Abstract
Purpose
The REal life assessmENt of safety And effeCTiveness of Razumab 2 (RE-ENACT 2) study evaluated the long-term effectiveness of biosimilar ranibizumab. We present the subgroup analysis of patients with retinal vein occlusion (RVO).
Methods
Data of patients who received pro re nata (PRN) biosimilar ranibizumab (November 2015 to December 2018, 17 centers) were analyzed. Endpoints were change from baseline in best corrected visual acuity (BCVA, Snellen’s/logMAR), central subfield thickness (CSFT), intraocular pressure (IOP), and proportions of patients having intraretinal fluid (IRF) and subretinal fluid (SRF) at weeks 4, 8, 12, 16, 20, 24, 30, 36, and 48.
Results
Of 101 patients, 48.51% were men, and the majority (79.21%) were treatment naïve and had received 3 (range 1–5) injections (53.5%). Significant improvements (P < 0.05) were observed from baseline to all timepoints for BCVA [baseline, 0.89 ± 0.06 (n = 94); week 48, 0.41 ± 0.08 (n = 14)] and CSFT [baseline, 527.58 ± 19.9 (n = 85); week 48, 307.47 ± 16.4 (n = 15)]. Changes in IOP (mmHg) were non-significant [baseline, 15.38 ± 0.4 (n = 94); week 48, 13.94 ± 0.6 (n = 16); P = 0.5575). Proportions of patients having IRF [baseline, 71.3% (n = 84) vs week 48, 0% (n = 15)] and SRF [baseline, 52.5% (n = 83) vs week 48, 0% (n = 15)] were decreased. Similar results for BCVA, CSFT, IOP, IRF, and SRF were observed for BRVO and CRVO subgroups. There were no new safety concerns.
Conclusions
Biosimilar ranibizumab demonstrated improvements in visual acuity and disease outcomes up to 48 weeks in patients with RVO without any new safety concerns.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Retinal vein occlusion (RVO) may result in irreversible vision loss despite availability of several treatment options. |
Branch RVO (BRVO) occurs more commonly than central RVO (CRVO), up to sevenfold in Indian patients. |
The 15-year cumulative incidence of BRVO and CRVO is 1.8% and 0.5%, respectively. |
Ranibizumab is approved as an anti-VEGF agent by the US Food and Drug Administration (FDA) and European Medicine Agency (EMEA) for the treatment of RVO. |
Biosimilar ranibizumab, Razumab™, approved by the Drug Controller General of India (DCGI) in 2015, provides a cost-effective alternative to innovator ranibizumab. |
This report presents the effectiveness of Razumab in patients with RVO treated in a real-world setting. |
Razumab (biosimilar ranibizumab) demonstrated improvements in visual acuity and disease outcomes in patients with RVO without new safety issues. |
Introduction
Retinal vein occlusion (RVO), characterized by a thrombus obstruction in the fundus of the eye, is the second most common retinal vascular disorder after diabetic retinopathy [1, 2]. RVO is classified on the basis of the location of venous occlusion as central retinal vein occlusion (CRVO)—obstruction at optic nerve head; branch retinal vein occlusion (BRVO)—obstruction at a branch of the central retinal vein; and hemiretinal vein occlusion (HRVO)—occlusion at disc involving retinal venous drainage (superior or inferior hemifield) [2]. BRVO occurs more commonly than CRVO, up to sevenfold in Indian patients [3], with a 15-year cumulative incidence of BRVO and CRVO at 1.8% and 0.5%, respectively, as per a large population-based study in the USA [4].
RVO may result in irreversible vision loss despite availability of several treatment options [5]. Among various treatment strategies attempted, laser therapy has been the gold standard for the treatment of RVO [6, 7]. The advent of intravitreal anti-vascular endothelial growth factor (VEGF) agents has reformed RVO management [6, 8, 9]. Ranibizumab has demonstrated efficacy and safety in the treatment of several retinal disorders including RVO [10,11,12,13], and is approved by the US Food and Drug Administration (FDA) and European Medicine Agency (EMEA) for the treatment of RVO. The US FDA approval of ranibizumab for the treatment of visual impairment due to macular edema secondary to RVO was based on the results from the pivotal phase III BRAVO (BRAnch retinal Vein Occlusion) trial [12] and CRUISE (Central Retinal vein OcclUsIon Study) trial [13].
Razumab™, the world’s first biosimilar ranibizumab developed by Intas Pharmaceuticals Ltd., Ahmedabad, India, is a cost-effective alternative, is easily accessible to patients, and was approved by the Drug Controller General of India in 2015 for the treatment of RVO, wet age-related macular degeneration (wet AMD), diabetic macular edema (DME), and myopic choroidal neovascularization (mCNV). The efficacy and safety of biosimilar ranibizumab have been also evaluated in a prospective study in Indian patients with chorioretinal vascular diseases including RVO [14].
The effectiveness of biosimilar ranibizumab for the treatment of retinal disorders in a real-world clinical setting was established in a previous retrospective, multicenter, observational REal life assessmENt of safety And effeCTiveness of Razumab (RE-ENACT) study [15,16,17]. The RE-ENACT 2 study was conducted with the intent to generate long-term data on the usage of biosimilar ranibizumab in a real-world clinical setting in Indian patients. The current report presents the subgroup analysis on patients with RVO from the RE-ENACT 2 study.
Methods
Study Design, Population, Endpoints, and Statistical Considerations
The RE-ENACT 2 study design was similar to the RE-ENACT study, which is published elsewhere [16]. The RE-ENACT 2 study analyzed the medical records of adult (aged 18 years or more) patients of either sex who had RVO and who had received one or more intravitreal biosimilar ranibizumab injections as per routine clinical care for the management of macular disorders between November 2015 and December 2018 at 17 centers across India. RVO is defined as intraretinal hemorrhages, dilated veins and/or cotton wool spots, and there may be evidence of macular edema. Retinal capillary ischemia facilitates the classification of RVO as non-ischemic and ischemic. The pathogenesis, clinical manifestations, and the treatment of RVO are predisposed to the location of occlusion. Patients were administered one intravitreal injection of biosimilar ranibizumab followed by pro re nata (PRN) treatment regimen. Biosimilar ranibizumab PRN treatment regimen/retreatment criteria included logMAR best corrected visual acuity (BCVA) of no greater than 0.5 or mean central subfield thickness (CSFT) of 250 µm or more in the study eye as compared to the previous follow-up visit. Patients were followed up every 4 weeks after biosimilar ranibizumab injection and the improvements in effectiveness parameters BCVA, CSFT, subretinal fluid (SRF) and intraretinal fluid (IRF) were recorded. Patients were evaluated at each follow-up visit for further treatment. The study included both treatment-naïve patients and patients previously treated with other anti-VEGF, steroids, or laser treatment. Patients were excluded if assessment of optical coherence tomography (OCT) was not available (i.e., dense cataract). This retrospective study was conducted after ethics committee (OM ethics committee, Ahmedabad, India) approval and in accordance with the protocol and the principles of the Helsinki Declaration.
The study assessment parameters were BCVA, CSFT, intraocular pressure (IOP), IRF, and SRF. The study endpoints were change in the BCVA, CSFT, IOP, and decrease in proportion of patients with IRF and SRF from baseline to weeks 4, 8, 12, 16, 20, 24, 30, 36, and 48. The BCVA measurement were done using Snellen’s chart or the logMAR chart. Spectral domain OCT (SD-OCT) was utilized to measure CSFT, IRF, and SRF. The BCVA and CSFT were analyzed using two-tailed paired t test, and IRF and SRF using χ2 test. All statistical analyses were done using SAS® 9.4.
Results
Patients Disposition and Demographics
A total of 101 patients with RVO were included in this subgroup analysis; 48.51% were men, and the majority (79.21%) were treatment naïve. The majority of the patients had received three biosimilar ranibizumab injections (53.5%); the median number of biosimilar ranibizumab injections in this study were 3 (range 1–5). The majority of the patients had hypertension (74.25%) and diabetes (26.73%). BRVO was the most common RVO type (n = 55, mean age 58.8 ± 11.1 years, men 32.7%) followed by CRVO (n = 32, mean age 55.3 ± 10.8 years, men 65.6%) and HRVO (n = 4, mean age 59 ± 10.8 years, men 75%). The treatment effects were observed up to 48 weeks. Table 1 presents the patient disposition and baseline characteristics.
Best Corrected Visual Acuity
A significant improvement (P < 0.05) in the mean ± SE BCVA was observed from baseline to all timepoints (baseline, 0.89 ± 0.06; week 48, 0.41 ± 0.08) indicating improved visual acuity. A slight decrease in BCVA improvements was observed from weeks 16 to 24 compared to previous weeks, though these improvements were significant when compared with baseline. Similarly, after week 30, there was a slight decrease in BCVA improvement though the improvements were significant from baseline (Fig. 1). Figure 2 presents the mean change in BCVA from baseline at each timepoint. The majority (57.9%) of the patients had received three biosimilar ranibizumab injections with significant improvements (P < 0.05) seen in BCVA from baseline to all timepoints. A maximum of five injections were administered and the improvements were observed up to 48 weeks. In the BRVO subgroup, the improvements (mean ± SE) in BCVA were significant (P < 0.05) from baseline (0.85 ± 0.07) to 48 weeks (0.3 ± 0.08). The improvements in BCVA were also significant (P < 0.05) for the CRVO subgroup from baseline (1.10 ± 0.1) to 48 weeks (0.6 ± 0.08) (Fig. 3). The mean change in BCVA did not differ significantly at all timepoints in a subgroup analysis of treatment-naïve vs previously treated patients (Table 2).
Central Subfield Thickness
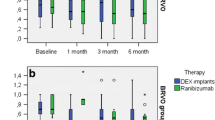
A significant (P < 0.05) decrease in CSFT scores (mean ± SE) indicating improved disease condition was observed from baseline (527.58 ± 19.9) to 48 weeks (307.47 ± 16.4) (Fig. 4). Improvements in CSFT were continuous till the 20th week, after which a slight decrease in the improvement was observed as compared with the previous weeks. But all the observations at all timepoints were significant when compared with baseline. There were significant (P < 0.05) improvements in CSFT from baseline to all timepoints in patients who received three biosimilar ranibizumab injections. Figure 5 presents the mean change in CSFT from baseline at each timepoint. The decrease in CSFT (mean ± SE) was significant (P < 0.05) from baseline to 48 weeks when analyzed for BRVO (499.84 ± 21.83 vs 313.33 ± 63.4) and CRVO subgroups (597.42 ± 37.7 vs 301.75 ± 16.4) (Fig. 6). For a subgroup analysis of treatment-naïve vs previously treated patients, the mean change in CSFT did not differ significantly at all timepoints (Table 2).
Intraretinal Fluid and Subretinal Fluid
A significant (P < 0.05) reduction in the proportion of patients having IRF or SRF from baseline to all timepoints was observed, indicating improved disease condition. The proportion of patients having IRF and SRF at baseline and at each timepoint is presented in Table 3. Similar results were reported for the subgroup analysis of treatment-naïve vs previously treated patients.
Intraocular Pressure
The changes in mean IOP scores observed from baseline [15.38 ± 0.4 mmHg (n = 94)] to 48 weeks [13.94 ± 0.6 mmHg, (n = 16)] were ± 1 mmHg at most of the timepoints and these changes were not significant. Similarly, the changes were not significant from baseline to all timepoints in patients who received three biosimilar ranibizumab injections. The changes in mean IOP from baseline to all timepoints were minimal and did not differ significantly when evaluated for a subgroup of treatment-naïve vs previously treated patients. The changes in IOP from baseline were minimal and they were not significant when analyzed for BRVO and CRVO subgroups.
Discussion
Ranibizumab, bevacizumab, and aflibercept have been extensively used for the treatment of RVO [18, 19]. A recent systematic review and network meta-analysis of 11 randomized controlled trials (n = 1130) demonstrated no significant differences among bevacizumab, ranibizumab, and aflibercept for the treatment of macular edema secondary to RVO [20]. The recent LEAVO study compared ranibizumab (0.5 mg/0.05 mL, n = 155), bevacizumab (1.25 mg/0.05 mL, n = 154), and aflibercept (2.0 mg/0.05 mL, n = 154) for the treatment of macular edema due to CRVO for 100 weeks and reported that alfibercept was noninferior (no worse than) whereas bevacizumab was not noninferior (may be worse or may not be worse) to ranibizumab for mean changes in vision. Also, bevacizumab treatment was not noninferior to aflibercept treatment in the post hoc exploratory analysis [21].
The pivotal BRAVO [12] and CRUISE [13] trials have demonstrated the efficacy and safety of ranibizumab in RVO treatment. Intas Pharmaceuticals Limited, Ahmedabad, India, has developed the world’s first biosimilar ranibizumab—Razumab™, with an objective to provide a cost-effective (up to 25%) alternative to innovator ranibizumab [22].
Biosimilar ranibizumab has been used by leading ophthalmologists in India and has demonstrated efficacy and safety managing macular disorders in prospective and retrospective studies in Indian patients. The current RE-ENACT 2 study further established the effectiveness of biosimilar ranibizumab in the treatment of macular disorders. The previous multicenter, retrospective RE-ENACT [15] (n = 561) study strengthened the effectiveness of biosimilar ranibizumab in macular disorders in Indian patients in a routine clinical setting with significant improvements in BCVA, central macular thickness, IRF, and SRF with the use of 4-weekly biosimilar ranibizumab injections (three injections) for a 3-month duration [16].
The RE-ENACT study evaluated the improvements in BCVA, CMT, IRF, and SRF with the use of biosimilar ranibizumab in patients with wet AMD, DME, and RVO for a short duration (12 weeks). The RE-ENACT 2 (n = 341) study evaluated the long-term (48 weeks) use (effectiveness measured by improvements in BCVA, CSFT, IRF, and SRF) of biosimilar ranibizumab in patients with wet AMD, DME, RVO, and additionally in patients with mCNV. Furthermore, in the RE-ENACT study, three biosimilar ranibizumab injections were given to all patients but the RE-ENACT 2 study evaluated the patients who had received one to five biosimilar ranibizumab injections overall. Biosimilar ranibizumab resulted in marked improvements in BCVA, CSFT, IRF, and SRF parameters in patients with RVO in this subgroup analysis of the RE-ENACT 2 study. The results for the RE-ENACT 2 study for pooled analysis of the wet AMD, DME, RVO, and mCNV population [23] and a subgroup analysis on the wet AMD population [24] are published elsewhere.
The visual acuity and disease condition improvement in the RE-ENACT 2 study, as measured by logMAR BCVA, was significant with biosimilar ranibizumab starting at week 4 and throughout 48 weeks, which was similar to the RE-ENACT study (weeks 4 to 12). These results are consistent with a study by Minami et al., which established the positive correlation of short-term effects of ranibizumab in predicting the long-term effects in patients with RVO [25]. The subgroup analysis in the current report demonstrated significant improvements in BCVA in patients with BRVO and patients with CRVO. The BRAVO [12] and CRUISE [13] studies also demonstrated similar results with significant improvements in BCVA sustained over 12 months in patients with BRVO and patients with CRVO, respectively. Several other studies [26] and case series [27] have demonstrated improved BCVA till 12 months. MARVEL [28] and ROCC [11] studies reported similar results with ranibizumab with significant improvements in BCVA at 6 months in patients with BRVO and patients with CRVO, respectively [29].
Biosimilar ranibizumab was also associated with significant reduction in CSFT in this subgroup analysis at all timepoints starting at week 4 to week 48. The subgroup analysis in patients with BRVO and patients with CRVO demonstrated similar results with a significant reduction in CSFT. The reduction in CSFT was observed as early as day 1 after ranibizumab administration in a case series (n = 3) by Verma et al., suggesting early effects of ranibizumab on macular edema related to RVO [30]. Ranibizumab was associated with significant reduction in CSFT in patients with BRVO and patients with CRVO at 6 months in a previous retrospective study by Son et al. [31] (n = 15, BRVO) and Yuan and colleagues (n = 26; BRVO 12, CRVO 13, HRVO 1) [32]. The BRIGHTER study showed a decrease in CSFT at 3, 6, 9, and 12 months with ranibizumab treatment in patients with BRVO (n = 183) that continued till 24 months [33].
The possible role of increased IOP in the pathogenesis of RVO was indicated by Frucht and colleagues [34]. Intravitreal injections are known to cause transient increase in IOP. However, information regarding long-term increase in IOP is varied in the literature [34]. Kampougeris and colleagues define a raise in IOP as an increase up to 5 mmHg from baseline [35]. In our study, the values of IOP were within the normal limits at all timepoints, and ± 1 mmHg changes in mean IOP scores were seen from baseline to most timepoints; these changes were not significant, consistent with the aforementioned statement. Furthermore, none of the patients had received any medications to reduce IOP before/after biosimilar ranibizumab administration. Similar to our study, Gu et al. reported that IOP remained stable with ranibizumab treatment (n = 32) with no statistical difference till 6 months with the mean IOP change at less than 1 mmHg [36]. One-year results from the COMRADE extension study revealed a relatively low IOP with ranibizumab, which was constant over time in patients with RVO when compared with dexamethasone implant, another commonly used agent for RVO treatment [37].
There is generally an accumulation of IRF and SRF in patients with RVO. The BRIGHTER study demonstrated a decrease in the proportion of patients with visible IRF and SRF with ranibizumab treatment at 6 months [38]. Post hoc analysis from the prospective randomized, controlled BRAVO and CRUISE trials showed resolution in SRF in almost all patients with BRVO/CRVO at 3 months after ranibizumab treatment [39]. SRF was effectively reduced through 24 months with ranibizumab treatment in the long-term CRYSTAL study [40]. Our study demonstrated a decrease in the proportion of patients with IRF and SRF throughout the study.
Overall, the current study demonstrated no significant differences in BCVA, CSFT, IOP, IRF, and SRF at most of the timepoints when evaluated for treatment-naïve vs previously treated patients. The major limitation of this study included its retrospective nature because of which complete information pertaining to severity of the disease, previous treatments, bilaterality ischemia, and adverse events could not be presented as it was not captured in medical records. Also, the data on effectiveness parameters were not available for all patients at all timepoints, and the number of patients at each timepoint is mentioned in the respective figures. Overall, no new safety concerns compared to the innovator ranibizumab were observed. The Early Treatment Diabetic Retinopathy Study (ETDRS) chart is used commonly in controlled clinical studies for measurement of visual acuity. However, the visual acuity was measured with logMAR/Snellen’s charts in this study, which is considered inferior to ETDRS charts [41].
Conclusion
The current subgroup analysis of patients with RVO from the RE-ENACT 2 study reinforces Razumab™, the world’s first biosimilar of ranibizumab, as an effective treatment option in managing RVO by reducing macular thickness and improving visual acuity. However, larger numbers of patients with complete details at longer follow-up are desirable for a definitive interpretation of the study results.
References
Morris R. Retinal vein occlusion. Kerala J Ophthalmol. 2016;28(1):4–13.
Kolar P. Definition and classification of retinal vein occlusion. Int J Ophthalmic Res. 2016;2(2):124–9.
Jonas JB, Nangia V, Khare A, Sinha A, Lambat S. Prevalence and associations of retinal vein occlusions: the Central India Eye and Medical Study. Retina. 2013;33(1):152–9.
Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126(4):513–8.
Gerding H, Mones J, Tadayoni R, Boscia F, Pearce I, Priglinger S. Ranibizumab in retinal vein occlusion: treatment recommendations by an expert panel. Br J Ophthalmol. 2015;99(3):297–304.
Esmaili DD, Boyer DS. Recent advances in understanding and managing retinal vein occlusions. F1000Res. 2018;16(7):467.
Golan S, Fisher N, Lowenstein A. Current treatment of retinal vein occlusion. Eur Ophthalmic Rev. 2011;5(1):62–8.
Lattanzio R, Torres Gimeno A, Battaglia Parodi M, Bandello F. Retinal vein occlusion: current treatment. Ophthalmologica. 2011;225:135–43.
Tah V, Orlans HO, Hyer J, et al. Anti-VEGF therapy and the retina: an update. J Ophthalmol. 2015;2015:627674.
Tan MH, McAllister IL, Gillies ME, et al. Randomized controlled trial of intravitreal ranibizumab versus standard grid laser for macular edema following branch retinal vein occlusion. Am J Ophthalmol. 2014;157(1):237–247.e231.
Kinge B, Stordahl PB, Forsaa V, et al. Efficacy of ranibizumab in patients with macular edema secondary to central retinal vein occlusion: results from the sham-controlled ROCC study. Am J Ophthalmol. 2010;150(3):310–4.
Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1102–1112.e1101.
Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1124–1133.e1121.
Sameera VV, Ayachit A, Joshi S, Guruprasad AS. Safety and efficacy of razumab—the new biosimilar in India: our experience. Kerala J Ophthalmol. 2016;28:180–5.
Sharma S, Khan MA, Chaturvedi A, RE-ENACT Study Investigators Group. Real-Life clinical effectiveness of Razumab® (the world's first biosimilar of ranibizumab) in retinal vein occlusion: a subgroup analysis of the pooled retrospective RE-ENACT study. Ophthalmologica. 2019;241(1):24–31.
Sharma S, Khan M, Chaturvedi A, RE-ENACT Study Investigators Group. Real-life clinical effectiveness of Razumab® (world’s first biosimilar ranibizumab) in wet age-related macular degeneration, diabetic macular edema, and retinal vein occlusion: a retrospective pooled analysis. Int J Ophthalmol Eye Res. 2018;6(4):377–83.
Sharma S, RE-ENACT Study Investigators Group, Khan MA, Chaturvedi A. Real life clinical effectiveness of Razumab® (world’s first biosimilar ranibizumab) in wet age-related macular degeneration: a subgroup analysis of pooled retrospective RE-ENACT study. Int J Ophthalmol Eye Res. 2018;6(2):368–73.
Campa C, Alivernini G, Bolletta E, Parodi MB, Perri P. Anti-VEGF therapy for retinal vein occlusions. Curr Drug Targets. 2016;17(3):328–36.
Rehak M, Wiedemann P. Retinal vein thrombosis: pathogenesis and management. J Thromb Haemost. 2010;8(9):1886–944.
Sangroongruangsri S, Ratanapakorn T, Wu O, Anothaisintawee T, Chaikledkaew U. Comparative efficacy of bevacizumab, ranibizumab, and aflibercept for treatment of macular edema secondary to retinal vein occlusion: a systematic review and network meta-analysis. Expert Rev Clin Pharmacol. 2018;11(9):903–16.
Hykin P, Prevost AT, Vasconcelos JC, et al. Clinical effectiveness of intravitreal therapy with ranibizumab vs aflibercept vs bevacizumab for macular edema secondary to central retinal vein occlusion: a randomized clinical trial. JAMA Ophthalmol. 2019;137(11):1256–64.
Intas Pharmaceuticals Limited. Intas launches RAZUMAB, globally the first biosimilar to Lucentis® (ranibizumab). https://www.prnewswire.com/in/news-releases/intas-launches-razumab-globally-the-first-biosimilar-to-lucentis-ranibizumab-508383021.html. Accessed 12 July 2019.
Sharma S, RE-ENACT 2 Study Investigators Group, Khan MA, Chaturvedi A. A multicenter, retrospective study (RE-ENACT 2) on the use of Razumab™ (world’s first biosimilar ranibizumab) in wet AMD, DME, RVO and myopic CNV. J Clin Exp Ophthalmol. 2019;10:826.
Sharma S, Khan M, Chaturvedi A. A multicenter, retrospective study (RE-ENACT 2) on the use of Razumab™ (world’s first biosimilar ranibizumab) in wet age-related macular degeneration. Ophthalmol Ther. 2020;9(1):103–14.
Minami Y, Nagaoka T, Ishibazawa A, Yoshida A. Correlation between short- and long-term effects of intravitreal ranibizumab therapy on macular edema after branch retinal vein occlusion: a prospective observational study. BMC Ophthalmol. 2017;17(1):90.
Campochiaro PA, Bhisitkul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology. 2013;120(4):795–802.
Spaide RF, Chang LK, Klancnik JM, et al. Prospective study of intravitreal ranibizumab as a treatment for decreased visual acuity secondary to central retinal vein occlusion. Am J Ophthalmol. 2009;147(2):298–306.
Narayanan R, Panchal B, Das T, et al. A randomised, double-masked, controlled study of the efficacy and safety of intravitreal bevacizumab versus ranibizumab in the treatment of macular oedema due to branch retinal vein occlusion: MARVEL Report No. 1. Br J Ophthalmol. 2015;99(7):954–9.
Pieramici DJ, Rabena M, Castellarin AA, et al. Ranibizumab for the treatment of macular edema associated with perfused central retinal vein occlusions. Ophthalmology. 2008;115(10):e47–54.
Verma L, Chakravarti A, Gupta A, Prakash S. One day wonder: fast resolution of macular edema following intravitreal ranibizumab in retinal venous occlusions. Indian J Ophthalmol. 2013;61(9):528–30.
Son BK, Kwak HW, Kim ES, Yu SY. Comparison of ranibizumab and bevacizumab for macular edema associated with branch retinal vein occlusion. Korean J Ophthalmol. 2017;31(3):209–16.
Yuan A, Ahmad BU, Xu D, et al. Comparison of intravitreal ranibizumab and bevacizumab for the treatment of macular edema secondary to retinal vein occlusion. Int J Ophthalmol. 2014;7(1):86–91.
Tadayoni R, Waldstein SM, Boscia F, et al. Sustained benefits of ranibizumab with or without laser in branch retinal vein occlusion: 24-month results of the BRIGHTER study. Ophthalmology. 2017;124(12):1778–877.
Frucht J, Shapiro A, Merin S. Intraocular pressure in retinal vein occlusion. Br J Ophthalmol. 1984;68(1):26–8.
Kampougeris G, Spyropoulos D, Mitropoulou A. Intraocular pressure rise after anti-VEGF treatment: prevalence, possible mechanisms and correlations. J Curr Glaucoma Pract. 2013;7(1):19–24.
Gu X, Yu X, Song S, Dai H. Intravitreal dexamethasone implant versus intravitreal ranibizumab for the treatment of macular edema secondary to retinal vein occlusion in a Chinese population. Ophthalmic Res. 2017;58(1):8–14.
Feltgen N, Hattenbach LO, Bertelmann T, et al. Comparison of ranibizumab versus dexamethasone for macular oedema following retinal vein occlusion: 1-year results of the COMRADE extension study. Acta Ophthalmol. 2018;96(8):e933–e941941.
Tadayoni R, Waldstein SM, Boscia F, et al. Individualized stabilization criteria-driven ranibizumab versus laser in branch retinal vein occlusion: six-month results of BRIGHTER. Ophthalmology. 2016;123(6):1332–444.
Bhisitkul R, Campochiaro P, Shapiro H, Rubio RG. Predictive value in retinal vein occlusions of early versus late or incomplete ranibizumab response defined by optical coherence tomography. Ophthalmology. 2013;120(5):1057–63.
Larsen M, Waldstein SM, Priglinger S, et al. Sustained benefits from ranibizumab for central retinal vein occlusion with macular edema: 24-month results of the CRYSTAL Study. Ophthalmol Retina. 2017;2(2):134–42.
Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice (an AOS thesis). Trans Am Ophthalmol Soc. 2009;107:311–24.
Acknowledgements
The authors thank Ms. Sudipta Ghosh (Biostatistics and Programming, Lambda Therapeutic Research Ltd.) for statistical analysis. The authors also thank Dr Tanishq Sharma (Intern at Shree Krishna Hospital, Anand, Gujarat) for providing logistic support for data collection.
RE-ENACT 2 Study Investigators Group
Dr. Manjunath Bhaskar Anandkumar, Department of Ophthalmology, Ganesh Netralaya, Sirsi, Karnataka, India, 581401, Email: manj009@gmail.com.
Dr. Sangita Jain, Department of Ophthalmology, Dev Bhumi Superspeciality Hospital, Dehradun, Uttarakhand, India, 248001 Email: sangitavrs@yahoo.co.uk.
Dr. Hemanth Murthy, Department of Ophthalmology, Retina Institute of Karnataka, Bangalore, Karnataka, India, 560018, Email: hemanthmurthy@yahoo.com.
Dr. Naveenam Srinivasa Murlidhar, Department of Ophthalmology, Retina Institute of Karnataka, Bangalore, Karnataka, India, 560018, Email: retina.nsm@gmail.com.
Dr. Raj Shri Hirawat, Department of Ophthalmology, Gomabai Netralaya, Neemuch, Madhya Pradesh, India, 458441, Email: drrajshreehirawat@gmail.com.
Dr. Aditya Sudhalkar, Department of Ophthalmology, Sudhalkar Eye Hospital, Baroda, Gujarat, India, 390001, Email: adityasudhalkar@yahoo.com.
Dr. Amarendra Deka, Department of Ophthalmology, Mission Netralaya, Shillong, Meghalaya, India, 793014, Email: dradeka@icloud.com.
Dr. Alay Banker, Department of Ophthalmology, Banker Retina Clinic & Laser Centre, Ahmedabad, Gujarat, India, 380009, Email: alay.banker@gmail.com.
Dr. Vatsal Parikh, Department of Ophthalmology, Drushti Eye And Retina Centre, Mumbai, Maharashtra, India, 400004, Email: vatsal@drushti.com.
Dr. Manisha Agarwal, Department of Ophthalmology, Dr. Shroff Charity Eye Hospital, New Delhi, India, 110002, Email: agarwalmannii@yahoo.co.in.
Dr. Charu Mithal, Department of Ophthalmology, Visitech Jasola Eye Centre, New Delhi, India, 110025, Email: drcharu_mithal@yahoo.co.in.
Dr. Rajender Pal Singh, Department of Ophthalmology, Visitech Jasola Eye Centre, New Delhi, India, 110025, Email: jasola.visitech@gmail.com.
Dr. Deepti Kulkarni, Department of Ophthalmology, Ameya Laser & Research Pvt. Ltd., Miraj, Maharashtra, India, 416410, Email: deepti.ameya@gmail.com.
Dr. Abhishek Desai, Department of Ophthalmology, Shri Ganpati Netralaya, Jalna, Maharashtra, India, 431203, Email: drabhisid@gmail.com.
Dr. Rushikesh Naigaonkar, Department of Ophthalmology, Shri Ganpati Netralaya, Jalna, Maharashtra, India, 431203, Email: rushikesh.naigaonkar@netralaya.org.
Dr. Nishikant Borse, Department of Ophthalmology, Insight Eye Clinic, Mumbai, Maharashtra, India, 400014, Email: nishikantborse@yahoo.com.
Dr. Simanta Pradeep Saikia, Department of Ophthalmology, Mission Netralaya, Shillong, Meghalaya, India, 793014, Email: jeetsaikia@yahoo.com.
Dr. Atul kumar Sahu, Department of Ophthalmology, RKN Eye Care, Varanasi, Uttar Pradesh, 221010, India, Email: atulkrsahu@gmail.com.
Dr. Shobhna Mange, Department of Ophthalmology, Shivam Retina Clinic, Surat, Gujarat, India, 395001, Email: shobha_72@yahoo.com.
Dr. Arup Chakraborty, Department of Ophthalmology, Amulya Jyoti Eye Foundation, Kolkata, West Bengal, India, 700029, Email: drarupchak@gmail.com.
Dr. Suprakash Roy, Department of Ophthalmology, Nivedita Eye Care And Microsurgery Centre, Bankura, West Bengal, India, 722141, Email: dr.suprakashroy@gmail.com.
Dr. Valensha Surong, Department of Ophthalmology, Mission Netralaya, Shillong, Meghalaya, India, 793014, Email: valenshasurong@yahoo.com.
Funding
RE-ENACT 2 Study Investigator Group received honorarium from Intas Pharmaceuticals Ltd. for their patient data contribution. Intas Pharmaceuticals Limited also paid the journal’s Rapid Service Fees.
Medical Writing and Editorial Assistance
The authors thank Mr. Shreekant Sharma (ISMPP, CMPP™, Intas Pharmaceuticals Limited) for providing writing assistance and Dr. Venugopal Madhusudhana (ISMPP, CMPP™ Intas Pharmaceuticals Limited) for additional editorial assistance for the development of this manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All the authors contributed to the study conception, design, analysis, and manuscript preparation. RE-ENACT 2 Study Investigator Group also contributed with patients’ data. All authors read and approved the final manuscript.
Disclosures
Drs. Shashikant Sharma, Mujtaba Khan, and Alok Chaturvedi are employees of Intas Pharmaceuticals Limited, Ahmedabad.
Compliance with Ethics Guidelines
This retrospective study was conducted after ethics committee (OM ethics committee, Ahmedabad, India) approval and in accordance with the protocol and the principles of the Helsinki Declaration.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12472949.
Members of the RE-ENACT 2 Study Investigators Group are listed in the Acknowledgements.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sharma, S., RE-ENACT 2 Study Investigators Group., Khan, M. et al. A Multicenter, Retrospective Study (RE-ENACT 2) on Razumab™ (World's First Biosimilar Ranibizumab) in Retinal Vein Occlusion. Ophthalmol Ther 9, 625–639 (2020). https://doi.org/10.1007/s40123-020-00277-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-020-00277-3