Abstract
Introduction
Fibrosis is one of the factors contributing to the development of primary acquired lacrimal duct obstruction (LDO). LIGHT (homologous to lymphotoxins, exhibiting inducible expression and competing with herpes simplex virus glycoprotein D for herpes virus entry mediator [HVEM]), a receptor expressed by T lymphocytes, has recently emerged as a new regulator of connective tissue remodeling and fibrotic response. The purpose of this study was to evaluate the role of LIGHT in the pathogenesis of LDO through: (1) assessment of expression of LIGHT and its two receptors, HVEM and LTβR (lymphotoxin β receptor), and (2) investigation of potential relationships between expression of LIGHT and its receptors and clinical and histopathologic features.
Methods
Lacrimal sacs of 30 patients undergoing endoscopic dacryocystorhinostomy because of LDO were assessed intraoperatively and histopathologically with respect to inflammation and fibrosis. Expression of LIGHT, HVEM and LTβR was assessed by immunohistochemistry using specific antibodies and evaluated semiquantitatively using a four-grade scoring system.
Results
All investigated molecules, LIGHT/TNFSF14, HVEM and LTβR, were expressed in biopsies from all patients. The most prominent expression was seen within inflammatory infiltrates. Expression of LIGH, HVEM and LTβR correlated significantly with the intensity of fibrosis and duration of the disease. In multivariate analysis only LIGHT showed a significant relationship with fibrosis (β coefficient = 0.759, p = 0.02). There was no significant correlation between expression of any molecule and other demographic or clinical features.
Conclusion
We assume that LIGHT along with its receptors may be a factor contributing to fibrosis and synechiae formation in the lacrimal sac. This assumption needs to be proven in a future study in a group of patients who fail to improve after the first operation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Primary acquired lacrimal duct obstruction (LDO) is a relatively common problem, particularly in elderly people. |
Fibrosis is one of the factors contributing to the development of primary acquired lacrimal duct obstruction (LDO). |
LIGHT is a cytokine belonging to the tumor necrosis factor (TNF) superfamily of molecules (TNF superfamily member 14, TNFSF14), which stimulates two different receptors: herpes virus entry mediator (HVEM) and lymphotoxin beta receptor (LTβR). |
We found significant correlation between the expression of LIGHT and severity of fibrosis assessed histopathologically (Spearman's r = 0.82, p < 0.05). There was also significant correlation between expression of HVEM and LTβR and the severity of fibrosis (Spearman's r = 0.71 and 0.74, respectively; p < 0.05 for both correlations). |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13161401.
Introduction
Primary acquired lacrimal duct obstruction (LDO) is a relatively common problem, in particular in elderly people. Clinically, LDO is manifested by epiphora, continuous and uncontrolled tearing, persistent mucopurulent discharge from lacrimal canaliculi and recurrent abscess formation in the medial canthal region, negatively influencing patients' quality of life. Dacryocystorhinostomy (DCR) aims to restore the patency of the tear drainage pathway by bypassing its obstructed fragment, which is usually located in the naso-lacrimal duct or lacrimal sac [1]. In addition to the surgical method and operative technique, the final outcome of the operation depends on the underlying pathology of LDO [2, 3].
Usually LDO develops as a primary/idiopathic disease because of non-specific chronic inflammation with or without fibrosis [4,5,6,7,8]. Chronic inflammation is responsible for many epithelial and subepithelial changes in the lacrimal duct walls, leading to narrowing and finally total obstruction of the duct. On histopathologic examination of biopsies taken during DCR, remodeling of the walls of the lacrimal drainage system may take different forms. The most common finding is inflammatory cell infiltrations of variable intensity. These inflammatory infiltrations consist mainly of mononuclear cells such as macrophages and/or lymphocytes, which in some cases may form submucosal follicles and cause thickening of the lacrimal sac wall [9]. Another frequent finding is connective tissue remodeling, which in some patients leads to development of ambient fibrous tissue and scar formation [10]. Chronic tear retention and stasis may lead to secondary infection and abscess formation.
It has been shown that the type and intensity of remodeling of the lacrimal sac wall may influence the results of surgical treatment of DCO. In particular, intensive fibrosis found in lacrimal sac biopsies is considered a negative prognostic factor [7, 10, 11].
LIGHT (homologous to lymphotoxins, exhibiting inducible expression, and competing with herpes simplex virus [HSV] glycoprotein D for herpes virus entry mediator [HVEM], a receptor expressed by T lymphocytes) is a cytokine belonging to the tumor necrosis factor (TNF) superfamily of molecules (TNF superfamily member 14, TNFSF14), which stimulates two different receptors: herpes virus entry mediator (HVEM) and lymphotoxin beta receptor (LTβR). LIGHT is known to regulate the function of T cells [12]. Recently, LIGHT has been shown to play an important role in the regulation of connective tissue remodeling and development of fibrosis [13].
Despite growing evidence from animal experiments that emphasizes the role of the LIGHT-HVEM/LTβR axis in the development of fibrosis, there is little evidence regarding the expression of LIGHT and its receptors in tissues from fibrotic conditions in humans. We have recently shown that LIGHT and its two receptors (HVEM and LTβR) are overexpressed in the skin of patients with systemic sclerosis, which is considered a model of fibrotic conditions in humans [13]. The present study aimed to assess the expression of LIGHT and its two receptors, HVEM and LTβR, in the wall of lacrimal sacs removed during endoscopic DCR performed in patients with primary acquired lacrimal duct obstruction. Moreover, we attempted to correlate the expression of the investigated molecules with intraoperatively collected data assessing the severity of lacrimal sac fibrosis and with the histopathology of lacrimal sac biopsies.
Methods
Patients
Thirty consecutive patients who underwent primary endoscopic DCR because of lacrimal duct stenosis were included in the study. Previous lacrimal duct surgery, any injury of the lacrimal sac region that might result in subsequent lacrimal duct obstruction, diagnosis of specific granulomatous inflammation or tumors of the lacrimal duct and any symptoms of chronic or recurrent inflammatory disease of the sinonasal mucosa were considered as exclusion criteria in the present study.
Stenosis of the lacrimal duct was confirmed by nasolacrimal probing, irrigation test and dye disappearance test [14].
There were 10 men and 20 women (6 pre- and 14 postmenopausal) aged from 21 to 94 years (mean age: 61 ± 17 years). Duration of the disease (tearing) ranged from 5 weeks to 10 years (mean: 2.7 ± 2.3 years). Chronic mucopurulent discharge from lacrimal canaliculi was present in 18 (60%) patients.
Detailed patient characteristics are shown in Table 1.
Surgical Procedure and Macroscopic Assessment of Lacrimal Sacs
All patients were operated on using the same transnasal endoscopic technique. After removal of the bone covering the lacrimal sac with a bony rongeur and chisel, the medial wall of the sac was fully exposed. Then, the lacrimal probe was introduced through the inferior lacrimal punctum and inferior lacrimal canaliculus to the sac. Tenting of the medial wall of the sac with the probe facilitated incision performed under direct visualization with a 0° optic. A longitudinal incision of the medial wall of the sac starting from the fundus superiorly down to the level of the inferior turbinate and the beginning of nasolacrimal duct was made creating anterior and posterior flaps. The first one was reflected anteriorly to cover the bare bone of the anterior rim of the osteotomy while the posterior was harvested with micro-scissors for histopathologic examination. In cases with severe fibrosis when tenting of the medial wall was impossible because of total overgrowth of the sac with inflammatory or fibrotic tissue, sharp excision of the entire medial sac wall along with adherent tissue and synechiae in the ostium of the common canaliculus using a sickle knife and side biting forceps was performed. Then, all biopsy samples were placed in 4% buffered formalin and sent for histopathologic examination. Intraoperatively, clinical characteristics of the sac as a consequence of an inflammatory process and/or fibrosis were carefully inspected and described in detail. This was done macroscopically using a three-level grading system: grade 1, minor fibrotic changes of the lacrimal sac, stenosis, usually present only in the inferior part of the sac and nasolacrimal duct, and most of the sac having a normal appearance; grade 2, predominant mucosal swelling, erythema present all over the lumen of the lacrimal sac and minor to moderate fibrosis; grade 3, intense fibrosis with the entire sac wall replaced with dense fibrotic and scar tissue (Fig. 1).
Thin wall of the lacrimal sac; normal-looking lumen of the lacrimal sac; no/mild fibrosis. Part of the medial wall is being taken for histopathologic examination; the lacrimal probe is visible inside the nose (right). The thick wall of the sac; the lumen of the sac is still present but partially filled with inflammatory/fibrotic tissue—moderate fibrosis (center). Complete overgrowth of the lumen of the lacrimal sac with inflammatory and fibrotic tissue. Tenting of the medial wall of the sac with the lacrimal probe is not possible (left)
Histopathologic Examination
Lacrimal sac biopsies were placed in 4% buffered formalin immediately after surgery and fixed for 24 h. Then, samples were embedded in paraffin according to the standard protocol. Thereafter, 3-µm tissue slides were obtained for histopathologic and immunohistochemical studies. Slides were stained with hematoxylin and eosin for assessment. Inflammatory cell infiltration and fibrosis were evaluated (Fig. 2). Fibrosis was evidenced using Masson’s trichrome staining in Ventana Benchmark and assessed semiquantitatively according to the following scoring system: 0 = no fibrosis (lack of collagen deposits), 1 = mild fibrosis (focal fibrotic expansion network of collagen, mainly in the perivascular areas), 2 = moderate fibrosis (focal dense increase of collagen bands) and 3 = severe fibrosis (diffuse, strong, dense collagen band expansion in the stroma).
Identification of subsets of mononuclear cells was done by immunohistochemistry using specific antibodies: anti-CD68 (monoclonal mouse anti-human CD68/FITC clone KP1, Dako-Agilent) for macrophages, anti-CD3 antibody (mouse monoclonal anti-CD3 concentrate clone F7.2.38 Dako Omnis, Agilent) for T cells and anti-CD20 antibodies (monoclonal mouse clone L26, Dako Omnis, Agilent) for B cells. The staining was done on an Omnis autostainer using protocols, preceded by antigen retrieval in PTLink (pH = 9.0). Positive controls were used (lymph node slides) for all mentioned staining. All histopathologic and immunohistochemical evaluations were performed by an experienced pathologist blinded to the origin of the samples and other clinical features.
Expression of LIGHT and Its Receptors, HVEM and LTβR
Expression of LIGHT and its two receptors (HVEM and LTβR) was assessed in paraffin-embedded lacrimal sac samples by immunohistochemistry using specific antibodies against LIGHT, TNFRSF14/HVEM or LTβR (Abcam, Cambridge, MA, USA), as appropriate. Following the deparaffinization and rehydration, epitope retrieval was carried out in EnVision Flex Target Retrieval Solution (Dako) at high pH (pH = 9.0). Endogenous peroxidases were blocked by incubating the sections in methanol and 3% hydrogen peroxidase for 20 min. The next slides were incubated with special types of antibodies against LIGHT protein (rabbit polyclonal antibody Abcam ab203578), TNFRSF14/HVEM protein (rabbit polyclonal antibody Abcam ab 47677) and anti-LTβR protein (rabbit polyclonal ab 186847, Abcam) in 1:100 dilution at 4 °C overnight. EnVision Flex (Dako) visualization reagent was applied for 30 min followed by DAB solution for 5 min. Immunohistochemical evaluation of each protein expression was performed by a pathologist. The intensity of immunostaining was evaluated in ten random fields under 20 × magnification. The results were expressed as the percentage of cells with strong positive staining as follows: no staining: negative (−); 30% positive cells: 1 +; 31–60% positive cells: 2 +; > 60% positive cells, 3 +. Appropriate positive and negative controls were performed. Positive controls for LIGHT, LTβR and NFRSF14/HVEM proteins were done basing on in thymus by sections. In the negative control, antibody application was avoided.
Expression of LIGHT and both of its receptors was evaluated semiquantitatively in different regions of the lacrimal sacs (epithelial cell layer, interstitial cells of the connective tissue, perivascular areas and endothelial cells). Total expression score was calculated as a mean of the scores for particular regions in a given subject.
Ethics Approval
This study was approved by the Ethics Committee at the Medical University of Bialystok, Poland (approval no. KB: R-I-002/105/2017). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Appropriate written informed consent was obtained from all the patients participating in the study.
Statistics
The between-group comparisons were performed using the Kruskal-Wallis, median test/chi-square, Mann-Whitney U test or Fisher test, as appropriate. p < 0.05 was considered significant. Correlations between continuous values were evaluated using Spearman’s rank test and considered significant if p values were < 0.05 and the Spearman's r coefficient was > 0.5.
In addition, to assess the relationships between the severity of fibrosis and expression of LIGHT, its receptors and other clinical features showing significant associations with fibrosis in single test analyses, appropriate multivariable regression models were created.
Values are given as mean and standard deviations (SD) unless stated otherwise.
Results
Patients
Eighteen patients underwent operations for acute or recurrent abscesses of the lacrimal sacs and the remaining 12 patients for non-inflammatory conditions (persistent tearing). There were no significant differences in the age (p = 0.47 in Kruskal–Wallis and p = 0.41 in median/chi-square test) between patients undergoing surgery for different reasons. However, patients with non-inflammatory conditions had significantly longer disease duration (duration of symptoms before surgery) (mean ± SD: 3.9 ± 2.2 years) compared with patients with inflammation of the lacrimal sacs (mean ± SD: 2.4 ± 2.2 years, p = 0.03 by Mann-Whitney test).
There was no significant correlation between age and duration of the disease (Table 2).
Histopathologic Assessment of Lacrimal Sacs and Relationship with Clinical Features
Histopathologic evaluation revealed non-specific inflammatory infiltrates of different intensities in all cases studied. In all of the lacrimal sac samples we observed the presence of inflammation and various degrees of fibrosis. These inflammatory infiltrates consisted mainly of mononuclear cells, mainly macrophages (CD68 positive, Fig. 3a and b) and T lymphocytes (CD3 positive) (Fig. 4). B lymphocytes (CD20 positive) were focal and present only in 3 out of 30 cases (33%). All biopsies also showed features of moderate to severe fibrosis, evaluated using Massons’ trichrome blue staining (Fig. 5). Mild fibrosis was present in 6/30 cases (20%), moderate fibrosis in 14/30 cases (47%) and severe fibrosis in the remaining ten cases (33%).
There was strong correlation between the severity of fibrosis assessed histopathologically and the severity of fibrosis assessed clinically (Spearman's r = 0.586, p < 0.05). Moreover, the severity of fibrosis assessed histopathologically correlated with the duration of the disease (Spearman's r = 0.821, p < 0.05).
There was no significant correlation between fibrosis assessed macroscopically or histopathologically and the age of the patients (r = − 0.216 and r = 0.103, respectively; p > 0.05 for both). No significant differences in the fibrosis score were found between males and females (both pre- and postmenopausal group) or between biopsies taken from the left or right site.
Expression of LIGHT and Its Receptors
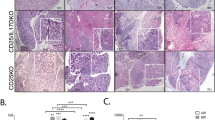
Expression of LIGHT and its receptors, LTβR and HVEM, was observed in all biopsies studied. All three molecules were expressed mainly in mononuclear cells within inflammatory infiltrates, but were also found in the columnar epithelium of the lacrimal sacs and with weaker expression also in the endothelial cells of the blood vessels (Figs. 6, 7, 8, 9, 10 and 11). LIGHT showed the most intense expression in the inflammatory cells (Fig. 6) (mean score ± SD: 2.27 ± 0.53) while the intensity of LTβR and HVEM was weaker in the inflammatory cells and columnar epithelium compared to LIGHT expression (compare Figs. 7 and 9) and similar in the endothelial cells and fibroblasts of the connective tissue (Figs. 8, 11 and 12) (mean score ± SD: 1.80 ± 0.71 and 1.3 ± 0.47, respectively).
There were significant correlations between the expression of LIGHT and expression of LIGHT receptors, HVEM and LTβR (R = 0.603 and 0.639, respectively, p < 0.05 for both) (Table 2).
Relationship Between Expression of LIGHT and Its Receptors and Severity of Fibrosis and Other Clinical Features
The results of correlations among LIGHT, its receptors and other demographic or clinical features are summarized in Table 2.
We found significant correlation between expression of LIGHT and the severity of fibrosis assessed histopathologically (Spearman's r = 0.82, p < 0.05). There was also significant correlation between the expression of HVEM and LTβR and the severity of fibrosis (Spearman's r = 0.71 and 0.74, respectively; p < 0.05 for both correlations).
Moreover, expression of LIGHT, HVEM and LTβR correlated with the duration of the disease.
There were no significant differences in the expression of LIGHT, HVEM or LTβR between males and females (both pre- and postmenopausal group) or between lacrimal sacs taken from different surgical sites (left versus right). There were no significant correlations between the expression of LIGHT or its receptors and patient age.
In multivariate analysis, including severity of fibrosis as the dependent variable and expression of LIGHT, both its receptors and duration of the disease, as predictors, only LIGHT showed a significant relationship with fibrosis (beta coefficient = 0.759, p = 0.02) (Table 3).
Discussion
In our study, histopathologic assessment revealed non-specific inflammatory infiltrates in all biopsies. These results are consistent with other reports showing that submucosal non-specific infiltration as well as various degrees of fibrosis is the most common histopathologic finding in biopsy specimens of the lacrimal sac wall taken during DCR procedures [9, 15].
It has been suggested that lymphocytic infiltration in the submucosa may lead to remodeling/thickening of the lacrimal sac wall, consequently promoting stenosis formation [5].
We have also shown that TNFSF14/LIGHT and its two receptors, LTβR and HVEM, are expressed in the wall of lacrimal sacs from patients with primary acquired LDO. All molecules were expressed mainly in mononuclear cells within inflammatory infiltrates. Indeed, in our study inflammatory infiltrates in lacrimal sacs consisted mainly of T lymphocytes (CD3-postive cells) and macrophages (CD68-postive cells), which are known to express TNFSF14/LIGHT.
Our results demonstrated that the expression of LIGHT and its two receptors correlated significantly with the severity of fibrosis of the lacrimal sac walls as assessed both intraoperatively and histopathologically.
Since fibrosis found in lacrimal sac biopsies is considered a negative prognostic factor of surgical treatment, evaluation of the relationship between fibrosis and LIGHT expression in the lacrimal sac wall may shed light on the processes responsible for the remodeling of the tear pathway during the inflammatory disease and consequently affecting the results of surgical treatment.
In this study we tried to maintain the homogeneity of the studied group of patients to limit the number of factors that may bias the results of surgery. Thus, the same surgical technique was used, the same main surgeon performed endoscopic DCR, no lasers and no antimetabolites, namely mitomycin-C, were used intraoperatively, and no canalicular intubation was performed at the end of the surgical procedure. As the observation time was relatively short (varied between 3 and 12 months), we cannot draw conclusions about the influence of the expression of LIGHT and its two receptors (HVEM and LTβR) in fibrotic tissue of the lacrimal sac on the clinical outcome of the surgery. The evaluation of the correlation between these factors will be the subject of our further research.
However, we managed to show that more intensive fibrosis was observed in the lacrimal sac during operations, and in histopathologic examination it was correlated with higher expression of LIGHT, HVEM and LTβR. Thus, we may assume that LIGHT along with its receptors may be a factor contributing to fibrosis and synechiae formation in the lacrimal sac both during the inflammatory process before surgical treatment and in postoperative restenosis of the newly created ostium. The latter needs to be proven in future studies in a group of patients who fail to improve after the first operation.
Our assumption is based on the results of studies using animal models. Herro showed that local injection of TNFSF14/LIGHT into the skin of mice induced accumulation of mononuclear inflammatory infiltrates and development of fibrosis [16]. Accordingly, silencing/deletion of LIGHT or either of its receptors, LTβR or HVEM, attenuated bleomycin-induced skin fibrosis/bleomycin-induced fibrosis.
Similarly, evidence from other studies indicates that silencing/deletion or neutralization of LIGHT reduces airway remodeling in an animal model of asthma or following recurrent rhinovirus infection. LIGHT might contribute to the development of fibrosis in different ways. Doherty has shown that LIGHT-deficient mice produce lower amounts of profibrotic cytokines, TGFbeta and interlukin-13, indicating that the LIGHT axis contributes to production of proinflammatory mediators [17]. Recently, DaSilva et al. demonstrated that LIGHT directly stimulates fibroblasts [18]. Fibrosis might be a result of chronic inflammation [19]. Interestingly, in our study both the severity of fibrosis and expression of LIGHT were significantly correlated with the duration of the symptoms.
The lack of a control group may be considered a drawback of our research. However, taking a biopsy from healthy subjects is not appropriate and harvesting tissue post-mortem for immunohistochemistry is difficult and requires additional safety precautions in the COVID-19 era. This limitation is partially compensated by evaluation of LIGHT expression in three clinically different stages of lacrimal sac disease—mild, moderate and severe fibrosis (Fig. 1).
Conclusion
In conclusion, this is the first study showing that TNFSF14/LIGHT and its two receptors are expressed in the walls of lacrimal sacs from patients with primary acquired LDO and that their expression correlated with the severity of fibrosis. In the next step of our research, evaluation of the correlation between the expression of LIGHT in fibrotic tissue samples taken from patients who fail to improve after the first endoscopic DCR seems warranted. The results of such a study would not only allow determining whether LIGHT expression can be considered a prognostic factor in endoscopic DCR but also open up the prospect of further research on LIGHT blocking to improve the effects of surgical treatment.
References
Marcet MM, Kuk AK, Phelps PO. Evidence-based review of surgical practices in endoscopic endonasal dacryocystorhinostomy for primary acquired nasolacrimal duct obstruction and other new indications. Curr Opin Ophthalmol. 2014;25:443–8.
Saratziotis A, Emanuelli E, Gouveris H, Tsironi E, Fountas K. Endoscopic dacryocystorhinostomy for acquired nasolacrimal duct obstruction: long-term results in 91 procedures. Rhinology. 2014;52:413–8.
Lin GC, Brook CD, Hatton MP, Metson R. Causes of dacryocystorhinostomy failure: external versus endoscopic approach. Am J Rhinol Allergy. 2017;1:181–5.
Ali MJ, Paulsen F. Etiopathogenesis of primary acquired nasolacrimal duct obstruction: what we know and what we need to know. Ophthalmic Plast Reconstr Surg. 2019;35:426–33.
Amin RM, Hussein FA, Idriss HF, Hanafy NF, Abdallah DM. Pathological, immunohistochemical and microbiologicalal analysis of lacrimal sac biopsies in patients with chronic dacrocystitis. Int J Ophthalmol. 2013;18:817–26.
Merkonidis C, Brewis C, Yung M, Nussbaumer M. Is routine biopsy of the lacrimal sac wall indicated at dacryocystorhinostomy? A prospective study and literature review. Br J Ophthalmol. 2005;89:1589–91.
Park J, Lee J, Baek S. Pathologic features and expression of heat shock protein 47 in the nasal mucosa and lacrimal sac: does it influence the surgical outcome of endoscopic endonasal dacryocystorhinostomy? Eye (Lond). 2018;32:1432–9.
Su PY. Comparison of endoscopic and external dacryocystorhinostomy for treatment of primary acquired nasolacrimal duct obstruction. Taiwan J Ophthalmol. 2018;8:19–23.
Salour H, Hatami MM, Parvin M, Ferdowsi AA, Abrishami M, Bagheri A, Aletaha M, Yazdani S. Clinicopathological study of lacrimal sac specimens obtained during DCR. Orbit. 2010;29:250–3.
Ozer O, Eskiizmir G, Unlü H, Işisağ A, Aslan A. Chronic inflammation: a poor prognostic factor for endoscopic dacryocystorhinostomy. Eur Arch Otorhinolaryngol. 2012;269:839–45.
Dave TV, Mohammed FA, Ali MJ, Naik MN. Etiologic analysis of 100 anatomically failed dacryocystorhinostomies. Clin Ophthalmol. 2016;28:1419–22.
Wang J, Anders RA, Wu Q, Peng D, Cho JH, Sun Y, Karaliukas R, Kang HS, Turner JR, Fu YX. Dysregulated LIGHT expression on T cells mediates intestinal inflammation and contributes to IgA nephropathy. J Clin Investig. 2004;113:826–35.
Gindzienska-Sieskiewicz E, Distler O, Reszec J, Jordan S, Bielecki P, Sieskiewicz A, Sulik A, Lukasik M, Bielecki M, Kowal K, Kowal-Bielecka O. Increased expression of the TNF superfamily member LIGHT/TNFSF14 and its receptors (HVEM and LTßR) in patients with systemic sclerosis. Rheumatology (Oxford). 2019;1:502–10.
Roh JH, Chi MJ. Efficacy of dye disappearance test and tear meniscus height in diagnosis and postoperative assessment of nasolacrimal duct obstruction. Acta Ophthalmol. 2010;88:73–7.
Anderson NG, Wojno TH, Grossniklaus HE. Clinicopathologic findings from lacrimal sac biopsy specimens obtained during dacryocystorhinostomy. Ophthalmic Plast Reconstr Surg. 2003;19:173–6.
Herro R, Antunes RDS, Aguilera AR, Tamada K, Croft M. The tumor necrosis factor superfamily molecule LIGHT promotes keratinocyte activity and skin fibrosis. J Invest Dermatol. 2015;135:2109–18.
Doherty TA, Soroosh P, Khorram N, Fukuyama S, Rosenthal P, Cho JY, Norris PS, Choi H, Scheu S, Pfeffer K, Zuraw BL, Ware CF, Broide DH, Croft M. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med. 2011;17:596–603.
Da Silva AR, Mehta AK, Madge L, Tocker J, Croft M. TNFSF14 (LIGHT) exhibits inflammatory activities in lung fibroblasts complementary to IL-13 and TGF-β. Front Immunol. 2018;19:576.
Ueha S, Shand FH, Matsushima K. Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis. Front Immunol. 2012;10:71.
Acknowledgements
We thank the participants of the study.
Funding
This study was supported by a research grant from the Medical University of Bialystok. The Rapid Service Fees were funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Pawel Bielecki, Ewa Gindzienska-Sieskiewicz, Joanna Reszeć, Bartosz Piszczatowski, Marek Rogowski, Otylia Kowal-Bielecka, Krzysztof Kowal and Andrzej Sieskiewicz declare no potential conflicts of interest.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee of the Medical University of Bialystok, Poland (approval number: KB: R-I-002/105/2017). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Appropriate written informed consent was obtained from all the patients participating in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bielecki, P., Gindzienska-Sieskiewicz, E., Reszeć, J. et al. Expression of LIGHT/TNFSF14 and Its Receptors, HVEM and LTβR, Correlates with the Severity of Fibrosis in Lacrimal Sacs from Patients with Lacrimal Duct Obstruction. Ophthalmol Ther 10, 63–74 (2021). https://doi.org/10.1007/s40123-020-00320-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-020-00320-3