Abstract
Introduction
Diabetic retinopathy is a leading cause of blindness in adults of working age. Patients with sight-threatening diabetic retinopathy (STDR) often have poor control of modifiable risk factors, including blood pressure and blood glucose. Patients in our eye department with STDR whose diabetes was managed only by their general practitioner (GP) were referred to a diabetes specialist. We have reviewed these referrals and assessed the control of modifiable risk factors in these patients at the time of referral.
Methods
A retrospective study was performed which identified 54 patients with STDR who had been referred from our eye department to a diabetes specialist between May 2013 and August 2014. Patient demographics, grades of retinopathy, glycated hemoglobin (HbA1c) levels, blood pressure, and lipid profiles were noted from the initial clinic visit and the first clinic appointment after 12 months. Initial management and any subsequent changes to management were recorded.
Results
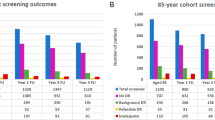
Of the 54 patients initially referred to the dedicated diabetic retinopathy clinic, data from 32 patients were available for analysis; 22 patients failed to attend the clinic. The majority of patients who presented to the clinic were found to have inadequate control of modifiable risk factors. At the initial clinic visit, nine of the 32 (28%) patients had a blood pressure that was less than the target of 130/80 mmHg and only two (6%) had a HbA1c level of less than the target of 48 mmol/L for type 2 diabetes and 58 mmol/L for type 1 diabetes, respectively. Changes were made to the management in 24 (75%) of the patients. Blood pressure management was changed in 18 (56%) patients. Overall, changes were made to blood pressure management and lipid and glycemic medication, including insulin.
Conclusion
The majority of patients with STDR were receiving suboptimal medical management. Collaboration between GPs, diabetes specialists, and ophthalmologists can lead to optimized medical management. All eye departments should develop protocols specifying when patients with diabetic retinopathy should be referred for to a diabetes specialist for input.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy is a leading cause of blindness in the UK, accounting for 14.4% of blindness in adults of working age [1]. Poor glycemic control and hypertension are both well-known factors that increase the rate of progression of diabetic retinopathy. Consequently, it is therefore not surprising that in patients with significant retinopathy these factors are not sufficiently under control [2–4]. Indeed, one study found that 65% of patients requiring laser treatment for diabetic maculopathy had suboptimal blood pressure control [5].
The 2010 Diabetes UK Task and Finish Group recommended that all patients with retinopathy requiring active management or complex monitoring should have their diabetes care provided by specialist teams [6]. However, data from the 2014/2015 UK National Diabetes Audit suggest that only 4.4% of patients are under specialist care for their diabetes and that the majority of patients in the UK are under the care of their primary care physician only [referred to as a general practitioner (GP) in the UK] [7].
In line with the aforementioned recommendations we referred patients managed at our eye center who were only under the care of their GP for their diabetes and found to have sight-threatening diabetic retinopathy (STDR) to a diabetologist as part of a pre-determined protocol involving assessment in a dedicated clinic by diabetic retinopathy specialists. The aim of the retrospective study reported here was to determine the benefit of physician input in these patients by identifying the interventions that were made and thus assess the modifiable risk factors for retinopathy progression at the point of referral.
Methods
Patients referred from the eye department to the dedicated retinopathy clinic between May 2013 and August 2014 were identified using the hospital computer system. Patients with STDR whose diabetes management was under the care of their GP only were referred for specialist input as part of a pre-determined protocol that had been set up previously. The case notes of the patients were reviewed. Data from referrals and documentation from resultant clinic appointments were analyzed.
Patient demographics were extracted and recorded alongside the patient’s co-morbidities and level of retinopathy in each eye at the time of referral (Table 1). The retinopathy was graded as background, preproliferative and proliferative, and a maculopathy graded as present if clinically significant macular edema was present [8] (Table 2). Current medical management was also noted, including drugs prescribed to control lipids, blood pressure, and blood glucose (Table 3).
Clinic letters were used to identify if changes had been made to the patient’s diabetes management following referral. The patient’s most recent body mass index (BMI) measurement, glycated hemoglobin (HbA1c), blood pressure, and lipid panel were noted. Case notes were re-reviewed 12 months after the initial presentation to the dedicated retinopathy clinic, and blood pressure, HbA1c, and any changes to management were recorded.
Data were compared to the National Institute for Health and Care Excellence (NICE) guidelines for each parameter [9]. The target HbA1c level was 6.5% (48 mmol/mol) in patients with type 2 (T2D) diabetes treated with lifestyle advice or metformin and <7.5% (58 mmol/mol) in patients with type 1 diabetes (T1D) or T2D treated with other agents. Target blood pressure was to be <130/80 mmHg. Serum lipid profile targets were a total cholesterol of <4 mmol/l, triglycerides of <1.7 mmol/l, and low-density lipoprotein (LDL) of <2 mmol/l.
Under UK guidelines the study was designated as service evaluation and formal ethical approval was not required.
Results
From the total of 54 patients referred to the dedicated diabetic retinopathy clinic, 32 patients reported to the clinic for an initial consultation, and 22 patients never attended the clinic at all. Following the initial clinic visit one patient was discharged from further follow-up and three patients failed to attend any further appointments. At baseline, antihypertensive medication was more commonly prescribed for patients with T2D than for those with T1D [12/22 (55%) vs. 3/10 (30%), respectively]. Changes were made to the medical management of 24 of the 32 (75%) patients who attended the clinic (Table 4). Of the 18 patients with macular edema who attended the clinic, ten (56%) had changes made to their hypertensive medication while only two (11%) had changes made to their glycemic therapy. In both of these latter patients, pioglitazone was stopped and replaced with either liraglutide or sitagliptin. Changes related to blood pressure control were made in 16 (76%) of the 21 patients with pre-proliferative/proliferative (grade R2/R3, respectively) retinopathy. These changes consisted of ten patients being started on an angiotensin converting enzyme (ACE) inhibitor, two patients being started on a calcium channel blocker, and four patients being started on combined therapy with both an ACE inhibitor and a calcium channel blocker.
Lipid profile measurements from the 12-month follow-up appointment were unavailable.
Discussion
We found that the management of modifiable risk factors in the majority of patients with sight-threatening diabetic retinopathy referred by our eye department to a dedicated diabetic retinopathy clinic was suboptimal. Changes to the medical management of these patients were made in 75% of the patients who attended the diabetic retinopathy clinic, which suggests that input from diabetes specialists is beneficial to patients with worsening retinopathy. Our results support a report published by Diabetes UK which recommends that patients with diabetes and retinopathy requiring active management or complex monitoring should be managed by specialist teams [6].
Eye specialist input in the treatment of diabetic retinopathy is ultimately limited without the optimization of medical diabetes treatment [10, 11]. Poor glycemic control, together with other cardiovascular risk factors, including high blood pressure, an abnormal lipid profile, and a high BMI, accelerate the progression of diabetic retinopathy and are associated with a poorer prognosis [2–4, 12–17]. Physicians are best placed to manage medical risk factors and early medical input in patients, and particularly in those with significant retinopathy it is vital to reduce morbidity and mortality. Ideally eye clinics would be combined with physician input, but such treatment strategies are complex to organize and represent an inefficient use of physician time [18].
A HbA1c level of >8.0% has been associated with STDR [19]. Numerous randomized control trials have shown that optimal long-term control of blood glucose reduces the risk of retinopathy. The Diabetes Control and Complications Trial (DCCT) and the UK Prospective Diabetes Study (UKPDS) both showed statistically highly significant reductions in the incidence and progression of retinopathy in patients who were randomized to tight blood glucose control [20]. Among the patients in our study, 84% had an HbA1c of >8% at their initial appointment at the diabetic retinopathy clinic, and changes were made immediately in 31% of these patients to hyperglycemic medication.
The use of pioglitazone has been associated with macular edema [21, 22]. Two patients in our study with diabetic maculopathy were on pioglitazone at presentation; this medication was stopped in both patients and an alternative glycemic agent prescribed. Stopping pioglitazone without the substitution of an alternative agent may lead to a deterioration in glycemic control [23].
The UKPDS found that tight control of blood pressure led to a 34% reduction in the rate of progression of retinopathy; more specifically, this trial found that for each 10 mmHg decrease in systolic blood pressure there was a 13% reduction in the risk of retinopathy [3, 5]. The UKPDS also showed that in patients with T2D, systolic blood pressure was strongly linked to the incidence of diabetic retinopathy [24]. The Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) found diastolic blood pressure to be a significant indicator of progression of diabetic retinopathy in patients with younger onset T1D. The WESDR also found that in patients with T1D and T2D, elevated diastolic blood pressure was associated with a significantly increased 4-year risk of developing macular edema [25–27].
In our study 72% patients had a blood pressure that surpassed the target of 130/80 mmHg at the initial clinic visit, and changes to blood pressure management were made in 57% (13/23) of these. A further three patients were referred for 24-h blood pressure monitoring. These findings suggest that blood pressure, a very easily measured parameter, may be a strong determining factor in identifying patients who would benefit from referral to the diabetologist. One study has suggested that blood pressure should be measured in all patients at every diabetes clinic appointment with the ophthalmologist [5]. However, in another study blood pressure recordings at an eye clinic were found to be significantly higher than comparative diabetes clinic measurements, possibly secondary to the white-coat effect [28]. Clearly, the benefits of one-off blood pressure measurements at eye clinics is of debatable value, and home and ambulatory blood pressure monitoring can provide objective data regarding individual blood pressure control.
Hypertension worsens with the progression of diabetes [29]. In our study, after 12 months of follow-up, blood pressure improvements were less consistent than those for glycemic control (Table 5). Only one additional patient had a combined target blood pressure. Three patients had an increase in their diastolic blood pressure such that it was no longer possible to achieve the target. The reasons for this increase are likely multifactorial and include the possibility of white-coat hypertension. Of 28 patients, seven (25%) had a drop in their diastolic blood pressure that was ≥10 mmHg at the follow-up visit as compared to the initial visit; 12 patients (43%) had an equivalent drop in their systolic blood pressure.
No patients in this study were prescribed fenofibrate either prior to or at the initial clinic visit. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study group highlighted that fenofibrate may reduce the rate of progression of diabetic retinopathy [15]. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study noted a 37% reduction in the need for laser treatment in patients with T2D who were taking fenofibrate [17]. Following this study, Australia’s Therapeutic Goods Association approved its use to slow the progression of diabetic retinopathy in patients with T2D [30]. The use of fenofibrate alongside statins is associated with an increased risk of myopathy and rhabdomyolysis [31]. NICE guidance recommends against the use of fibrates for the prevention of cardiovascular disease in patients with T1D and T2D [32], but in the UK there is no current guidance on the use of fenofibrate in patients with diabetic retinopathy.
Statins have been shown not to have a significant effect on the progression of diabetic retinopathy despite being effective in treating hyperlipidemia [33–35]. Of the 32 patients in our study, 72% had changes made to their management which involved the prescription of statins with the aim to reduced overall systemic risk.
A pre-determined protocol was used to determine which patients should be referred to the diabetologist, which resulted in all patients with STDR being referred to the dedicated diabetic physician clinic. An alternate strategy of only referring patients with suboptimal diabetes control or management could be considered, as recommended by the UK Royal College of Ophthalmologists; however, key information, such as HbA1c and current medical management, is often unknown or unavailable [36, 37]. Ultimately, ophthalmologists need to weigh advice from both the Royal College of Ophthalmologists and Diabetes UK against their knowledge of the capacity of local services when deciding which patients would benefit most from specialist input in their diabetes care. Improved collaboration with GPs and local protocols could prevent unnecessary referrals and enhance monitoring of modifiable risk factors in patients with STDR such that early specialist input can be achieved as required.
Failure to attend outpatient appointments is a particularly prevalent issue in the diabetic population and is associated with poorer outcomes [38, 39]. Of the 54 patients referred by our eye department to the diabetic retinopathy clinic, 22 failed to attend their initial clinic appointment. One study found that the presence of major diabetic complications was associated with improved clinic attendance [40]. Only three of the 32 patients who presented to the diabetic retinopathy for their initial appointment failed to attend their first follow up appointment after 12 months, possible due to an increased understanding of the importance of improvements to their systemic control.
A strength of the study was our ability to access data from both the eye department and the diabetes clinics. Limiting factors include the retrospective nature of our study and our small sample size. The longest period of follow-up data was from the first clinic appointment up to 12 months following the initial visit. The availability of additional follow-up data was limited due to the high turnover of patients in the clinic. Patients who require longer term follow-up are referred to a general diabetes clinic for ongoing management, and those who are on maximal medical therapy are discharged to the care of their GP with the option of re-referral if required.
Conclusion
We have shown that a large proportion of patients with STDR have sub-optimal medical management of their diabetes. A collaborative approach between primary care physicians, diabetes specialists, and ophthalmologists is needed to provide optimal therapies. Strategies to maximize attendance should be carefully considered. We would recommend that all eye departments should develop protocols for referring patients with STDR for specialist diabetes input.
References
Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open. 2014;4(2):e004015.
[No authors listed] Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53.
UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–13.
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Sivaprasad S, Jackson H. Blood pressure control in type II diabetics with diabetic retinopathy. Eye. 2006;21(6):708–11.
Diabetes UK. Specialist diabetes services for adults with diabetes. From the 2010 Diabetes UK Task and Finish Group report. 2010. https://www.diabetes.org.uk/About_us/What-we-say/Healthcare-professional-staffing-competency/Specialist-diabetes-team-role-and-members/. Accessed 13 June 2016.
National Diabetes Inpatient Audit 2015. 2016. http://content.digital.nhs.uk/searchcatalogue?productid=20443&q=%22National+diabetes+audit%22&sort=Relevance&size=10&page=1#top. http://content.digital.nhs.uk/catalogue/PUB20206/nati-diab-inp-audi-15-nat-rep.pdf.
Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796–806.
National Institute for Health and Care Excellence (NICE). Type 2 diabetes in adults: management [NG28]. 2015. https://www.nice.org.uk/guidance/ng28. Accessed 13 Feb 2016.
Aiello L, Ayala AR, Antoszyk AN, et al. Assessing the effect of personalized diabetes risk assessments during ophthalmologic visits on glycemic control: a randomized clinical trial. JAMA Ophthalmol. 2015;133(8):888–96.
Frank RN. Systemic therapies for diabetic retinopathy. Ophthalmology. 2014;121(12):2295–6.
The Kroc Collaborative Study Group. Diabetic retinopathy after two years of intensified insulin treatment: Follow-up of the Kroc Collaborative Study. JAMA. 1988;260(1): 37–41.
Dahl-Jørgensen K, Brinchmann-Hansen O, Hanssen KF, Ganes T, Kierulf P, Smeland E, et al. Effect of near normoglycaemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. Br Med J. 1986;293(6556):1195–9.
Reichard P, Nilsson B-Y, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Eng J Med. 1993;329(5):304–9.
Margolis KL, O’Connor PJ, Morgan TM, Buse JB, Cohen RM, Cushman WC, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabet Care. 2014;37(6):1721–8.
Chew EY, Klein ML, Ferris FL, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy: Early Treatment Diabetic Retinopathy Study (ETDRS) report 22. Arch Ophthalmol. 1996;114(9):1079–84.
Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TME, Moffitt MS, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687–97.
Ho ETL. Improving waiting time and operational clinic flow in a tertiary diabetes center. BMJ Qual Improv Rep. 2014;2. doi: 10.1136/bmjquality.u201918.w1006
Raman R, Verma A, Pal SS, Gupta A, Vaitheeswaran K, Sharma T. Influence of glycosylated hemoglobin on sight-threatening diabetic retinopathy: A population-based study. Diabet Res Clin Pract. 2011;92(2):168–73.
Frank RN. Diabetic retinopathy and systemic factors. Middle East Afr J Ophthalmol. 2015;22(2):151–6.
Niemeyer NV, Janney LM. Thiazolidinedione-induced edema. Pharmacotherapy. 2002;22(7):924–9.
Ryan Jr EH, Han DP, Ramsay RC, Cantrill HL, Bennett SR, Dev S, et al. Diabetic macular edema associated with glitazone use. Retina. 2006;26(5):562–70.
Oshitari T, Asaumi N, Watanabe M, Kumagai K, Mitamura Y. Severe macular edema induced by pioglitazone in a patient with diabetic retinopathy: a case study. Vasc Health Risk Manag. 2008;4(5):1137–40.
Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–63.
Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105(10):1801–15.
Klein R, Moss SE, Klein BE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. XI. The incidence of macular edema. Ophthalmology. 1989;96(10):1501–10.
Klein R, Klein BEK. Blood pressure control and diabetic retinopathy. Br J Ophthalmol. 2002;86(4):365–7.
Al-Husainy S, Farmer J, Gibson JM, Dodson PM. Is measurement of blood pressure worthwhile in the diabetic eye clinic? Eye. 2004;19(3):312–6.
Sowers JR, Epstein M. Diabetes mellitus and associated hypertension, vascular disease, and nephropathy an update. Hypertension. 1995;26(6 Pt 1):869–79.
Sharma N, Ooi JL, Ong J, Newman D. The use of fenofibrate in the management of patients with diabetic retinopathy: an evidence-based review. Austr Fam Phys. 2015;44(6):367–70.
Shek A, Ferrill MJ. Statin-fibrate combination therapy. Ann Pharmacother. 2001;35(7–8):908–17.
National Institute for Health and Care Excellence (NICE). Clinical guideline [CG181]. Cardiovascular disease: risk assessment and reduction, including lipid modification. 2014. https://www.nice.org.uk/Guidance/cg181. Accessed 22 July 2016.
Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–96.
Zhang J, McGwin G. Association of statin use with the risk of developing diabetic retinopathy. Arch Ophthalmol. 2007;125(8):1096–9.
Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78.
Prabhu M, Kakhandaki A, Chandra KRP, Dinesh MB. A hospital based study regarding awareness of association between glycosylated haemoglobin and severity of diabetic retinopathy in type 2 diabetic individuals. J Clin Diagn Res JCDR. 2016;10(1):NC01–4.
Ophthalmologists TRCo. Diabetic Retinopathy Guidelines. 2012. https://www.rcophth.ac.uk/wp-content/uploads/2014/12/2013-SCI-301-FINAL-DR-GUIDELINES-DEC-2012-updated-July-2013.pdf. Accessed 18 April 2016.
Dyer PH, Lloyd CE, Lancashire RJ, Bain SC, Barnett AH. Factors associated with clinic non-attendance in adults with type 1 diabetes mellitus. Diabet Med. 1998;15(4):339–43.
Forster AS, Forbes A, Dodhia H, Connor C, Du Chemin A, Sivaprasad S, et al. Non-attendance at diabetic eye screening and risk of sight-threatening diabetic retinopathy: a population-based cohort study. Diabetologia. 2013;56(10):2187–93.
Hammersley MS, Holland MR, Walford S, Thorn PA. What happens to defaulters from a diabetic clinic? Br Med J (Clin Res Ed). 1985;291(6505):1330–2.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
S. Mamtora, T. Sandinha, P. E. Carey and D. H. W. Steel have nothing to disclose.
Compliance with ethics guidelines
Under UK guidance the study was designated as service evaluation and formal ethical approval was not required.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/6517F06067C51742.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mamtora, S., Sandinha, T., Carey, P.E. et al. Optimizing Medical Management in Patients with Sight-Threatening Diabetic Retinopathy. Ophthalmol Ther 6, 105–114 (2017). https://doi.org/10.1007/s40123-016-0069-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-016-0069-z