Abstract
For several years, the gold standard for surgical treatment of glaucoma has been trabeculectomy. Although very successful at reducing intraocular pressure (IOP), there are several potential complications of trabeculectomy, including sight-threatening ones. This has stimulated much research aimed at the development of new and effective procedures to lower IOP with an enhanced safety profile. Minimally invasive glaucoma surgery (MIGS) procedures prioritise patient safety but also demonstrate efficacy in reducing IOP. We performed an online search of peer-reviewed literature using PubMed, entering keywords relevant to this clinical discipline. In summary, there is a lack of long-term safety and efficacy data, a lack of comparative data and a lack of data on standalone (i.e. without simultaneous cataract surgery) procedures. Most implants are not yet FDA approved. Although not exhaustive, since it does not discuss MIGS procedures that are not implants, this article summarises the range of different MIGS implants that are available to the ophthalmic surgeon.
Similar content being viewed by others
Introduction
Glaucoma remains the leading cause of irreversible blindness worldwide. It is estimated that 64.3 million people have glaucoma [1]. The only modifiable risk factor is raised intraocular pressure (IOP), with therapy aimed at reducing this by various means [2]. This includes topical hypotensive agents, laser trabeculoplasty and glaucoma drainage surgery. Adherence to drops and their multiple side effects limits their use and efficacy, whereas the effects of laser trabeculoplasty wear off over time, requiring multiple repeat procedures [3, 4]. Drainage surgeries such as trabeculectomy and aqueous shunts demonstrate excellent efficacy but have less than ideal risk profiles [5]. Despite their efficacy, tube and trabeculectomy patients have similar rates of vision-threatening complications such as endophthalmitis or choroidal haemorrhage; ocular surface scarring and ocular surface disease can lead to poor patient quality of life [6].
Minimally invasive glaucoma surgery (MIGS) offers a safer, less invasive means of reducing IOP than traditional surgery, with a goal of reducing dependency on topical agents. MIGS can usually be combined with cataract surgery, and most clinical studies have analysed results of combined surgery. With MIGS, there is a trade-off between enhanced safety and less efficacy compared to traditional surgery. MIGS procedures are currently targeted at patients with mild-to-moderate glaucoma.
Generally speaking, MIGS procedures are ab interno, microincisional and conjunctiva-sparing. They share a common approach of minimal tissue trauma and minimal disruption of the normal anatomy and physiology [7]. For the patient, MIGS provides IOP control in the mid- to low teens with rapid visual rehabilitation and less dependence on topical treatment. This article summarises the current literature on the range of MIGS implants available. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by the author.
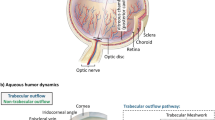
There are three main groups of implants. These include implants that increase trabecular outflow by bypassing the juxtacanalicular trabecular meshwork (TM), increasing uveoscleral outflow via suprachoroidal pathways, or by creating a subconjunctival drainage pathway. Table 1 summarises the different implants and their mechanisms of action; the ones highlighted in bold are discussed in this review.
Trabecular Meshwark Bypass Stents
iStent (Glaukos Corp., Laguna Hills, CA, USA)—this is a heparin-coated, nonferromagnetic titanium device with a snorkel shape that is used for implantation into Schlemm’s canal (SC). It can be implanted alone (single or multiple), or in combination with cataract surgery (Fig. 1).
The iStent Study Group [8] randomised controlled trial (RCT) compared cataract surgery alone to combined surgery with one iStent (n = 239; 116 in a combined/study group and 123 in a cataract surgery only group). The primary outcome measure was defined as IOP (measured in mmHg) ≤21 mmHg on no topical hypotensive medications at 12 months. The secondary outcome measure was subjects with an IOP reduction from baseline of greater ≥20% without the use of a topical hypotensive medication at 12 months.
In the study group (combined surgery group), 72% had IOP <21 mmHg at 12 months and 61% at 24 months, which was statistically significant. In the cataract surgery group, the success rate was 50% at 12 and 24 months. Mean IOP reduction was 8.4 ± 3.6 mmHg at 12 and 24 months in the study group. There was a mean decrease in medications, 1.4 ± 0.8, in the study group and 1.0 ± 0.8 in the controls (p = 0.005) at 12 months. In the study group, 15% were taking glaucoma medications at 12 months compared to 35% in the cataract group (p = 0.001).
Although there is more emphasis on using MIGS for earlier stages of glaucoma, the use of iStent in more advanced cases of glaucoma, including post-drainage surgery eyes, has also been studied [9]. At 36 months, the mean IOP in cases with no previous surgery was 15.4 ± 2.2 mmHg, with 13% of eyes on topical treatment. The mean IOP in the group with previous surgery was 14.2 ± 2.3 mmHg, with 44% of eyes on medications.
There is evidence of enhanced IOP reduction with multiple implants. In a prospective study (n = 119) of iStent-only cases, at 18 months follow-up, the IOP was 15.9 ± 0.9 mmHg with one stent, 14.1 ± 1.0 mmHg with two stents and 12.2 ± 1.1 mmHg with three stents [10]. Implantation of each additional stent realised a significant further reduction in IOP (p = 0.001).
This has led to the development of a second-generation iStent, the iStent Inject. This is preloaded with two stents. In one study, implantation of this device resulted in IOP ≤18 mmHg in 66% of cases at 12 months, with an encouraging safety profile [11].
Hydrus (Ivantis Inc., Irvine, CA, USA)—this is a crescent-shaped trabecular bypass device made of nitinol (an alloy of nickel and titanium), a shape-memory alloy. This means that, when deformed, it returns to its original shape after being heated. Being 8 mm long, it straddles 3 clock hours of SC, with the aim being to access more collector channels and dilate the SC. It acts as a scaffold so that it does not block the collector channel ostia.
A RCT of 100 cases randomised to cataract surgery alone or combined cataract surgery with Hydrus has been completed [12]. There was a washout of glaucoma drops and the results were presented without the influence of glaucoma medications. At 24 months, a significantly greater proportion of the combined surgery cases reached the endpoint of a 20% reduction in diurnal IOP (80% versus 46%, p = 0.0008). The IOP was also significantly lower in the combined surgery group (16.9 ± 3.3 versus 19.2 ± 4.7 mmHg, p = 0.0093), and there was a significant reduction in cases without ocular hypotensive medications in the combined surgery group (73% versus 38%, p = 0.0008).
The safety in the Hydrus group was similar to that in the control. Six of 50 (12%) Hydrus patients had focal peripheral anterior synechiae, but this did not affect device efficacy adversely. The Hydrus stent would have the same indications and relative contraindications as the iStent in theory, but more data are needed to support this.
Suprachoroidal Implants
The CyPass (Alcon, Fort Worth, TX, USA), a suprachoroidal/supraciliary implant, is discussed here. The iStent supra (ab interno) and the SOLX Gold microshunt (ab externo) (SOLX, Waltham, MA, USA) are other examples of suprachoroidal shunts, but there are no published clinical trials on them. SOLX Gold could be used in the presence of corneal opacities precluding a clear view on gonioscopy.
The CyPass is a polyamide implant, 6.35 mm in length and 510 μm in external diameter, that forms a connection between the anterior chamber and the supraciliary space. The collar of the device rests in the anterior chamber angle (Fig. 2), and microholes placed along its length allow for circumferential egress of aqueous into the supraciliary space. Implant position can be confirmed by gonioscopy and/or anterior segment OCT [13].
CyPass with Cataract Extraction and Intraocular Lens Implant (CE/IOL)
Hoeh et al. conducted a multicenter prospective study of 57 uncontrolled (≥21 mmHg) POAG patients and 41 controlled (<21 mmHg) POAG patients undergoing CyPass implantation and CE/IOL. The safety profile was encouraging. The mean medicated IOP in both groups combined was 21.1 ± 5.91 mmHg (the baseline IOP for each group was not stated). IOP at 6 months was 15.6 ± 0.53 mmHg with 0.9 ± 0.15 medications in the uncontrolled group; i.e. a 37% reduction in IOP (p < 0.001) and a 50% reduction in medications (p < 0.001). The resulting IOP in controlled patients was 15.6 ± 0.68 mmHg on 0.6 ± 0.07 medications; i.e. a 71.4% reduction in medications (p < 0.001) [14].
In the CYCLE study, 136 cases were followed up prospectively for 2 years [15]. At baseline, the uncontrolled group of 51 eyes had IOP ≥21 mmHg and the controlled group of 85 eyes had IOP <21 mmHg. CyPass implantation and CE/IOL were performed in each case.
At 24 months, 82 subjects remained in the study. No sight-threatening adverse events occurred. Transient hypotony (15.4%) and microstent obstruction (8.8%) were the most common complications. Fifteen subjects (11%) required glaucoma drainage surgery. In the uncontrolled group (n = 23), mean ± SD IOP was 15.8 ± 3.8 mmHg after 24 months (change, −37% ± 19%), which was statistically significant (p < 0.0001) at months 6, 12, and 24. For the controlled group (n = 59), mean ± SD IOP at 24 months was 16.1 ± 3.2 mmHg (change, 0% ± 28%). Mean decrease from baseline was statistically significant at months 6 (p = 0.0188) and 12 (p = 0.0356). At 24 months, the mean ± SD number of medications was 1.0 ± 1.1 in the uncontrolled group and 1.1 ± 1.1 in the controlled group. Mean decrease from baseline medication use was statistically significant for both groups at months 6, 12 and 24.
The COMPASS Trial was a two-year RCT of 505 subjects randomized to CE/IOL + CyPass or CE/IOL alone [16]. The treatment group demonstrated IOP lowering of 7.4 mmHg compared to 5.4 mmHg in the control group (p < 0.001), with 85% of treated patients being drop-free at 24 months. There were no vision-threatening AEs in the CyPass group and visual acuity was at least 20/40 in 98% of all cases studied.
CyPass Implantation Alone
In contrast to the COMPASS Trial, the DUETTE study followed patients (n = 65) for 1 year following standalone CyPass implantation [17]. This was a multicentre, single-arm interventional study of 65 eyes with OAG and IOP uncontrolled at >21 mmHg on topical therapy. Baseline IOP was reduced from 24.5 ± 2.8 mmHg with 2.2 ± 1.1 medications to 16.4 ± 5.5 mmHg with 1.4 ± 1.3 medications at 12 months, i.e. a 34.7% reduction in IOP. IOP spikes to >30 mmHg lasted beyond 1 month in 11% of cases; 12.2% had cataract progression at 12 months; four eyes had hyphaema that resolved by month 1. 17% of patients went on to require a trabeculectomy; 6% exited the study by choice, and 4.6% were lost to follow-up.
Viscopass is a term used to describe CyPass implantation combined with the injection of 60 μL of ophthalmic viscosurgical device (OVD) at the end of the lumen to increase the size of the aqueous drainage area created by the CyPass Micro-Stent. A clinical trial comparing this with CyPass alone is underway.
Subconjunctival Filtration
The implants in this group use subconjunctival filtration to establish a nonphysiological route for aqueous outflow akin to traditional trabeculectomy and aqueous shunt surgery. Therefore, they require subconjunctival mitomycin C (MMC) injection pre-insertion to optimise bleb function and survival.
XEN Gel Stent
Subconjunctival filtration represents a familiar route for aqueous outflow, and is the basis of trabeculectomy and aqueous shunt procedures. The XEN gel stent (Allergan, Dublin, Ireland) is an ab interno gelatin stent that is implanted via a clear corneal incision, avoiding conjunctival dissection. It is 6 mm in length and composed of porcine gelatin crosslinked with glutaraldehyde. Three models have been evaluated with inner diameters of 45, 63 and 140 μm [18], with 45 μm being recommended by the manufacturer (Fig. 3). Due to Poiseuille’s law of laminar flow, the length and inner diameter of the tube determine the rate of flow, and thus the flow resistance—which prevents hypotony. Preclinical tests established that the implant does not occlude inside the lumen and the implant material does not cause a tissue reaction in the eye [18].
There are few published studies on the XEN 45 implant. Two pilot studies [19, 20] have been published. One study investigated the insertion of one XEN implant (the 63 or 140 model) +MMC combined with cataract surgery, and demonstrated a reduction of IOP from 22.4 (±4.2) mmHg to 15.4 (±3.0) mmHg at 12 months; there was a reduction in glaucoma medications from 2.5 ± 1.4 to 0.9 ± 1.0 [20].
In another pilot study using XEN 140 model implantation alone +MMC (n = 49 eyes), 40% had unqualified success at 12 months (IOP ≤18 mmHg and ≥20% reduction in IOP), 89% had qualified success, and most cases involved a previous failed trabeculectomy [21].
In those studies, there were no serious adverse events such as tube erosion or prolonged hypotony, but some patients had injection of ophthalmic viscosurgical device into the anterior chamber, particularly in the XEN 140 group.
A small prospective interventional study of 13 cases (XEN 45 +MMC)—mostly combined procedures (n = 10), with a follow-up period of 12 months—was performed recently [21]. IOP dropped from 16 ± 4 mmHg pre-op to 9 ± 5, 11 ± 6, 12 ± 5, 12 ± 4 and 12 ± 3 mmHg at 1 week, 1, 3, 6 and 12 months (p = 0.004, 0.026, 0.034, 0.01 and 0.01, Wilcoxon signed-rank test) consecutively. Mean number of medications dropped from 1.9 ± 1 preoperatively to 0.3 ± 0.49 (p = 0.003) at 1 year. 42% of eyes achieved complete success (IOP reduction of at least 20% from baseline without medications) and 66% achieved qualified success. Complications included choroidal detachment in 2 eyes and implant extrusion in 1 eye, and 2 eyes underwent trabeculectomy.
Obviously, larger studies are required to evaluate long-term efficacy and late complications. Results of the phase 4 APEX trial using XEN 45 will be available later this year.
InnFocus
The InnFocus microshunt (Santen Pharmaceutical Company Ltd, Osaka, Japan) is an ab externo drainage device. It involves more steps akin to trabeculectomy compared to other MIGS. The material is SIBS (polystyrene-block-isobutylene-block-styrene) which has been developed specifically for medical implants. It is a biocompatible and biostable thermoplastic elastomer (Fig. 4).
In one study (n = 23 eyes) of microshunt +MMC, 80% had IOP ≤14 mmHg at 3 years, but some cases were standalone and others were combined procedures. The mean IOP for the entire group was 10.7 ± 1.5 mmHg at 3 years; the qualified success rate was 95%, with a reduction in medications from 2.6 ± 0.9 to 0.8 ± 1.2 [22].
Transient hypotony and transient choroidal effusion occurred in 13% and 8.7%, respectively, but with spontaneous resolution. There were no serious long-term adverse events, leaks, erosions, migrations or infections.
The results of the RCT of InnFocus microshunt versus trabeculectomy are awaited.
Discussion
MIGS implants targeting different aqueous outflow pathways offer an improved safety profile for glaucoma surgery while preserving modest efficacy (Table 2). An important advantage for patients in addition to the safety element is that comorbid cataract can be treated simultaneously with MIGS implants. The procedures targeting the subconjunctival space appear to be more efficacious in terms of IOP reduction. However, there is a lack of comparative studies between the different implants. Although RCTs have been conducted for some of the implants discussed, well-designed randomised clinical trials with an extended follow-up are needed to evaluate the long-term efficacy and late complications of these implants.
MIGS technology has potential advantages that could improve the management of glaucoma. These include reducing the medication burden, which enhances patient quality of life [3], bypassing or delaying the need for more invasive surgery and preserving the conjunctiva if a more-invasive intervention were to be required later on. There are limited data on the cost-effectiveness of MIGS, but one study showed the cost-effectiveness of iStent compared to branded medications [23]. More studies like this are required for a wider range of MIGSs to demonstrate their cost-effectiveness compared to medical treatment.
However, there are several limitations to the current state of MIGS. There is a lack of high-quality data, a lack of study standardization, a lack of cost-effectiveness data, a lack of long-term data and incomplete knowledge of ideal patient selection. Furthermore, many studies have been performed for cases combined with cataract surgery, meaning that they lack robust evidence for the effect of MIGS alone [24]. It is also unclear which established procedures should be compared to the MIGS devices.
Standardisation and improvements in the quality of future MIGS studies will help clinicians to negotiate this ever-expanding area more knowledgably and help them to optimise the selection of the appropriate device for the right patient. With the correct approach to investigating and evaluating new technologies, there is much potential for future generations of MIGS to improve the quality of care for glaucoma patients.
Change history
13 September 2017
An erratum to this article has been published.
References
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90.
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79.
Lemij HG, Hoevenaars JG, van der Windt C, Baudouin C. Patient satisfaction with glaucoma therapy: reality or myth? Clin Ophthalmol. 2015;9:785–93.
Leahy KE, White AJ. Selective laser trabeculoplasty: current perspectives. Clin Ophthalmol. 2015;9:833–41.
Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC, Tube Versus Trabeculectomy Study Group. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–14.
Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL, Tube versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153:789–803.
Saheb H, Ahmed IIK. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96–104.
Samuelson TW, Katz LF, Wells JM, Duh YJ, Giamporcaro JE, US iStent Study Group. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459–67.
Neuhann TH. Trabecular micro-bypass stent implantation during small incision cataract surgery for open angle glaucoma or ocular hypertension: long term results. J Cat Ref Surg. 2015;41(12):2664–71.
Katz LJ, Erb C, Carceller GA, et al. Prospective randomised study of one, two, or three trabecular bypass stents in open angle glaucoma subjects on topical hypotensive medication. Clin Ophthalmol Auckl NZ. 2015;9:2313–20.
Voskanyan L, Garcia-Feijoo J, Belda JI, et al. Prospective masked evaluation of the iStent inject system for open-angle glaucoma: Synergy Trial. Adv Ther. 2014;31(2):189–201.
Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology. 2015;122:1283–93.
Saheb H, Ianchulev T, Ahmed II. Optical coherence tomography of the suprachoroid after CyPass Micro-Stent implantation for the treatment of open-angle glaucoma. Br J Ophthalmol. 2014;98:19–23.
Hoeh H, Ahmed IK, Grisanti S, et al. Early postoperative safety and surgical outcomes after implantation of a suprachoroidal micro-stent for the treatment of open-angle glaucoma concomitant with cataract surgery. J Cataract Refract Surg. 2013;39:431–7.
Höh H, Grisanti S, Grisanti S, et al. Two-year clinical experience with the CyPass micro-stent: safety and surgical outcomes of a novel supraciliary micro-stent. Klin Monbl Augenheilkd. 2014;231(4):377–81.
Vold S, Ahmed IIK, Craven ER, et al. Two-year COMPASS results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123(10):2103–11.
García-Feijoo J, Rau M, Grisanti S, et al. Supraciliary micro-stent implantation for open-angle glaucoma failing topical therapy: 1-year results of a multicenter study. Am J Ophthalmol. 2015;159:1075–81.
Lewis RA. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cataract Refract Surg. 2014;40:1301–6.
Sheybani A, Lenzhofer M, Hohensinn M, et al. Phacoemulsification combined with a new ab interno gel stent to treat open-angle glaucoma: pilot study. J Cat Ref Surg. 2015;41(9):1905–9.
Sheybani A, Dick B, Ahmed IIK. Early clinical results of a novel ab interno gel stent for the surgical treatment of open-angle glaucoma. J Glaucoma. 2016;25(7):e691–6. doi:10.1097/IJG.0000000000000352.
Galal A, Bilgic A, Eltanamly R, Osman A. XEN glaucoma implant with mitomycin C 1-year follow-up: result and complications. J Ophthalmol. 2017;2017:5457246. doi:10.1155/2017/5457246 (Epub 2017 Mar 1).
Batlle JF, Fantes F, Riss I, et al. Three year follow-up of a novel aqueous humor microshunt. J Glaucoma. 2016;25(2):e58–65.
Tan SZ, Au L. Manchester iStent study: 3-year results and cost analysis. Eye (Lond). 2016;30(10):1365–70.
Chen PP, Lin SC, Junk AK, Radhakrishnan S, Singh K, Chen TC. The effect of phacoemulsification on intraocular pressure in glaucoma patients: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122:1294–307.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Ejaz Ansari has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by the author.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/21E8F060670ED283.
An erratum to this article is available at https://doi.org/10.1007/s40123-017-0106-6.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ansari, E. An Update on Implants for Minimally Invasive Glaucoma Surgery (MIGS). Ophthalmol Ther 6, 233–241 (2017). https://doi.org/10.1007/s40123-017-0098-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-017-0098-2