on the polyps, which is important because both refractory and recurrent PCV frequently present with drug resistance. The possible mechanism by which PBI induces polyp regression or closure is thought to involve the inhibition of proliferating angiogenic cytokine-producing inflammatory cells, endothelial cells, and other cell types during lesion formation, as observed in AMD [15, 16]. As we know, proliferating choroidal endothelial cells, which are essential for the progression of PCV, are sensitive to proton radiation. It is noteworthy that inflammation is another driving force behind the progression of PCV, and may account for the resistance to anti-VEGF agents [29, 30]. The anti-inflammatory and antifibrotic effects of PBI could prevent the progression of polypoidal lesions and reduce complications [9]. Our results suggest that PBI not only exerts a synergistic effect with IVC by various mechanisms but also exerts unique effects on polyps lesions.
As we know, the BVNs from which polyps commonly sprout also provide nourishment for the polyps. However, BVNs usually persist or even gradually enlarge, which has been considered to correlate with recurrent polyps or persistent exudation [9, 18, 19, 31]. Arteriogenesis, the main driving force behind the development of BVNs, is not merely a VEGF-dependent process [32]. Therefore, the arteriogenesis of BVNs is one explanation for the lack of response to anti-VEGF treatments. Unlike previous studies, a novel finding of our study is that the area of BVNs was reduced to varying degrees in all eyes during the follow-up period. Compared with baseline, the area of BVNs had decreased by over 50% in 63.6% (7/11) of patients at the final visit. The exact mechanisms involved remain to be clarified. Radiation is considered to inhibit the proliferation of choroidal endothelial cells through the irreparable damage to DNA and proteins caused by reactive oxygen species [33]. We suspect that these shrunken BVNs prompt the regression of polyps and, more importantly, inhibit the recurrence of polyps in the long run.
In previous studies, PBI was used to treat CNV at doses ranging from 8 to 24 Gy, administered in one or two equal fractions, with radiation-related change rates of 15.7–48% [14]. On the basis of reported experiences with CNV [11], a dose of 14 Gy in one fraction was selected for PCV in this study. With this fractionation scheme, radiation retinopathy was observed in 5 eyes (45.5%), but no systemic adverse events were detected during the 1-year follow-up period. The discrepancies in these findings may be attributable to the different irradiation doses, strategies used, populations examined, and follow-up periods.
The limitations of this study were its noncomparative design, small sample size, and short-term follow-up. Although more than half the patients showed a greater than 50% reduction in the area of BVNs after therapy, the change was not statistically significant, which is attributed to the small sample size. It was also difficult to adjust the size or position of the PBI spot used, which was 8 mm in diameter and centered on the fovea. The dose was uniform to within 5% over the 8-mm-diameter circle. A size limit of 6 mm allows for alignment uncertainty. Further studies are required to investigate the optimal therapeutic dose of PBI and its optimal timing, and the best types and frequency of anti-VEGF therapy, for refractory or recurrent PCV. The diverse therapeutic histories of the patients in our study could also have affected the efficacy of PBI. Head-to-head comparisons between anti-VEGF monotherapy and its combination with PBI may be required to identify the most effective treatment for refractory or recurrent PCV. Further research is also required to confirm the long-term efficacy and safety of PBI therapy combined with IVC.
Conclusion
The results of our study suggest that PBI combined with IVC therapy causes polyp regression, reduces the area of BVNs, and improves visual acuity in patients with refractory or recurrent PCV. Therefore, PBI may be a useful adjuvant therapy for these patients.
References
Yannuzzi LA, Sorenson J, Spaide RF, et al. Idiopathic polypoidal choroidal vasculopathy (IPCV). 1990. Retina. 2012;32:1–8.
Cheung C, Lai T, Ruamviboonsuk P, et al. Polypoidal choroidal vasculopathy: definition, pathogenesis, diagnosis, and management. Ophthalmology. 2018;125:708–24.
Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453–64.
Koh A, Lai T, Takahashi K, et al. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol. 2017;135:1206–13.
Kawashima Y, Oishi A, Tsujikawa A, et al. Effects of aflibercept for ranibizumab-resistant neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253:1471–7.
Saito M, Kano M, Itagaki K, et al. Switching to intravitreal aflibercept injection for polypoidal choroidal vasculopathy refractory to ranibizumab. Retina. 2014;34:2192–201.
Wong CW, Cheung CM, Mathur R, et al. Three-year results of polypoidal choroidal vasculopathy treated with photodynamic therapy: retrospective study and systematic review. Retina. 2015;35:1577–93.
Kang HM, Kim YM, Koh HJ. Five-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013;155:438-447.e1.
Introini U, Casalino G, Triolo G, et al. Stereotactic radiotherapy for polypoidal choroidal vasculopathy: a pilot study. Ophthalmologica. 2015;233:82–8.
Zambarakji HJ, Lane AM, Ezra E, et al. Proton beam irradiation for neovascular age-related macular degeneration. Ophthalmology. 2006;113:2012–9.
Moyers MF, Galindo RA, Yonemoto LT, et al. Treatment of macular degeneration with proton beams. Med Phys. 1999;26:777–82.
Yonemoto LT, Slater JD, Friedrichsen EJ, et al. Phase I/II study of proton beam irradiation for the treatment of subfoveal choroidal neovascularization in age-related macular degeneration: treatment techniques and preliminary results. Int J Radiat Oncol Biol Phys. 1996;36:867–71.
Adams JA, Paiva KL, Munzenrider JE, et al. Proton beam therapy for age-related macular degeneration: development of a standard plan. Med Dosim. 1999;24:233–8.
Flaxel CJ, Friedrichsen EJ, Smith JO, et al. Proton beam irradiation of subfoveal choroidal neovascularisation in age-related macular degeneration. Eye (Lond). 2000;14:155–64.
Kirwan JF, Constable PH, Murdoch IE, et al. Beta irradiation: new uses for an old treatment: a review. Eye (Lond). 2003;17:207–15.
Finger PT, Gelman YP, Berson AM, et al. Palladium-103 plaque radiation therapy for macular degeneration: results of a 7-year study. Br J Ophthalmol. 2003;87:1497–503.
Teo K, Yanagi Y, Lee SY, et al. Comparison of optical coherence tomography angiographic changes after anti-vascular endothelial growth factor therapy alone or in combination with photodynamic therapy in polypoidal choroidal vasculopathy. Retina. 2018;38:1675–87.
Takahashi K, Ohji M, Terasaki H, et al. Efficacy and safety of ranibizumab monotherapy versus ranibizumab in combination with verteporfin photodynamic therapy in patients with polypoidal choroidal vasculopathy: 12-month outcomes in the Japanese cohort of EVEREST II study. Clin Ophthalmol. 2018;12:1789–99.
Lee WK, Iida T, Ogura Y, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial. JAMA Ophthalmol. 2018;136:786–93.
Wykoff CC, Brown DM, Maldonado ME, et al. Aflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial). Br J Ophthalmol. 2014;98:951–5.
Cheung C, Gan A, Yanagi Y, et al. Association between choroidal thickness and Drusen subtypes in age-related macular degeneration. Ophthalmol Retina. 2018;2:1196–205.
Jackson TL, Chakravarthy U, Kaiser PK, et al. Stereotactic radiotherapy for neovascular age-related macular degeneration: 52-week safety and efficacy results of the INTREPID study. Ophthalmology. 2013;120:1893–900.
Jackson TL, Chakravarthy U, Slakter JS, et al. Stereotactic radiotherapy for neovascular age-related macular degeneration: year 2 results of the INTREPID study. Ophthalmology. 2015;122:138–45.
Ciulla TA, Danis RP, Klein SB, et al. Proton therapy for exudative age-related macular degeneration: a randomized, sham-controlled clinical trial. Am J Ophthalmol. 2002;134:905–6.
Chen L, Kim IK, Lane AM, et al. Proton beam irradiation for non-AMD CNV: 2-year results of a randomised clinical trial. Br J Ophthalmol. 2014;98:1212–7.
Dugel PU, Petrarca R, Bennett M, et al. Macular epiretinal brachytherapy in treated age-related macular degeneration: MERITAGE study: twelve-month safety and efficacy results. Ophthalmology. 2012;119:1425–31.
Osmanovic S, Moisseiev E, Mishra KK, et al. Phase I/II randomized study of proton beam with anti-vascular endothelial growth factor for exudative age-related macular degeneration: one-year results. Ophthalmol Retina. 2017;1(3):217–26.
Nakashizuka H, Mitsumata M, Okisaka S, et al. Clinicopathologic findings in polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008;49:4729–37.
Kumar S, Nakashizuka H, Jones A, et al. Proteolytic degradation and inflammation play critical roles in polypoidal choroidal vasculopathy. Am J Pathol. 2017;187:2841–57.
Maruyama-Inoue M, Sato S, Yamane S, Kadonosono K. Intravitreal injection of aflibercept in patients with polypoidal choroidal vasculopathy: a 3-year follow-up. Retina. 2018;38:2001–9.
Dansingani KK, Gal-Or O, Sadda SR, Yannuzzi LA, Freund KB. Understanding aneurysmal type 1 neovascularization (polypoidal choroidal vasculopathy): a lesson in the taxonomy of ’expanded spectra’- a review. Clin Exp Ophthalmol. 2018;46:189–200.
Coscas G, Lupidi M, Coscas F, et al. Toward a specific classification of polypoidal choroidal vasculopathy: idiopathic disease or subtype of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:3187–95.
Kishan AU, Modjtahedi BS, Morse LS, et al. Radiation therapy for neovascular age-related macular degeneration. Int J Radiat Oncol Biol Phys. 2013;85:583–97.
Acknowledgements
The authors would like to thank all the participants and staff for their valuable contributions to this research.
Funding
This study, including the journal’s Rapid Service Fees, was supported by Clinical Research Plan of Shanghai Hospital Development Center (SHDC2020CR2041B), Science and Technology Commission of Shanghai Municipality (20Y11911100).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Gezhi Z. Xu: concept and design, critical revision for important intellectual content; Jiade D. Lu: concept and design, critical revision for important intellectual content; Jingli Guo: proton-beam irradiation therapy and statistical analysis; Jiade D. Lu: data analysis and manuscript preparation; Jiayao Y. Sun: proton-beam irradiation therapy; Lin Kong: concept and design, critical revision for important intellectual content; Michael F. Moyers: concept and design, critical revision for important intellectual content; Wei Ren: proton-beam irradiation therapy; Wei Lu: concept and design, data acquisition, critical revision for important intellectual content; Wenyi Y. Tang: data analysis and manuscript preparation; Xianxin X. Qiu: data acquisition; Ying Xing: proton-beam irradiation therapy.
Disclosures
Jingli Guo, Xianxin Qiu, Wenyi Tang, Gezhi Xu, Michael. F. Moyers, Wei Ren, Ying Xing, Jin Gao, Jiayao Sun, Jiade Lu, Lin Kong and Wei Liu all confirm that they have nothing to declare.
Compliance with Ethics Guidelines
The study protocol was approved by the institutional review board. All procedures were performed in compliance with the principles of the Declaration of Helsinki. Written informed consent was obtained from every patient.
Data Availability
All data will be made available on request.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Guo, J., Qiu, X., Tang, W. et al. One-Year Efficacy and Safety of Proton-Beam Irradiation Combined with Intravitreal Conbercept for Refractory or Recurrent Polypoidal Choroidal Vasculopathy: A Pilot Study. Ophthalmol Ther 11, 187–199 (2022). https://doi.org/10.1007/s40123-021-00409-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-021-00409-3