Abstract
Age-related macular degeneration (AMD) is the leading cause of visual impairment in the western world, causing significant reduction in quality of life. Despite treatment advances, the burden of visual impairment caused by AMD continues to rise. In addition to traditional low vision rehabilitation and support, optical and electronic aids, and strategies to enhance the use of peripheral vision, implantable telescopic devices have been indicated as a surgical means of enhancing vision. Here we examine the literature on commercially available telescopic devices discussing their design, mode of action, surgical procedure and published outcomes on visual acuity, quality of life, surgical complication rates and cost effectiveness data where available.
Funding Article processing charges were funded by VisionCare Inc.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is the leading cause of visual impairment in the western world, causing significant reduction in quality of life [1]. A meta-analysis of population based studies in 2014 projected that by 2020, 196 million people will have AMD, rising to 288 million in 2040 [2]. Though significant advances in treatment for neovascular AMD have been made with the introduction of anti-vascular endothelial growth factor (VEGF) intravitreal injections [3, 4], a sizeable proportion do not respond. Evidence of active exudation is present on optical coherence tomography (OCT) or angiography in between 25% and 35% of eyes on regular anti-VEGF treatment at 12 months [5,6,7,8]. Additionally, there are no effective treatments for dry AMD available which makes up 90% of cases [9].
Therefore, in spite of these developments the burden of irreversible vision loss from AMD continues to rise. Traditionally, in the United Kingdom (UK), these patients are referred to a low vision clinic. Good visual rehabilitation can help people with AMD make best use of the sight they retain and help them maintain an active life [10]; however, a review of low vision rehabilitation service provision highlights a paucity of well designed high quality research on the effectiveness and in particular cost effectiveness of current low vision services [11].
The majority of low vision rehabilitation in the UK occurs within the National Health Service (NHS) hospital eye service [12]. NHS low vision clinics are primarily optometrist-led and aim to help patients make the best use of their remaining vision by providing suitable low vision aids for tasks identified as being important to the patient. A range of optical magnifiers can be loaned to patients depending on their individual needs. Electronic magnifiers and assistive technology are not provided by the NHS in England, but is demonstrated to the patient where appropriate.
Margrain has reported 88% of those referred to a specialist low vision clinic can read standard sized newsprint using appropriate low vision aids [13, 14]; however, there is debate whether this improves patient’s quality of life. A randomised controlled trial (RCT) comparing standard NHS low vision services to an enhanced service (one additional home visit) for patients with AMD failed to show an improvement in vision-related quality of life in either group despite up to 94% of participants reporting continued use of their magnifiers [15].
Though improved acuity can be obtained using magnifiers, elderly patients can find them difficult to handle particularly when high magnification is needed. A variety of intra-ocular implants have been developed aimed at improving visual acuity in patients with AMD by creating a magnified retinal image [9, 16], without the need for a hand-held magnifier. The method and amount of magnification and resultant visual field produced by these prosthetic devices varies considerably, but in general the magnified retinal image affords patients improved visual acuity at the expense of some peripheral field loss and depth perception. Given this change in visual status, candidates for implantation should be carefully selected and a period of postoperative rehabilitation is often recommended to translate better visual acuity into meaningful improvements in day to day tasks requiring detailed vision, such as reading, seeing faces or watching television.
Though peer reviewed reports on such devices are available, the strength of evidence across devices differs substantially with respect to study design, sample size, length of follow-up, endpoints (whether purely clinical or including functional measures) and effect size.
A systemic literature review was carried out in Embase (1974–2018) and Ovid Medline (1946–2018) using the following keywords: “Age-related macular degeneration”, “macular degeneration”, “intraocular lens”, “intra ocular lens”, “intra-ocular lens”, “telescope”, “telescopic lens” and “telescopic implant”. Related keywords were linked using the Boolean operator AND. All studies describing outcomes of telescopic intra-ocular implants in age-related macular degeneration were included. Studies describing telescopic effects created by an intra-ocular or contact lens used in tandem with a spectacle lens and non-magnifying fresnel lens were excluded. Duplicated results, unpublished abstracts and non-English texts were also excluded.
With the increasing burden of vision loss from AMD, we examine the literature on commercially available prosthetic devices discussing their design and mode of action, the surgical procedure and published outcomes on visual acuity, quality of life, surgical complication rates and cost effectiveness data. Key evidence is summarized in Table 1. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
IOL System (Soleko, Pontecorvo, Italy)
The Intra-ocular Lens for Visually Impaired People (IOL-VIP) uses two separate polymethyl methacrylate (PMMA) IOLs to produce the effect of a Galilean telescope: a high minus (≈ − 66D) IOL implanted into the capsular bag (eyepiece) and a high powered plus (≈+ 55DD) IOL in the anterior chamber (objective) during cataract surgery, with a resultant magnification of ≈ 1.3× [17] and an 80° field of view [18]. The device has been implanted in one or both eyes [17]. It has been suggested that displacing the position of the IOLs can induce a prismatic affect to displace the image away from the diseased macula; however, no evidence has been presented to this affect and the effect is said to be unpredictable [17]. More recently, the IOL-VIP Revolution is a second generation single piece device has become available, which is implanted in the capsular bag.
A pilot study of 40 phakic eyes of 35 patients [17] with a 20 month mean follow-up (range 7–35) and a further case series of 13 phakic eyes of 10 patients [19] followed up for 1 year have been published. Both were single site studies and used the first generation IOL-VIP. A 1.3× external monocular was used to simulate the affect of the magnification preoperatively. Patients completed twelve 30 min computer based training sessions aimed at promoting the use of a preferred retinal locus (PRL) preoperatively and continued with five 30 min sessions per week for 12 weeks postoperatively.
A similar mean change in visual acuity following implantation and rehabilitation was found in each study group: + 5 lines (preoperative best corrected visual acuity ≈ 20/400, postoperative best correct visual acuity ≈ 20/125) [17] and almost + 7 ETDRS lines (preoperative best corrected visual acuity ≈ 20/500, postoperative best correct visual acuity ≈ 20/100) [19]. It was not clear whether preoperative visual acuity was measured before or after preoperative PRL training and the effect of the preoperative training alone was not described. Data on near acuity change were not presented in either study and no formal assessments of quality of life were described; however, subjective reports of improved quality of life were referred to [17, 19].
Implantation of IOL-VIP has been associated with an average increase in postoperative refractive error by around + 8.00DS and + 9.00DS and it has been suggested more favourable results are seen in myopic eyes and eyes with an axial length of greater than 23 mm [19].
Across both studies surgical complications were low and the device well tolerated. Mean endothelial cell loss was 7% [17] and 11.1% [19]. Following three episodes of pupillary block the procedure was modified to include an intra-operative peripheral iridotomy. Fundus examination postoperatively was undisturbed. At the time of writing no published evidence for IOL-VIP Revolution was found.
LMI (Optolight Vision Technology, Herzlia, Israel)
The Lipshitz Mirror Implant (LMI) is a modified conventional IOL with two miniature mirrors arranged in a Cassegrain telescope formation indicated for phakic eyes [20]. This produces a dual optical system similar to a multifocal IOL or contact lens whereby light passing through the central optic is magnified 2.5 times, while light passing through the peripheral or “normal” IOL portion remains unmagnified [20]. In this fashion it should not interfere with peripheral vision whilst providing magnified central vision. In principle the LMI can be implanted in both eyes; however, published outcomes only comment on monocular implantation. No specific rehabilitation programme has been described.
A pilot study of six patients with less than 20/200 vision who underwent unilateral implantation in the worse eye and were followed up for 6 months reported a mean 3.66 line improvement in LogMAR (converted from Snellen measurements) and a 50.83 letter mean increased in single letter near acuity as measured by the Early Treatment Diabetic Retinopathy Study (ETDRS) near vision chart at 20 cm [20].
Quality of life was assessed using a non-validated questionnaire designed for the study at 6 months. Patients were asked to rate their difficult with six tasks: reading small print, reading large print, telling time and counting money, using keyboard or dialing the phone, watching television and independent mobility in a public space. Mean quality of life score changed from 11.16 preoperatively to 4.50 at 6 months which was described as a “statistically significant improvement”. Unfortunately, the method of scoring was not described and so it is difficult to fully quantify the meaning of this change.
No intraoperative complications were reported. Mean endothelial cell loss was less than 6%. All patients experienced glare postoperatively and two patients complained of shadowing which resolved by 3 months. Implantation of the LMI is not thought to limit examination of the fundus significantly [20].
OriLens (Optolight Vision Technology, Herzlia, Israel)
Optolight have also developed a pseudophakic version of the LMI that can be implanted in the sulcus as a piggy-back lens over an existing IOL and provides the same mirror optics of the LMI [21]. There are no peer reviewed studies on the safety and effectiveness of the Orilens presently, however an RCT is currently underway in the UK with results expected in 2018 [16].
Iol-AMD (London Eye Hospital Pharma)
The Iol-AMD, like the IOL-VIP uses the principle of a Galilean telescope to produce between 1.25× and 1.3× magnification in the implanted eye (phakic or pseudophakic), with an anticipated visual field reduction of about 30% [22]. Following removal of the natural lens (or explantation of an existing IOL) two soft hydrophobic IOLs, one high negative and one high positive, are injected separately via 3 mm corneal incisions into the capsular bag and ciliary sulcus, respectively [22, 23]. It is reported that decentration of the optic of the positive IOL relative to the negative IOL can create up to 3° of retinal eccentricity in order to project the retinal image onto healthier paracentral retina if required.
To date, a feasibility pilot study has been published, which saw the Iol-AMD implanted in 18 eyes of 12 patients with AMD and vision worse than 20/80 who were followed up for 4 months. Mean decimal distance visual acuity changed from 0.12 at baseline to 0.2 at 4 months, equivalent to a two-line improvement in LogMAR visual acuity. A similar gain in near visual acuity was noted.
Despite the Iol AMD having a similar design to the IOL-VIP system, its visual acuity improvements were modest in comparison to that reported for the IOL-VIP (two lines of LogMAR acuity versus 5–7 lines). Interestingly, patients undergoing Iol AMD implantation were not given pre- or postoperative rehabilitation so as not to confound the effect of the implant, whereas patients who received the IOL-VIP had extensive pre- and postoperative rehabilitation, and the effect of this alone was not described [17, 22]. Additionally, in contrast to the IOL-VIP, the Iol AMD did not induce the same significant hyperopia, rather a mean change in refraction of − 1.50DS (spherical equivalent) was found. Therefore, spectacle magnification postoperatively may influence the more favourable results see with the IOL-VIP.
Microperimetry and fixation stability were assessed in three patients. Evidence of a smaller bivariate contour ellipse area 4 months after implantation (indicating more stable fixation) was presented. One case of a 5° superior shift in PRL 4 months after implantation was presented as evidence of the induced prismatic effect of the IoL AMD; however, we would suggest the results presented do not make clear whether the shift was induced by the implant or by an eccentric movement of the patient’s eye. The gain in visual acuity of 1 LogMAR line recorded in this case is smaller than the test–retest variability of a normal eye [24]. Therefore, the evidence for the benefit of the prismatic effect of the Iol AMD is not compelling.
Mean reduction in endothelial cell count at 4 months was 18% and no significant postoperative complications were reported. No difficulties imaging the fundus postoperatively were reported.
Quality of life was not assessed in the current study and the authors recommend further evaluation of safety and efficacy of the device with particular attention to functional endpoints.
Scharioth Macula Lens (Medicontur Ltd.)
The Scharioth Macula Lens (SML) is a one-piece hydrophilic acrylic foldable “add-on” IOL designed to improve the near vision of pseudophakic patients with AMD [25] at a 15 cm working distance, without restricting the peripheral visual field. It is implanted into the ciliary sulcus in addition to the patient’s existing IOL through a minimum incision of 2.2 mm. This can be carried out alongside conventional phacoemulsification and posterior capsular IOL insertion or at a later date.
The SML is a refractively neutral 13 mm diameter lens, with a 1.5 mm central optic zone containing a + 10.00DS addition which translates to approximately 2× magnification at a working distance of 15 cm depending on the anatomy of an individual eye [25]. In a similar fashion to multifocal contact lenses for presbyopia, the SML utilizes reflex near vision miosis. On viewing a near target pupil miosis will cause the image formed through the optic zone to dominate. As light also induces miosis, the affect will be greater in bright light [26].
The SML is recommended for patients with vision between 0.1 and 0.4 decimal (20/200–20/50) and is implanted in the better seeing eye. Prior to implantation, patients should demonstrate an improvement in near acuity with a + 6.00DS spectacle lens in the better eye.
To date, two studies examining the effectiveness of the SML have been published; however, a multicentre trial is currently ongoing with results expected soon [25, 26]. Each study enrolled eight patients who were followed up for 1 [25] and 6 [26] months. On comparison of preoperative near acuity with a + 6.00DS spectacle addition and postoperative near acuity with the SML, Scharioth reported patients were on average 2.1 lines better off with the SML at 15 cm than with a + 6.00DS spectacle addition at 1 month (Radner reading chart). Mean distance visual acuity remained unchanged at 0.24 decimal (≈ 20/80) pre and postoperatively [25].
Nekolova reported a mean preoperative near acuity with a + 6.00DS spectacle addition of J2 (approximately 0.5 M/N5 print). Near acuity with the SML was comparable at 1 month (J2.5 at 15 cm), but had declined at 3 and 6 months to J4.5 and J4, respectively (approximately 0.75 M, N6). When compared to unaided preoperative measurements, near acuity improved from J13 (approximately 2.0 M, N18) to J4 (approximately 0.75 M, N6). Distance visual acuity remained unchanged at 0.27 decimal (≈ 20/80) [26, 27].
All patients were instructed to read high contrast text for 10 min at least twice a day using only the implant (no glasses or external magnifiers). Patients reported difficulty adapting to the 15 cm reading distance and 5/8 patients required occlusion of the fellow eye when reading up to 1 month postoperatively. No formal assessment of the impact on implantation on quality of life was reported, though difficulty with reading speed and working distance was reported.
Neither study reported on corneal endothelial cell loss and no postoperative complications were encountered. Visualization of the fundus and OCT examination were not hindered following implantation. We await the findings of the ongoing multicentre clinical trial.
The Implantable Miniature Telescope (IMT, VisionCare Ophthalmic Technologies)
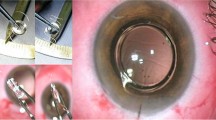
The first incarnation of the IMT and surgical technique were described by Lipshitz and colleagues in 1997 [28] with early clinical results published by Alio in 2004 [29]. VisionCare Inc. (Saratoga, CA, USA) has since developed the technology. The IMT takes the form of a fixed focus Galilean telescopic system comprised of high precision quartz glass wide angle optics [30]. Mounted on a PMMA IOL, it is implanted in the capsular bag following removal of the natural lens. The device is 4.4 mm long, 3.6 mm in diameter and weighs 60 mg in aqueous. Its size necessitates a 10–11 mm limbal or scleral tunnel incision to allow adequate vertical clearance without damage to the endothelium. A peripheral iridectomy is performed and 6–8 sutures are required to close the wound. A detailed description of the surgical technique has been previously published by Colby and Chang [31].
The IMT is currently indicated for phakic eyes; however, a multicentre trial is currently underway in the US investigating the safety and effectiveness of implanting the IMT in pseudophakic patients (Registered at ClinicalTrials.gov: study number NCT03011554).
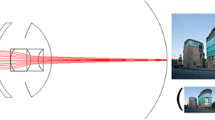
The IMT, in combination with the cornea produces 2.7× magnification of the retinal image projecting it over an approximately 55° area of the central and peripheral retina, which translates to a ≈ 20° field of view. It has an optimum focusing distance of 3 m and a depth of focus between 1.5 and 10 m. Conventional spectacles are used to enhance viewing conditions at distance, intermediate and near. A 2.2× version of the device has also been described [30, 32, 33]; however, it is current practice to use 2.7× version [34].
When implanted in one eye, the magnified view improves central vision whilst the fellow eye is used for navigation and depth perception. Despite the field of view being larger than an equivalent external telescopic device, the significant reduction in visual field means the IMT is not suitable for bilateral implantation, or for single eyed patients. This change in visual status necessitates that all patients complete six sessions of postoperative rehabilitation [34].
A full description of the treatment paradigm has been published [34]. Patients should have bilateral end-stage macular degeneration, no other co-morbidity that could further reduce visual field and visual acuity of between 20/160 and 20/800 in each eye and refraction of the target eye should fall within − 6.00DS and + 4.00DS (spherical equivalent). A simulator replicating the magnification, visual field and light transmission of the IMT in situ is used to predict the increase in visual acuity expected and to assess tolerance to the reduced visual field, loss of binocularity and reduced light transmission cause by the implant. In the presence of a significant increase in visual acuity, tolerance to the simulation, appropriate expectations and confirmation of surgical suitability, the IMT is implanted unilaterally.
Appropriate eye selection has been shown to be a critical factor in determining patient satisfaction and functional success [35]. Briefly, the predicted visual acuity of the implanted eye during simulation should be at least 2 LogMAR lines better than that of the best-correct visual acuity in the fellow eye. In the absence of this difference, there will be no incentive to use the implant eye and the patient is very likely to continue to use their fellow eye for activities of daily living requiring detailed vision [35]. If both eyes satisfy this criterion, ocular dominance with respect to mobility and aiming are assessed to select the eye for implantation. This eye selection algorithm is based upon the results of a multicentre open label prospective trial. Initially 206 patients with a mean baseline visual acuity of 20/320 were implanted with either the 2.7× or 2.2× device across 28 sites in the United States. 1- and 2-year results were published [30, 32] followed by an extension study where 63 patients from the original cohort were followed up for 5 years [33].
At 1 year, mean improvement in ETDRS distance and near acuity was 3.5 lines and 3.2 lines, respectively, with a greater improvement seen in eyes with the 2.7× device [30]. These gains were largely maintained at 2 years [32]. At 5 years, mean gain in acuity from baseline had reduced to 2.4 lines, with those under the age of 75 at implantation retaining 2.6 lines as compared to 2.1 in those over 75 [33].
Notably, as this study evaluated safety and efficacy of the device, the US Food and Drug Administration (FDA) stipulated that where a patient had best corrected visual acuity of better than 20/200 in one or both eyes, the device be implanted in the worse seeing eye. Where best corrected visual acuity was worse than 20/200 in both eye, the eye to be implanted was chosen by the investigator and patient. Despite this, visual acuity goals were met in 90% of cases [35].
Vision-related quality of life was assessed with the 25-item National Eye Institute Visual Function Questionnaire (NEI VFQ-25) [36]. The NEI VFQ-25 is a well-validated instrument, used extensively in ophthalmology and low vision interventional studies and has been shown to be responsive to change with time [15, 37,38,39,40,41,42]. Respondents are asked to rate their general health, vision, ocular pain and their difficulty with a range of daily activities. A total (composite) score is generated together with a range of subscale scores related to general health, general vision, near activities, distance activities, colour vision, social functioning, mental health, role difficulties, dependency, ocular pain, driving and peripheral vision where higher scores indicate better self reported vision related quality of life. A 5-point change in score it thought to be indicative of the clinically meaningful change [43].
NEI VFQ-25 mean composite score rose by 6 points (p < 0.0001) from baseline to 1 year postoperatively. Not only were there clinically and statistically significant gains in vision based domains, specifically general vision (14 points, p < 0.0001), near activities (11 points, p < 0.0001) and distance activities (8 points, p < 0.0001), but psychosocial domains also exhibited similar meaningful improvements: social functioning (9 points, p < 0.0001), mental health (9 points, p < 0.0001), role difficulties (7 points, p = 0.0002) and dependence (10 points, p < 0.0001). As expected, a significant reduction in peripheral vision was reported (6 points, p < 0.0001) and general health declined, perhaps unsurprising in an elderly population (5 points, p = 0.03). Changes in colour vision and ocular pain were not significant and the majority of patients did not answer questions related to driving, as they did not have sufficient vision to drive at the outset of the study.
The device was well accepted and tolerated with inflammatory deposits and pigment on the device being the most commonly reported complications (25% and 11% of cases, respectively) [30, 32]. These were not thought to be visually significant. Mean endothelial cell loss at 1 year was 25% and was correlated with post surgical oedema [30], with a further loss of 2.4% at 2 years [32]. There was no evidence of ongoing corneal trauma following implantation and no significant difference in endothelial cell density between implanted eyes and contralateral pseudophakic fellow eyes [30]. The authors suggest the initial loss is related to the incision size and geometry of the device [32]. Endothelial loss was typically greater during the first three cases than for future cases suggesting a surgical learning curve should be anticipated [32].
A total of 12 explants from 206 cases were recorded (5.6%). Corneal transplantation was required in two eyes within 1 year, both of which had experienced surgical complications [30]. Two additional corneal transplants were reported in a 5-year follow-up extension study [33]. The remaining eight explants were due to “patient dissatisfaction”; however, the specific reasons were not cited [33].
Performing fundus OCT after implantation presents a challenge due the minimization of the fundus image through the device and poor fixation by patients; however, recommended modifications to the Spectralis SD-OCT have been published to overcome these issues [44]. Successful anti-VEGF injection with subsequent OCT monitoring [45] and the application of thermal laser photocoagulation for choroidal neovascularization [30, 46] have also been described.
Cost-effectiveness of the 2.7× IMT implant has been established [47]. When compared to no intervention, the average cost utility ratio was $19,001 per Quality Adjusted Life Year (QALY). In the UK interventions costing < £20,000/QALY) are considered to be cost-effective by NICE [47, 48].
Following publication of these favorable results, the IMT was granted FDA approval in July 2010 for patients over the age of 75 and on completion of the 5-year follow-up extension study the age limit was lowered to 65 years [34].
Discussion
As the burden of visual impairment from macular degeneration rises, telescopic implants may offer hope to some patients; however, gaps in the evidence still exist. Much of the evidence for commercially available implants is based on small, single site, single surgeon studies. Though we are aware of one ongoing Orilens RCT, there have been no RCT results published to date.
Notably, long-term follow-up data are lacking for LMI, IoL-AMD and SML. The IOL-VIP has published outcomes at a mean follow-up of 20 months (7–35 months) [17] while 5-year follow-up data are available for the IMT [33].
Pleasingly all telescopic implants were well tolerated within the eye. Arguably, implantation of the IMT commands a higher level of surgical skill than is required for the procedures associated with other devices due to the device size and large incision needed. Notably, an injectable version of the IMT device is currently being investigated in a single site trial in Ireland and if positive will reduce the incision from 12 to 6.5–7.0 mm (Personal communication, VisionCare Inc., 2nd Oct 2017).
In September 2016, the National Institute for Clinical Excellence (NICE) in the UK published Interventional Procedure Guidance for “Miniature lens system implantation for advanced age-related macular degeneration” noting that devices can improve visual acuity and quality of life in the short term [49]. The report examined evidence from the IMT, IOL-VIP and the LMI and conceded that the majority of the evidence is based on the IMT device. In addition, NICE recommends these procedures be carried out by experienced cataract surgeons and stressed the importance of assessing the patient’s ability to cope with the change in visual status prior to surgery. They recommend continued audit and review of clinical outcomes of patients having telescopic implants. Presently, the IMT is the only implant approved by the Food and Drug Administration (FDA).
It is important to evaluate the influence of telescopic devices on visual field and binocularly, given the considerable variation between devices. The field of view restriction stated for each device refers to manufactures specifications rather than a post-implantation objective visual field assessment and there are no published mobility related outcomes such as navigational performance or incidence of falls; however, self reported peripheral vision was significantly worse in patients with the IMT after 12 months. Implants that limit peripheral field significantly and interrupt binocularity may not be suitable for those with existing mobility problems.
With the exception of the IMT, functional effectiveness has been based on improved visual acuity without demonstrating an associated improvement in vision related quality of life on a standardized outcome measure. The use of subjective patient reported outcomes has grown tremendously in ophthalmology in the last 2–3 decades and it is no longer seen as sufficient in interventional trials to rely on objective clinical measures only [50]. The absence of these data may reflect the small scale of the studies presented and we would encourage authors to include it in future studies.
Difficulty reading is the most commonly reported complaint of patients attending low vision services [51, 52]. Though the majority of implant studies reported improvements in near acuity, measures of reading performance were not included any study. There is debate as to the most appropriate method of assessing reading performance, for example reading speed, comprehension or critical print size (the smallest sized print a person can read at maximum speed) [51]. A lack of correlation between maximum reading speed and visual acuity in patients with geographic atrophy has been described [53], therefore near acuity alone may not provide sufficient information to predict fluency of reading. It is generally accepted that spot reading (i.e. reading a label) requires a reading speed of 40 words per minute (wpm), fluent reading requires 80 wpm and highly fluent reading at a speed of 160 wpm [54], hence including reading speed data in future trials could elucidate those reading tasks devices likely to improve, a beneficial aid to setting appropriate expectations for future patients. It should be noted that fluent reading post-implantation may not be possible especially where pre-implantation is very poor.
Appropriate expectations are of utmost importance with any device. Where there is the possibility of simulating the outcome, patients must be satisfied with the improvement without harbouring hopes of a greater improvement or different result. Patients and professionals must appreciate that these devices provide an enlarged retinal image to differing degrees. They cannot restore vision to pre-AMD levels and they do not treat the underlying cause of visual impairment. In addition to producing a magnified image, the IoL AMD and IOL VIP may create a prismatic effect to shift the magnified image away from diseased macula onto healthier peripheral retina; however, there is limited published evidence in support of this. It is vital that the strength of the evidence available for each device and to offer realistic outcomes to patients based on the available evidence.
It is also worth considering a patient’s mental state with respect to selection for implantation and overall satisfaction with the outcome. Depression is a significant co-morbidity in around 30% of patients with AMD [55]. Recently, 1323 consecutive attendees of 16 NHS funded low vision clinics were screened for depressive symptoms. Forty-three percent of patients exhibited such symptoms and of those, 75% were not receiving treatment [56]. Though identifying mental health issues may be considered outside the remit of an ophthalmological assessment, it may be prudent to consider mental health state within a detailed history of any patient considering a telescopic device. This is of particular importance where a significant postoperative rehabilitation demand exists, as depression and low mood may affect motivation to attend and engage with rehabilitation sessions and ultimately satisfaction with the outcome. Interestingly, patients undergoing IMT implantation and subsequent post-surgery rehabilitation showed significant improvements in psycho-social domains of the NEI VFQ questionnaire after 12 months. There are no longer-term NEI VFQ data available.
Though originally developed for patients with macular degeneration, the use of implants in patients with other forms of central vision loss has been considered.
Six elderly patients with Stargardt’s disease were included in the IMT cohort, and of these, three (50%) had an explant due to dissatisfaction (VisionCare Inc, personal communication, Oct 2nd 2017). It could be hypothesized that patients who have lived with significant central visual impairment for many decades find it more challenging to adapt to the new visual status created by IMT implantation than someone who has lived a relatively short time with a central visual impairment. Different fixation patterns in eyes with AMD and Stargardt’s may also help account for the less than favourable results in Stargardt’s patients. AMD patients tend to fixate at the edge of the scotoma whereas Stargardt’s patients often use a more eccentric position, appreciably further from the edge of the scotoma border where the resolution of the retina is lower [53] and so the benefit of magnification is minimised. As a result, the IMT is specifically contraindicated in Stargardt’s disease and is only licensed for use in patients with AMD.
Furthermore the potential of the Iol AMD, LMI and SML for patients with other forms of central vision loss has been discussed, in particularly macular holes [20, 22], diabetic maculopathy [20, 22, 25], myopic maculopathy [25], retinitis pigmentosa and Usher’s syndrome, Stargardt’s disease, glaucoma, albinism, solar retinitis and toxoplasmosis [20]. Aside from two non-AMD patients included in the 2008 LMI study [20] (one myopic degeneration and one macular dystrophy) and patients with myopic degeneration receiving the IOL-VIP [19], the effectiveness of telescopic devices in patients with other conditions has not been fully investigated. Caution is warranted in overstating the effectiveness of implants in patients with conditions other than age related macular degeneration, especially where their effects have not been investigated.
Additional guidance on appropriate candidate and eye selection has been retrospectively published for the IMT based on clinical trial data [35]. Presently, it is not possible to characterize fully the population of patients who stand to benefit from the other currently available technologies; however, this has been identified as an area for future work by many authors. Should more robust evidence become available, the range of magnification offered by implants may allow implants to be indicated for patients depending on the extent of their vision loss, i.e. implants with lesser magnification indicated for patients with better preoperative visual acuity while implants providing higher imagination indicated for patients with more significant visual acuity loss. However where there is a risk of disease progression and further vision loss, the benefit of low levels of magnification may be eliminated. It would also be interesting to evaluate the impact of other aspects of visual function such as scotoma size or contrast sensitivity on patient suitability.
Concerns about the claimed effectiveness of telescopic implants have been raised by a well respected group of low vision professionals [57] who have called for the relative benefits of cataract extraction, device implantation and the often-intensive rehabilitation programmes required post-implantation to be estimated. Authors have made attempts to address some of these concerns. Qureshi et al. intentionally omitted pre and post rehabilitation from their study protocol so as not to confound the effect of the Iol-AMD [22]. Aiming to estimate the effect of cataract extraction only, Orzalesi et al. considered a subgroup of 12 patients who had previous fellow eye cataract extraction for lens opacities similar or worse than those in their eye implanted with the IOL-VIP. Visual acuity improved two lines in eyes that received a traditional IOL only compared to six lines in eyes with the IOL VIP system [17]. Hudson and colleagues compared change of visual acuity in IMT implanted eyes to non-implanted fellow eyes as a control to estimate any rehabilitation effect [30]. Though implanted eyes displayed a mean improvement of 3.5 LogMAR lines compared to 0.8 lines in fellow eyes, 13% of fellow eyes gained 3 lines of LogMAR respectively at 12 months, an effect that was attributed to the rehabilitation.
Despite these attempts, only an RCT comparing implantation plus rehabilitation, to rehabilitation only as a control can provide definitive evidence of effectiveness. One could argue the choice of appropriate control is hampered somewhat by the lack of good quality evidence of effectiveness of low vision services [11]. The results of the Orilens RCT will be an important contribution to the field.
Undoubtedly, low vision optometrists and/or rehabilitation practitioners provide an essential role in working closely with implant patients to convert acuity gains into functional improvements in activities of daily living. We believe the introduction of telescopic implants for AMD patients should be viewed as an adjunct to existing low vision therapy rather than a replacement. As the magnification provided by implants is relatively low, patients are likely to benefit from the prescription of additional external magnification for visually demanding tasks. Implants are compatible with optical aids, as well as adaptive technology such as electronic magnifiers, and the additional on screen magnification provided by accessibility features or magnification software programmes on tablets, desktops and laptops. In each instance, patients should need less magnification than before implantation, thus providing a larger field of view.
Conclusions
In summary, on the basis of NICE guidance, number of patients implanted, length of follow-up, safety profile, gain in distance and near acuity and self reported vision related quality of life, the available published evidence for the IMT is stronger than that of any other commercially available device. Additionally, it is the only device demonstrated to be cost effective by way of a health economics assessment [47]. Potential shortcomings include its visual field restriction, more complex surgery and the lack of evidence for pseudophakic patients, though two trials are under way exploring the feasibility of smaller incision surgery and pseudophakic IOL/IMT exchange.
When considering telescopic implants, not only is there a need to carefully consider the available evidence, but also the expectations and goals of each individual patient in relation to the expected outcome for any device under consideration. Only then will successful outcomes be achieved.
References
Wiliams RA, et al. The psychosocial impact of macular degeneration. Arch Ophthalmol. 1998;116:514–20.
Wong WL, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–16.
Brown DM, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: 2-year results of the ANCHOR study. Ophthalmology. 2009;116(57–65):e55.
Rosenfeld PJ, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Heier JS, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Broadhead GK, Hong T, Chang AA. Treating the untreatable patient: current options for the management of treatment-resistant neovascular age-related macular degeneration. Acta Ophthalmol. 2014;92:713–23.
Martin DF, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: 2-year results. Ophthalmology. 2012;119:1388–98.
Ehlken C, et al. Switch of anti-VEGF agents is an option for nonresponders in the treatment of AMD. Eye. 2014;28:538–45.
Singer MA, et al. Improving quality of life in patients with end-stage age-related macular degeneration: focus on miniature ocular implants. Clin Ophthalmol. 2012;6:33–9.
Ryan B. Fragmented vision. Survey of low vision services in the UK. London: Royal National Institute for the Blind; 1999.
Binns AM, et al. How effective is low vision service provision? A systematic review. Surv Ophthalmol. 2012;57:34–65.
Culham LE, et al. Low vision services for vision rehabilitation in the United Kingdom. Br J Ophthalmol. 2002;86:743–7.
Margrain TH. Minimising the impact of visual impairment. Low vision aids are a simple way of alleviating impairment. BMJ. 1999;318:1504.
Margrain TH. Helping blind and partially sighted people to read: the effectiveness of low vision aids. Br J Ophthalmol. 2000;84:919–21.
Reeves BC, Harper RA, Russell WB. Enhanced low vision rehabilitation for people with age related macular degeneration: a randomised controlled trial. Br J Ophthalmol. 2004;88:1443–9.
Teh BL, et al. Optimizing cataract surgery in patients with age-related macular degeneration. Surv Ophthalmol. 2017;62:346–56.
Orzalesi N, et al. The IOL-Vip System: a double intraocular lens implant for visual rehabilitation of patients with macular disease. Ophthalmology. 2007;114:860–5.
Felipe A, et al. Optical analysis to predict outcomes after implantation of a double intraocular lens magnification device. J Cataract Refract Surg. 2007;33:1781–9.
Amselem L, et al. Clinical magnification and residual refraction after implantation of a double intraocular lens system in patients with macular degeneration. J Cataract Refract Surg. 2008;34:1571–7.
Agarwal A, et al. Mirror telescopic intraocular lens for age-related macular degeneration: design and preliminary clinical results of the Lipshitz macular implant. J Cataract Refract Surg. 2008;34:87–94.
Jacob S. New Technology For AMD. The cataract surgeon’s solution to a retinal problem. Eurotimes 2012;17/18(123/1).
Qureshi MA, et al. Injectable intraocular telescope: pilot study. J Cataract Refract Surg. 2015;41:2125–35.
Tabernero J, et al. An aspheric intraocular telescope for age-related macular degeneration patients. Biomed Opt Express. 2015;6:1010–20.
Rosser DA, et al. How sensitive to clinical change are ETDRS LogMAR visual acuity measurements? Investig Ophthalmol Vis Sci. 2003;44:3278–81.
Scharioth GB. New add-on intraocular lens for patients with age-related macular degeneration. J Cataract Refract Surg. 2015;41:1559–63.
Nekolova J, et al. Scharioth Macula Lens: a new intraocular implant for low-vision patients with stabilized maculopathy-first experience. Olomouc: Biomedical papers of the Medical Faculty of the University Palacky; 2017.
Colenbrander A, Runge P. Can Jaeger numbers be standardized. Investig Ophthalmol Vis Sci. 2007;48:3563.
Lipshitz I, et al. An intraocular telescopic lens for macular degeneration. Ophthalmic Surg Lasers. 1997;28:513–7.
Alio JL, et al. Intraocular telescopic lens evaluation in patients with age-related macular degeneration. J Cataract Refract Surg. 2004;30:1177–89.
Hudson HL, et al. Implantable miniature telescope for the treatment of visual acuity loss resulting from end-stage age-related macular degeneration: 1-year results. Ophthalmology. 2006;113:1987–2001.
Colby KA, et al. Surgical placement of an optical prosthetic device for end-stage macular degeneration: the implantable miniature telescope. Arch Ophthalmol. 2007;125:1118–21.
Hudson HL, et al. Implantable telescope for end-stage age-related macular degeneration: long-term visual acuity and safety outcomes. Am J Ophthalmol. 2008;146:664–73.
Boyer D, et al. Long-term (60-month) results for the implantable miniature telescope: efficacy and safety outcomes stratified by age in patients with end-stage age-related macular degeneration. Clin Ophthalmol. 2015;9:1099–107.
Hau VS, London N, Dalton M. The treatment paradigm for the implantable miniature telescope. Ophthalmol Ther. 2016;5:21–30.
Primo SA. Implantable miniature telescope: lessons learned. Optometry. 2010;81:86–93.
Mangione CM, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8.
Bressler NM, et al. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol. 2003;121:1621–4.
Clemons TE, et al. National Eye Institute Visual Function Questionnaire in the Age-Related Eye Disease Study (AREDS): AREDS Report No. 10. Arch Ophthalmol. 2003;121:211–7.
Lindblad AS, Clemons TE. Responsiveness of the National Eye Institute Visual Function Questionnaire to progression to advanced age-related macular degeneration, vision loss, and lens opacity: AREDS Report no. 14. Arch Ophthalmol. 2005;123:1207–14.
Bass EB, et al. Patients’ perceptions of the value of current vision: assessment of preference values among patients with subfoveal choroidal neovascularization—The Submacular Surgery Trials Vision Preference Value Scale: SST Report No. 6. Arch Ophthalmol. 2004;122:1856–67.
Miskala PH, et al. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: quality-of-life findings: SST report no. 12. Ophthalmology. 2004;111:1981–92.
Hawkins BS, et al. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: ophthalmic findings: SST report no. 11. Ophthalmology. 2004;111:1967–80.
Globe DR, et al. The impact of visual impairment on self-reported visual functioning in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1141–9.
Pappas A, Fortin M, Joondeph BC. SD-OCT imaging in eyes implanted with a miniature telescope. Retina Today. 2015;10(5).
Joondeph BC. Anti-vascular endothelial growth factor injection technique for recurrent exudative macular degeneration in a telescope-implanted eye. Retin Cases Brief Rep. 2014;8:342–4.
Garfinkel RA, Berinstein DM, Frantz R. Treatment of choroidal neovascularization through the implantable miniature telescope. Am J Ophthalmol. 2006;141:766–7.
Brown GC, et al. Comparative effectiveness and cost-effectiveness of the implantable miniature telescope. Ophthalmology. 2011;118:1834–43.
NICE National Institue for Health and Care Excellence. The guidelines manual. Process and methods [PMG6] Section 7: assessing cost effectiveness. 2012. https://www.nice.org.uk/process/pmg6/chapter/assessing-cost-effectiveness. Accessed 3 Aug 2017
NICE National Institue for Health and Care Excellence. Miniature lens system implantation for advanced age-related macular degeneration. Interventional procedures guidance [IPG565]. 2016. https://www.nice.org.uk/guidance/ipg565. Accessed 3 Aug 2017
Massof RW, Rubin GS. Visual function assessment questionnaires. Surv Ophthalmol. 2001;45:531–48.
Rubin GS. Measuring reading performance. Vis Res. 2013;90:43–51.
Elliott DB, et al. Demographic characteristics of the vision-disabled elderly. Investig Ophthalmol Vis Sci. 1997;38:2566–75.
Sunness JS, et al. Fixation patterns and reading rates in eyes with central scotomas from advanced atrophic age-related macular degeneration and Stargardt disease. Ophthalmology. 1996;103:1458–66.
Whittaker SG, Lovie-Kitchin J. Visual requirements for reading. Optom Vis Sci Off Publ Am Acad Optom. 1993;70:54–65.
Casten RJ, Rovner BW, Tasman W. Age-related macular degeneration and depression: a review of recent research. Curr Opin Ophthalmol. 2004;15:181–3.
Nollett CL, et al. High prevalence of untreated depression in patients accessing low-vision services. Ophthalmology. 2016;123:440–1.
Colenbrander A, et al. Rehabilitation and intraocular telescopes. Ophthalmology. 2008;115:1437–8 (author reply 1438).
Acknowledgements
Funding
Article processing charges were funded by VisionCare Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Hannah M P Dunbar is a paid consultant of VisionCare Inc. Felipe E Dhawahir-Scala is a consultant advisor for DORC and Alcon.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6115856.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dunbar, H.M.P., Dhawahir-Scala, F.E. A Discussion of Commercially Available Intra-ocular Telescopic Implants for Patients with Age-Related Macular Degeneration. Ophthalmol Ther 7, 33–48 (2018). https://doi.org/10.1007/s40123-018-0129-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-018-0129-7