Abstract
Introduction
The EyeCOPE study characterized noninfectious intermediate posterior, or panuveitis (NIIPPU) before biologic agents were widely available.
Methods
This retrospective, observational study included adults with NIIPPU attending a routine ophthalmological visit. Data were collected from the study visit and medical records.
Results
Of 565 patients, 58.8% were female, and the mean age was 41.3 years; 33.8% had idiopathic uveitis and 45.8% had panuveitis. The median time from symptom onset to diagnosis and treatment was 27.0 and 30.5 days, respectively. Patients received immunosuppressants and systemic/local corticosteroids. Most patients experienced substantial decline in ocular function (mean best corrected visual acuity, 0.4 logMAR). Mean total work productivity impairment among employed patients was 31.0%. Most patients reported ocular complications (70.8%) such as vision loss and cataracts.
Conclusions
Despite treatment, most patients with NIIPPU experienced a decline in ocular function and ocular complications. There is an unmet need for additional NIIPPU treatment, such as targeted monoclonal antibodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Noninfectious intermediate, posterior, or panuveitis (NIIPPU) can lead to high-risk ocular complications including glaucoma, macular edema, cataract, and vision loss; however, few studies have evaluated the epidemiological and clinical characteristics of NIIPPU and its associated economic burden. |
The EyeCOPE study aimed to characterize NIIPPU before biologic agents were a widely available treatment option. |
What was learned from this study? |
Of 565 patients included in the analysis, 34% had idiopathic uveitis and 46% had panuveitis. Most patients reported a decline in ocular function, and 71% experienced ocular complications; about 27% of patients were unemployed. |
The EyeCOPE study demonstrated the importance of early identification and treatment of patients with NIIPPU. |
The EyeCOPE study highlighted the unmet need for additional NIIPPU therapies, including the use of targeted monoclonal antibodies. |
Digital Features
This article is published with digital features, including a summary slide to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14541582.
Introduction
Uveitis refers to a group of intraocular inflammatory diseases classified by subtype according to etiology, anatomic location, disease course, severity, and the presence of underlying systemic diseases [1,2,3,4]. The etiology of the inflammatory process of uveitis is categorized as infectious, noninfectious, and masquerade [2, 5]. Infectious uveitis is specific, and the condition may be characteristic of the underlying infection; noninfectious uveitis is an immune-mediated inflammatory process, which is less well understood and may be multifactorial [5]. Masquerade syndromes do not result from immune-mediated uveitis [6]. Anatomically, uveitis has been classified as anterior, intermediate, posterior, or panuveitis by the Standardization of Uveitis Nomenclature (SUN) Working Group, and is defined by the primary site of inflammation [3]. Disease onset can be sudden or insidious, with symptoms described as acute, recurrent, or chronic [1]; the severity of inflammation is graded for cells and flare in the anterior chamber (AC), and vitreous haze (VH) for inflammation in the vitreous [3].
The epidemiology of uveitis varies widely depending on geographic location; however, it is a significant cause of visual blindness worldwide [7]. Despite the availability of effective treatment options, up to 35% of all patients with uveitis experience substantial visual impairment or legal blindness [8]. Noninfectious intermediate, posterior, or panuveitis (NIIPPU) is estimated to affect 23/100,000 people in the United States [9], and those with the condition are at a high risk of ocular complications including glaucoma, macular edema, cataract, and vision loss [10]. As recurring flares can lead to cumulative eye damage and increased risk of impaired vision [11], NIIPPU is associated with substantial economic burden driven by complications of the disease [12]. Although epidemiological data suggest that NIIPPU accounts for between 15 and 40% of noninfectious uveitis cases [7, 13], limited data on the epidemiology and disease characteristics for this patient population present a challenge in effective management of this condition, and increased availability of these data could assist in the development of successful management strategies and tackling unmet patient needs. To our knowledge, few studies evaluating the epidemiological and clinical characteristics of the disease and its associated economic burden have been published to date [12, 14]; here, we report a large-scale, retrospective, observational, multicenter, international study in patients with NIIPPU.
The EyeCOPE study aimed to characterize NIIPPU before biologic agents were a widely available treatment option for the disease, and the primary objective was to describe the demographic data (age, sex, race) and disease characteristics (anatomical and etiological) of patients with NIIPPU attending a routine clinical visit at ophthalmology centers of participating countries. Secondary objectives included collection of data relating to study site characteristics, time from NIIPPU symptom onset to diagnosis, NIIPPU treatment patterns, burden of disease based on healthcare resource utilization and patient-reported quality of life (QoL)/work productivity, and proportion of patients with previous/current ocular complications. This study was not designed to gather comprehensive safety data, and no safety data are reported.
Methods
Study Design and Participants
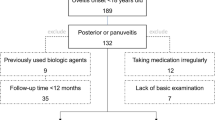
EyeCOPE was a retrospective, observational, multicenter, international study conducted in 14 countries across various regions, including Western and Eastern Europe, Latin America, and the Middle East. Participating sites were experienced in NIIPPU treatment, and researchers conducted the study in accordance with applicable legal and regulatory requirements. Treatment, procedures, and diagnostic methods followed physicians’ routine clinical practice. An enrollment period of approximately 12 months was planned.
Participants aged > 18 years with a diagnosis of NIIPPU who attended a routine clinical visit at one of the participating ophthalmological centers were eligible for inclusion. Those participating in an interventional clinical trial were excluded. Institutional review board/ethics committee approval was obtained, and patients voluntarily signed a patient authorization form to use and disclose personal health information (or informed consent, where applicable) to participate in the study. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Since this study was exploratory in nature and there were no pre-specified statistical hypotheses to test, a power calculation was not performed; however, it was assumed that approximately 500 patients would be sufficient to provide statistically stable estimates for the study endpoints. Owing to the purely observational nature of the EyeCOPE study, selection bias was controlled where possible.
Data Collection
Patient data were documented in an electronic data recording form (eDRF). Ophthalmological examinations, diagnostic measures, and findings and observations routinely performed in patients enrolled in this study were entered into the eDRF by study personnel, according to the research plan. Patient-reported outcome responses were transferred by study site personnel to the eDRF.
Study site characteristics collected included type of site; site location; average number of total patients, uveitis patients, and NIIPPU patients examined per year; number of ophthalmologists specializing in uveitis; number of certified ophthalmologists; and availability of optical coherence tomography (OCT) systems.
Patient data were collected during the single study visit and retrospectively from medical records. Demographic data included age, sex, race, employment status (paid employment, unemployed but seeking work, unemployed owing to disability, retired, student, sick leave), and previous/current occupation. Ophthalmological evaluations were conducted for the left and right eye and included number of eyes, best corrected visual acuity (BCVA; logMAR), AC cell grade, presence of active inflammatory chorioretinal and/or inflammatory retinal vascular lesions, VH grade, intraocular pressure, and central retinal thickness (CRT).
Patient-reported assessment of burden of disease was surveyed using three rating scales. Firstly, the EuroQoL five-dimension, five-level questionnaire (EQ-5D-5L; Supplementary Table 1) was used to assess mobility, self-care, usual activities, pain/discomfort, anxiety/depression, and self-rated (global) health status. Secondly, the Visual Function Questionnaire (VFQ-25; Supplementary Table 2) was employed to measure self-reported vision-targeted health status, including general vision/eye health; ocular pain; near/distance activities; driving, color, and peripheral vision; and vision-specific social functioning, mental health, role difficulties, and dependency. Finally, the Work Productivity and Activity Impairment–Uveitis 2.0 Questionnaire (WPAI-UV; Supplementary Table 3) was used to quantify the amount of absenteeism, presenteeism, and daily activity impairment attributable to uveitis.
NIIPPU-specific medical history, NIIPPU type (etiological/anatomical), previous and current ocular complications, NIIPPU-specific medications (previous and current use of predefined systemic and local corticosteroid therapies; local corticosteroids included eye drops, subtenon and subconjunctival injections, intravitreal injections, and intravitreal implants), healthcare resource utilization (visits for uveitis), and time from onset of NIIPPU symptoms to NIIPPU diagnosis and first treatment were also collected retrospectively from patient medical records, when available. NIIPPU flares (defined as AC cell grade ≥ 2+, or VH grade ≥ 2+ in ≥ 1 eye and new active inflammatory lesions [16]) were assessed.
Statistical Analyses
The analysis was based on the main analysis set (MAS), which comprised all enrolled patients who fulfilled inclusion criteria. All analyses were performed using SAS® version 9.4 software (SAS Institute Inc., Cary, NC, USA).
Participant characteristics were summarized using descriptive statistics (valid N, missing N, mean, standard deviation [SD], median, maximum [max], and minimum [min]). Two-sided 95% confidence intervals were calculated. Qualitative data are presented as absolute and relative means of frequency distributions.
NIIPPU-specific medication was analyzed for previous and current use with respect to the visit date. Other NIIPPU-specific medication was coded according to the World Health Organization Drug Dictionary including anatomical therapeutic chemical (ATC) classification up to level 4, and frequency tables were provided for NIIPPU-specific medication according to ATC system level 2. For this analysis, patients receiving > 1 medication at ATC level 2 were counted only once.
Healthcare resource utilization variables (number of consultations, visits, and days admitted to hospital) are presented on a monthly basis.
For BCVA, reported decimal notation was converted to logMAR notation using the visual acuity conversions listed in Supplementary Table 4. The following conversions were applied for the low-vision range: 2.0 logMAR, can count fingers; 2.3 logMAR, can perceive hand movement; and 2.6 logMAR, can perceive light; the lowest of the three values was used [15].
The transformed variables “time from onset of NIIPPU symptoms to NIIPPU diagnosis” and “time from onset of NIIPPU symptoms to first treatment” were calculated as the date of NIIPPU diagnosis minus the date of onset of NIIPPU symptoms and the date of first NIIPPU treatment minus the date of onset of NIIPPU symptoms, respectively.
For patient-reported burden of disease assessments, the EQ-5D-5L utility index was not computed if the weight of any dimension was missing, items left blank in the VFQ-25 were considered missing and not used to calculate scale scores, and outcomes utilizing missing items were not calculated for the WPAI-UV. No sensitivity analyses were planned.
Results
A total of 568 patients were enrolled in the EyeCOPE study, of whom 565 (99.5%) fulfilled the inclusion criteria and were included in the MAS population. Patients were enrolled between August 9, 2017, and June 15, 2018, from 48 sites across 14 countries (Argentina, Brazil, Chile, Colombia, Croatia, Czech Republic, Israel, Kazakhstan, Romania, Serbia, Slovakia, Switzerland, Turkey, and Ukraine). A list of participating sites is available in Supplementary Table 5.
Site Characteristics
Participating site characteristics and ophthalmologists’ specializations are summarized in Supplementary Tables 6 and 7, respectively. The majority of sites were academic centers or university hospitals (76.6%), and all were located within urban areas. On average, more than half of the sites had examined ≥ 10,000 patients with any ocular condition (57.4%), 100–999 patients with uveitis (52.2%), and at least 100 patients with NIIPPU (53.2%) per year. Overall, 91.7% of the sites had access to an OCT unit; the Spectralis® OCT system (Heidelberg Engineering) was the most common (39.6%). The mean (SD) number of certified ophthalmologists and ophthalmologists specializing in uveitis at each site was 13.4 (13.7) and 2.5 (2.2), respectively.
Patient Demographics
A summary of baseline demographics of patients with NIIPPU collected during the study visit is shown in Table 1. The majority of patients were female (58.8%) and White (79.3%), with a mean (SD) age of 41.3 (14.5) years. Of those in paid employment (58.2%), the majority were working full-time (89.7%); more than a quarter of patients (26.9%) were unemployed (unemployed but seeking work/homemaker, 15.4%; unemployed owing to disability, 11.5%), with other patients being retired (9.7%), students (6.2%), and/or on sick leave (5.3%; multiple categories possible). NIIPPU was cited as a reason for sick leave and unemployment due to disability in 83.3% and 70.8% of MAS patients, respectively. Patients reported manual (46.3%) and non-manual (53.7%) previous/current occupations.
Disease Characteristics
Baseline disease characteristics of patients with NIIPPU based on medical history are listed in Table 1. Physicians reported that both eyes were affected by NIIPPU in more than three quarters of patients (79.8%), with almost equal proportions being affected in the left (10.3%) or right (9.9%) eye only. The most frequently reported NIIPPU types were idiopathic (33.8%), Behçet disease (16.6%), and Vogt–Koyanagi–Harada disease (15.4%), and panuveitis was the most common anatomical location (45.8%). Forty-five percent of patients had known underlying systemic immune-mediated or systemic disease, among which Behçet disease (33.9%), Vogt–Koyanagi–Harada disease (28.3%), and sarcoidosis (13.0%) occurred in ≥ 10% of patients.
Ophthalmology Evaluations
Table 2 and Fig. 1 provide an overview of the ophthalmology evaluation data collected during the study. A small proportion of patients had had an eye removed (left eye, 1.1%; right eye, 1.2%). Mean BCVA (range) was 0.4 (−0.2 to 2.6) logMAR for both eyes. Mean intraocular pressure was 15.1 (range 1–50) mmHg for the left eye and 15.2 (range 2–40) mmHg for the right eye. Mean CRT was 269.4 (range 2.5–880.0) µm for the left eye and 264.1 (range 1.0–946.0) µm for the right eye (Table 2). For the majority of patients, an AC cell grade of 0 was reported (left eye, 79.4%; right eye, 80.5%; Fig. 1a). VH grade was 0 for the majority of patients (left eye, 61.8%; right eye, 64.7%; Fig. 1b). Active inflammatory chorioretinal and/or inflammatory retinal vascular lesions were documented for 14.4% (left eye) and 14.9% (right eye) of patients (Fig. 1c).
Summary of AC cell gradea (a), VH gradeb (b), and presence of active inflammatory chorioretinal and/or inflammatory retinal vascular lesionsb (c). MAS population with left (n = 559) and right (n = 558) eye. Patients with valid data were included and percentages were calculated based on non-missing values. aMissing data from one left eye. bMissing data from two left eyes. AC anterior chamber, MAS main analysis set, NEI National Eye Institute, SUN Standardization of Uveitis Nomenclature, VH vitreous haze
NIIPPU Management
NIIPPU management steps based on medical history are summarized in Table 3. A median (min, max) time from onset of NIIPPU to diagnosis and time from onset of NIIPPU to first treatment of 27.0 (−394, 5927) days and 30.5 (−394, 5945) days, respectively, was reported based on patient medical history. The negative data points indicate that some patients obtained NIIPPU diagnosis prior to symptom onset. For NIIPPU flares, a median (min, max) of 1.0 (0, 10) flare was recorded within the 12 months before the study visit, with a duration of 61.0 (0, 3748) days immediately preceding the current flare. A highest mean (SD) and median (range) daily dose of 95.2 (221.6) mg and 40.0 (0.0–1250.0) mg, respectively, of systemic prednisone or prednisone equivalent corticosteroid was administered during this flare. The median (min, max) time since onset of current NIIPPU flare was 54.0 (0, 4590) days.
NIIPPU Treatment
In total, 95.0% of patients with NIIPPU had previously received systemic treatment, based on medical history; prednisone (48.7%), azathioprine (30.3%), methylprednisolone (27.3%), cyclosporine A (24.6%), and methotrexate (21.8%) were the most common (Fig. 2a). A total of 66.5% of patients were currently taking systemic therapies; no patients reported the use of tacrolimus or leflunomide. Most patients (85.3%) had also previously taken local corticosteroids, with dexamethasone (51.0%) the most frequently administered (Fig. 2b). In addition, a total of 28.5% of patients were currently taking local corticosteroids.
Summary of treatment patterns in NIIPPU patients (MAS population). Systemic therapies (a) and topical and injectable corticosteroid therapies (b). Methods of application were eye drops, subtenon and subconjunctival injection, intravitreal injection, and intravitreal implant. Prednisone was used as topical only. Participants previously using systemic therapies, n = 565; local corticosteroid therapies, n = 565. Participants currently using systemic therapies, n = 565; local corticosteroid therapies, n = 565. a“Other” includes immunosuppressants (including immunobiologicals, e.g., monoclonal antibodies), corticosteroids for systemic use, immunostimulants, anti-inflammatory and anti-rheumatic products, anti-anemic preparations, anti-diarrheals, intestinal anti-inflammatory/anti-infective agents, anti-gout preparations, ophthalmologicals, anti-bacterials for systemic use, drugs for acid-related disorders, anti-neoplastic agents, and anti-protozoals. b“Other” includes ophthalmologicals (prednisolone acetate, triamcinolone acetonide, triamcinolone, fluorometholone, betamethasone diproprionate, betamethasone and chloramphenicol, loteprednol etabonate, prednisolone, rimexolone, dexamethasone, loteprednol, difluprednate, betabioptal, dexamethasone sodium phosphate, methylprednisolone acetate). MAS main analysis set; NIIPPU noninfectious intermediate, posterior, or panuveitis
Healthcare Resource Utilization
The burden of disease based on healthcare resource utilization from medical history (Supplementary Tables 8 and 9) and patient-reported quality of life and work status/productivity (Supplementary Table 10) are summarized. Most of the patients (94.0%) used a healthcare resource at least once in the 12 months preceding enrollment (Supplementary Table 8). The mean number of visits to a healthcare professional per month was 0.5 (range 0.1–3.3), mean number of visits to the emergency room per month was 0.1 (range 0.1–1.7), and mean number of days spent in the hospital per month was 1.0 (range 0.1–4.3; Supplementary Table 9). Based on the EQ-5D-5L questionnaire, health-related quality of life was rated as “rather good,” with a mean utility index of 0.8 (range: −0.1 to 1.0), and mean self-rated global health status of 71.7 (range 0–100; Supplementary Table 10). Approximately half of patients (54.5%) were in paid employment; the mean total work productivity impairment among employed patients (n = 277) was 31.0%, ranging from 0 to 100%. Total activity impairment was assessed in 557 patients, irrespective of their employment status; mean total activity impairment was 32.0%, ranging from 0 to 100%.
Ocular Complications
Over three quarters (77.7%) of NIIPPU patients reported previous ocular complications, based on medical history; macular edema (52.2%), cataract (49.7%), and vision loss (45.1%) were the most common (Fig. 3). Current ocular complications were reported by 70.8% of patients, with vision loss (41.0%), cataract (40.8%), and macular edema (32.3%) the most common (Fig. 3).
Discussion
This retrospective, observational study provides a comprehensive and detailed overview of the baseline demographics and disease characteristics of an international, adult patient population diagnosed with NIIPPU attending a routine clinical visit at ophthalmology centers of participating countries. Over the planned 12-month enrollment period, data were collected from a total of 565 patients with NIIPPU.
In line with previously described demographics, there was a slight female preponderance in the current study population, and mean patient age reflected the typical age profile of this disease (between 20 and 50 years of age) [7, 11, 14, 17]. Although most patients were below the age of retirement, 26.9% were unemployed, and among those patients unemployed owing to disability, the majority retired early because of NIIPPU. To put these figures into perspective, the U.S. National Bureau of Labor Statistics cited an unemployment rate of 3.7% for 2019 in the general population [18], which is considerably lower than in the patient population in this study; however, because of differences in population numbers and definitions of unemployment, comparisons should be interpreted with caution.
A defined NIIPPU etiology was reported for approximately two thirds of patients, most commonly Behçet disease and Vogt–Koyanagi–Harada disease. Prevalence of Behçet disease and Vogt–Koyanagi–Harada disease can vary depending on geographic region. The baseline disease characteristics in this study were consistent with previously reported high prevalence of Behçet disease in Turkey and Vogt–Koyanagi-Harada disease in the Middle East [19, 20]. The remainder of cases were reported as idiopathic. Although the etiology of uveitis varies among studies, the high prevalence of idiopathic uveitis is consistent [14, 16, 17, 21,22,23]. In the EyeCOPE study, panuveitis was the most frequently reported anatomic location, followed by intermediate and posterior uveitis, and similar findings were observed in a study of patients with uveitis in northern California [24]; however, the order of prevalence varies in other published work [7, 14, 25]. The variability of etiology by geography, coupled with variations in genetics and environmental factors within patient populations, may account for the observed differences in prevalence [7].
Many patients visited their ophthalmologist promptly after the onset of symptoms; however, an ensuing lag time between symptom onset and a NIIPPU diagnosis and subsequent treatment initiation was observed. This is possibly due to a lack of inflammatory signs when seeking healthcare, and thus no need for treatment, or a lack of disease awareness by ophthalmologists, as indicated by the relatively low numbers of ophthalmologists specializing in uveitis at participating sites. Shorter intervals are warranted, though the exact clinical impact cannot be fully appreciated. A few aberrant patient outliers were noted during these analyses and are likely due to one patient being treated after more than 16 years (5945 days) after initial diagnosis and others receiving a diagnosis of NIIPPU prior to symptom onset. The causes in each of these cases warrant further investigation.
Despite previous and/or current use of treatment, the majority of patients experienced a substantial decline in ocular function during the course of the disease, as reflected in the mean BCVA (logMAR, 0.4) in the current study. This decline may result from treatment-related adverse events or unmet treatment needs; for example, 32.3% of patients reported a current complication of macular edema, the recalcitrant nature of which may explain the observed decline in mean BCVA. In the MUST clinical trial follow-up, only 15.2% and 7.3% of patients with the implant or systemic treatment, respectively, had macular edema at 7 years [26]. However, differences in inclusion criteria and geographic locations compared with the current study may have contributed to differences in the numbers of patients with ocular complications. Furthermore, based on the ophthalmologic examinations performed, disease activity appeared to be well controlled in the majority of cases at the time of study visit, with an absence of AC cells in most patients, and only a few patients with chorioretinal lesions or VH.
NIIPPU is typically treated by immunosuppression throughout the course of the disease, initially with high-dose corticosteroids, followed by lower doses for chronic treatment. The often long-term disease course of NIIPPU requires immunosuppressive agents for either an additive effect or steroid-sparing effect, while keeping corticosteroid dosage preferably at ≤ 7.5 mg prednisone (or equivalent) [27, 28]. Inability to reduce corticosteroid dose despite one or more immunosuppressive agents supports the use of biologic therapy in NIIPPU. In some situations it may even raise the question of whether biologic agents should be used as first-line treatment [28]. Not unexpectedly, data collected during this study showed that patients with NIIPPU continued to experience ocular complications despite receiving NIIPPU-specific corticosteroids, which could have been a result of the adverse effects associated with corticosteroid treatment. In addition, some diseases (e.g., Behçet disease) are corticosteroid-resistant and require early intervention for a favorable outcome [28]. In the current study, NIIPPU treatment consisted predominantly of systemically or locally administered corticosteroids. Patients also reported using nonsteroidal immunosuppressants such as azathioprine and immunobiological drugs (e.g., monoclonal antibodies), although the percentage of patients receiving immunosuppressants for treatment of NIIPPU was lower than expected for patients with posterior uveitis. Interestingly, greater use of azathioprine was observed in this study compared with methotrexate. Although a recent clinical study reported that methotrexate and mycophenolate mofetil had similar effects in patients with NIIPPU [29], there may be geographic and population differences (ie, incidence of Behçet disease and Vogt–Koyanagi–Harada disease) that contributed to differences in the preferred treatment. Specifically, Behçet disease has been associated with a poor functional prognosis and may be difficult to treat [30]. Azathioprine has been demonstrated to be effective in patients with Behçet disease and may be used as a steroid-sparing agent [31].
Steroid-induced systemic and ocular adverse effects (e.g., increased intraocular pressure) are well documented and limit their potential for long-term application [27, 32]. In an effort to address this limitation, the MUST trial compared the effectiveness of systemic therapy with corticosteroids (plus immunosuppression when necessary) versus implant therapy (fluocinolone acetonide) in 255 patients with NIIPPU [33]. The implant approach was designed to avoid systemic side effects, but while the two treatments produced comparable improvements in visual acuity over 24 months, there were minimal differences in systemic adverse events between groups [33]. The incidence of ocular complications was higher in the implant group than with systemic therapy, with the occurrence of cataracts in the systemic treatment group (44.9%) consistent with that observed in the current study (40.8%) [33]. As such, the use of nonsteroidal NIIPPU therapies is increasingly recommended, including conventional immunosuppressive therapies (antimetabolites, calcineurin inhibitors, and alkylating agents [27]) and monoclonal antibodies (e.g., anti-tumor necrosis factor-α agents [28, 34,35,36]), which may control multiple signs of uveitic inflammation [37]. In this retrospective study, biologics were not a widely available treatment option in the patient population, particularly for those patients with disease duration > 5 years. Because recurring flares are associated with visual impairment [10], the median number of flares in a 12-month period reported in this study reaffirms the unmet need for long-term effective treatments.
Patient-assessed quality of life and visual function was rated “rather good,” but general health was rated less favorably, possibly reflecting the patients’ awareness of experiencing a chronic disease that may require long-term medical intervention. Patients receiving treatment for chronic health conditions may report a lower quality of life, which can be exacerbated by comorbid conditions. A cross-sectional study of patients aged ≥ 21 years with diabetes showed that those with diabetes-related eye conditions reported a lower quality of life in the physical health domain than those with diabetes but no eye conditions (physical component summary: 37.6 vs. 42.4, respectively) [38]. In addition, after controlling for a large number of variables, patients with diabetes-related comorbid eye conditions were significantly more likely to require polypharmacy (defined as ≥ 6 medication classes; odds ratio = 1.27; p = 0.006) than those with diabetes but no eye conditions [38]. According to the WPAI, more than half of patients (54.5%) enrolled in EyeCOPE were in paid employment; however, a discrepancy in the number of employed patients was observed between the answers provided in the employment section of the eDRF compared with the WPAI. There may be several reasons for this discrepancy: (1) the employment section was assessed by the physician, while the WPAI was assessed by the patient; (2) the physician and patient interpreted employment differently; and (3) the employment section provided more differentiated answer options.
The large patient population and international design of this study aim to ensure a comprehensive overview of patient characteristics in real-life conditions; as such, we believe these results are generalizable to the overall population. In terms of age and sex distribution, the observed patient population appears to reflect the known epidemiological characteristics of NIIPPU; however, despite the international design of the study, most patients were White, and comparisons with other ethnic groups should be made with caution.
A few limitations are associated with this study. Firstly, a prospective, longitudinal study may have yielded a more robust methodology compared with the observational, retrospective design of the study reported here. Secondly, the majority of patients were recruited from large academic sites in urban areas; therefore, a bias toward a patient population with better access to optimal NIIPPU treatment is likely. Furthermore, variations in disease management across widely different geographic sites may affect outcomes. Finally, self-reported outcomes are inherently prone to self-presentational and recall bias.
NIIPPU is a severe disease affecting mainly adults of working age and is associated with a large number of complications. Demographic data collected during the EyeCOPE study were largely in line with published epidemiologic data. Although most patients were diagnosed and treated soon after the initial appearance of NIIPPU symptoms, efforts should be made to reduce the time not only between diagnosis and steroid treatment, but also to the introduction of first-line immunosuppression. The substantial decline in ocular function observed in the majority of patients with NIIPPU in the EyeCOPE study, despite administration of systemic treatment, may be due to inadequate treatment, such as failure to administer immunosuppressant agents in a timely manner, persistent inflammation despite treatment in some patients, and corticosteroid-related complications. Ocular complications may persist even when inflammation is controlled, and can impact employment.
Conclusion
In summary, the results from the EyeCOPE study highlight the need to raise awareness of NIIPPU among ophthalmologists in order to identify and treat patients earlier, and to use nonsteroidal long-term management strategies, including monoclonal antibodies that target the root cause of NIIPPU.
References
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–16.
Deschenes J, Murray PI, Rao NA, Nussenblatt RB, International Uveitis Study Group. International Uveitis Study Group (IUSG): clinical classification of uveitis. Ocul Immunol Inflamm. 2008;16(1):1–2.
Zierhut M, Deuter C, Murray PI. Classification of uveitis – current guidelines. Eur Ophthalmic Rev. 2007;77–8.
Read RW. Uveitis: advances in understanding of pathogenesis and treatment. Curr Rheumatol Rep. 2006;8(4):260–6.
Karkhur S, Hasanreisoglu M, Vigil E, et al. Interleukin-6 inhibition in the management of non-infectious uveitis and beyond. J Ophthalmic Inflamm Infect. 2019;9(1):17–30.
Grange LK, Kouchouk A, Dalal MD, et al. Neoplastic masquerade syndromes in patients with uveitis. Am J Ophthalmol. 2014;157(3):526–31.
Engelhard SB, Patel V, Reddy AK. Intermediate uveitis, posterior uveitis, and panuveitis in the Mid-Atlantic USA. Clin Ophthalmol. 2015;9:1549–55.
Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80(4):332–6.
Thorne JE, Suhler E, Skup M, et al. Prevalence of noninfectious uveitis in the United States: a claims-based analysis. JAMA Ophthalmol. 2016;134(11):1237–45.
Dick AD, Tundia N, Sorg R, et al. Risk of ocular complications in patients with noninfectious intermediate uveitis, posterior uveitis, or panuveitis. Ophthalmology. 2016;123(3):655–62.
Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88(9):1159–62.
Chu DS, Johnson SJ, Mallya UG, Davis MR, Sorg RA, Duh MS. Healthcare costs and utilization for privately insured patients treated for non-infectious uveitis in the USA. J Ophthalmic Inflamm Infect. 2013;3(1):64–74.
Barisani-Asenbauer T, Maca SM, Mejdoubi L, Emminger W, Machold K, Auer H. Uveitis—a rare disease often associated with systemic diseases and infections—a systematic review of 2619 patients. Orphanet J Rare Dis. 2012;7:57.
Lopalco G, Venerito V, Sota J, et al. Epidemiological profile of non-infectious uveitis from the rheumatologist’s perspective: a survey from two tertiary referral centres in Italy. Clin Exp Rheumatol. 2018;36(6 suppl 115):68–73.
Ischemic Optic Neuropathy Decompression Trial Research Group. Ischemic optic neuropathy decompression trial: twenty-four-month update. Arch Ophthalmol. 2000;118(6):793–8.
Jaffe GJ, Dick AD, Brezin AP, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375(10):932–43.
Hosseini SM, Shoeibi N, Ebrahimi R, Ghasemi M. Patterns of uveitis at a tertiary referral center in Northeastern Iran. J Ophthalmic Vis Res. 2018;13(2):138–43.
U.S. Bureau of Labor Statistics. Employment Situation Summary. https://www.bls.gov/news.release/empsit.nr0.htm. Accessed 14 Feb 2020.
Azizlerli G, Kose AA, Sarica R, et al. Prevalence of Behcet’s disease in Istanbul. Turkey Int J Dermatol. 2003;42(10):803–6.
Cunningham ET Jr, Rathinam SR, Tugal-Tutkun I, Muccioli C, Zierhut M. Vogt-Koyanagi-Harada disease. Ocul Immunol Inflamm. 2014;22(4):249–52.
Nguyen QD, Merrill PT, Jaffe GJ, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet. 2016;388(10050):1183–92.
Goto H, Mochizuki M, Yamaki K, Kotake S, Usui M, Ohno S. Epidemiological survey of intraocular inflammation in Japan. Jpn J Ophthalmol. 2007;51(1):41–4.
Ohguro N, Sonoda KH, Takeuchi M, Matsumura M, Mochizuki M. The 2009 prospective multi-center epidemiologic survey of uveitis in Japan. Jpn J Ophthalmol. 2012;56(5):432–5.
Gritz DC, Wong IG. Incidence and prevalence of uveitis in northern California: the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491–500.
Oruc S, Kaplan AD, Galen M, Kaplan HJ. Uveitis referral pattern in a Midwest university eye center. Ocul Immunol Inflamm. 2003;11(4):287–98.
Writing Committee for the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study Research Group, Kempen JH, Altaweel MM, et al. Association between long-lasting intravitreous fluocinolone acetonide implant vs systemic anti-inflammatory therapy and visual acuity at 7 years among patients with intermediate, posterior, or panuveitis. JAMA. 2017;317(19):1993–2005.
Jabs DA. Immunosuppression for the uveitides. Ophthalmology. 2018;125(2):193–202.
Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. 2014;121(3):785-96e3.
Rathinam SR, Gonzales JA, Thundikandy R, et al. Effect of corticosteroid-sparing treatment with mycophenolate mofetil vs methotrexate on inflammation in patients with uveitis: a randomized clinical trial. JAMA. 2019;322(10):936–45.
Esatoglu SN, Hatemi G. Update on the treatment of Behcet’s syndrome. Intern Emerg Med. 2019;14(5):661–75.
Saadoun D, Wechsler B, Terrada C, et al. Azathioprine in severe uveitis of Behcet’s disease. Arthritis Care Res (Hoboken). 2010;62(12):1733–8.
Becker MD, Smith JR, Max R, Fiehn C. Management of sight-threatening uveitis: new therapeutic options. Drugs. 2005;65(4):497–519.
Kempen JH, Altaweel MM, Holbrook JT, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118(10):1916–26.
Cordero-Coma M, Salom D, Diaz-Llopis M, Lopez-Prats MJ, Calleja S. Golimumab for uveitis. Ophthalmology. 2011;118(9):1892e3-4.
Cordero-Coma M, Sobrin L. Anti-tumor necrosis factor-alpha therapy in uveitis. Surv Ophthalmol. 2015;60(6):575–89.
Goto H, Zako M, Namba K, et al. Adalimumab in active and inactive, non-infectious uveitis: global results from the VISUAL I and VISUAL II trials. Ocul Immunol Inflamm. 2018;27(1):40–50.
Suhler EB, Adan A, Brezin AP, et al. Safety and efficacy of adalimumab in patients with noninfectious uveitis in an ongoing open-label study: VISUAL III. Ophthalmology. 2018;125(7):1075–87.
Meraya AM, Alwhaibi M. Health related quality of life and healthcare utilization among adults with diabetes and kidney and eye complications in the United States. Health Qual Life Outcomes. 2020;18(1):85–95.
Acknowledgements
The authors thank all patients for their participation in this study.
Funding
This study and the journal’s Rapid Service Fee was supported by AbbVie Inc.
Medical Writing Assistance
The authors thank all study investigators. Medical writing assistance, under the direction of the authors, was provided by Adele Blair, PhD, of CMC AFFINITY, McCann Health Medical Communications, in accordance with Good Publication Practice (GPP3) guidelines. This assistance was funded by AbbVie Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
All authors were involved in the conception of the study, data analysis and interpretation, and manuscript writing. All authors approved the final version of the manuscript.
List of Investigators
International EyeCOPE Study Group principal investigators: Ayse Cigdem Altan, Radgonde Amer, Matthias Becker, Florian Balta, Daniel Branisteanu, Gustavo Budmann, Thiago George Cabral Silva, Muge Pinar Cakar Ozdal, Sonja Cekic, Alina Popa Cherecheanu, Carmen Chiotan, Vivian Cristina Costa Afonso, Cristóbal Couto, Ciprian Danielescu, Daniel Vitor de Vasconcelos Santos, Assel Doshakanova, Luis Jose Escaf, Jarmila Fabiánová, Justus Gerhard Garweg, Aida Geamanu, Tamar Hareuveni, Jarmila Heissigerová, Aleksandra Ilic, Svetlana Jovanovic, Sanda Jurja, Sibel Kadayifcilar, Esra Kardes, Tea Čaljkušić Mance, Hernando Antonio Muñeton Abadia, Ana Oros, Hilal Eser Ozturk, Yilmaz Ozyazgan, Aleksandra Radosavljevic, Carlos Mario Rangel, Ali Osman Saatci, Ariel Schlaen, Maja Vinković, Nenad Vukojević, Fatime Nilufer Yalcindag, Melike Balikoglu Yilmaz, Suzan Guven Yilmaz, Oleksandra Zborovska, and Gordana Zlatanovic. International EyeCOPE Study Group sub-investigators: Halil Ateş, Berna Başarır, Bora Eldem, Sandra Garcia, Patricio Gerardo Schlottmann, Juan Guillermo Gaviria, Mahmut Kaya, Sandra Lortz, Angelica Maria Prada, Livia Melcioiu, Luiz Guilherme Marchesi Mello, Merih Oray, Sarah Pereira de Freitas Cenachi, Emine Temel, Didar Uçar, Tamara Mišljenović Vučerić, Yuslay Fernández Zamora, and Cristina Zorila.
Disclosures
Michaela Brichova: Personal fees—AbbVie Czech Republic (advisory boards). Ilknur Tugal-Tutkun: Personal fees—AbbVie Turkey (consulting, lectures). Mykola Panchenko: Speaker for AbbVie. Natali Gormezano: Employee of AbbVie Inc., São Paulo, Brazil. Franziska Koenigsbauer: Employee of AbbVie Deutschland GmbH & Co. KG, Ludwigshafen, Germany. Pablo Franco: Personal fees—Novartis Argentina, Bayer Argentina, and AbbVie Argentina. Cristina Muccioli, Murat Hasanreisoglu and Michal Kramer declare they have nothing to disclose.
Compliance with Ethics Guidelines
Institutional review board/ethics committee approval was obtained (Supplementary Table 11). Patients voluntarily signed a patient authorization form to use and disclose personal health information (or informed consent, where applicable) to participate in the study. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The members of the International EyeCOPE Study Group are mentioned in the Acknowledgments section
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kramer, M., Brichova, M., Tugal-Tutkun, I. et al. Noninfectious Intermediate, Posterior, or Panuveitis: Results from the Retrospective, Observational, International EyeCOPE Study. Ophthalmol Ther 10, 565–580 (2021). https://doi.org/10.1007/s40123-021-00351-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-021-00351-4