Abstract
Introduction
This study is a retrospective case series to evaluate the outcomes and complications of Baerveldt glaucoma implant surgery (BGI) in patients without prior cataract or incisional glaucoma surgery.
Methods
Patients who underwent 350-mm2 BGI through the Glaucoma Service of the University of Illinois at Chicago between 2010 and 2015 were included in this study. Outcome measures included age, sex, ethnicity, operated eye, preoperative diagnosis, preoperative, and sequential postoperative intraocular pressure (IOP), visual acuity, glaucoma medications, and postoperative complication and interventions. Statistical analyses were performed using the two-sided Student t test for continuous variables.
Results
Thirty-seven patients were studied. IOP was consistently and statistically significantly lower at 3 months (17.4 ± 6.4, p = 3 × 10−7), 6 months (13.9 ± 5.1, p = 2 × 10−11), 1 year (12.2 ± 4.0, p = 9 × 10−10), and 2 years (14.6 ± 3.3, p = 0.0004) postoperatively compared to IOP at baseline (27.5 ± 8.1). Fewer glaucoma medications were used at 3 months (2.8 ± 1.3, p = 0.04), 6 months (2.6 ± 1.2, p = 0.02), 1 year (2.7 ± 1.7, p = 0.04), and 2 years (2.0 ± 1.2, p = 0.03) postoperatively compared to baseline (3.4 ± 1.1). A total of six cases (16%) had failure. A total of five patients (15%) had postoperative complications. Mean Snellen visual acuity was not statistically different at 6 months (0.5 ± 0.6, p = 0.88) or 1 year (0.4 ± 0.4, p = 0.57) postoperatively from baseline (0.5 ± 0.6).
Conclusions
Primary BGI is effective at reducing IOP and the medication burden in patients suffering glaucomatous optic neuropathy. Further randomized prospective studies are needed to compare various procedures in the primary surgical management of patients with uncontrolled glaucoma.
Funding
This study was funded by an unrestricted grant from Research to Prevent Blindness.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Incisional glaucoma surgery is generally indicated when medical therapy and laser treatments fail to control intraocular pressure (IOP) adequately. Trabeculectomy remains the most commonly performed incisional glaucoma surgery worldwide [1]. However, recent surveys of the American Glaucoma Society have shown an increase in the proportion of practitioners using tube shunts and a decrease in the popularity of trabeculectomy [2]. Medicare data has also demonstrated an increase in the number of tube shunts with a decline in trabeculectomies from 1995 to 2004 [3]. The Tube Versus Trabeculectomy study demonstrated that Baerveldt glaucoma implant (BGI; Advanced Medical Optics, Santa Ana, CA, USA) surgery had a higher success rate compared to trabeculectomy, with fewer postoperative complications in patients with prior glaucoma or cataract surgery. In a recent retrospective study, Panarelli et al. [4]. have shown similar rates of surgical success and post-operative complications in patients undergoing trabeculectomy with mitomycin C (MMC) and in those undergoing Baerveldt glaucoma implant surgery during a 3-year follow-up.
This retrospective case series evaluated the outcomes and complications of Baerveldt glaucoma implant surgery in patients without prior cataract or incisional glaucoma surgery.
Methods
The Institutional Review Board of the University of Illinois at Chicago Human Subject Research Committee approved this study. Informed consent was obtained from all patients for being included in the study. Patients who underwent 350-mm2 Baerveldt glaucoma implant surgery through the Glaucoma Service of the University of Illinois at Chicago between 2010 and 2015 were identified using Current Procedural Terminology codes. Patients were excluded if they were younger than 18 or older than 85 years of age, had undergone prior incisional ocular surgery, had an IOP less than 18 mmHg immediately before the surgery, or had fewer than 6 months of postoperative follow-up. Exclusion criteria also included preoperative no light perception vision; pregnant or nursing women; active iris neovascularization or proliferative retinopathy; iridocorneal endothelial syndrome; and epithelial or fibrous down-growth. Information obtained from the medical records of each patient included age, sex, ethnicity, operated eye, preoperative diagnosis, preoperative and sequential postoperative IOP, visual acuity (VA), glaucoma medications, and postoperative complication and interventions.
Consistent with prior reports, we defined failure was defined as IOP >21 mmHg or less than 20% reduction below baseline on two consecutive follow-up visits after 3 months, IOP ≤5 mmHg on two consecutive follow-up visits after 3 months, reoperation for glaucoma, or loss of light perception vision [1, 4]. All eyes that had not failed by the above criteria and were not on supplemental medical therapy were considered complete success. Eyes were considered a qualified success if they did not meet failure criteria, yet required supplemental medical therapy. Reoperation for glaucoma was defined as additional surgery requiring a return to the operating room. Laser cyclodestructive therapy was considered a reoperation for glaucoma and therefore a failure. A vitreous tap with injection of intravitreal antibiotics was considered a reoperation for a surgical complication and, therefore, a failure. Interventions performed at the slit-lamp, such as needling procedures or reformation of the anterior chamber, were not considered reoperations for glaucoma or a complication and, therefore, not classified as failures. Persistent diplopia and corneal edema, as well as dysesthesia, were defined as the postoperative development of these complications and their continued presence at the 6-month follow-up visit or after. Eyes that tested Seidel positive within the first month of follow-up were classified as wound leaks, and those occurring after 1 month were categorized as bleb leaks. Patients who underwent additional glaucoma surgery were censored from analysis of complications after the reoperation for glaucoma. Cataracts were considered to have progressed if there was loss of 2 or more lines of Snellen VA attributable to cataract, or if cataract surgery was performed.
Statistical analyses were performed using the two-sided Student t-test for continuous variables. Snellen VA measurements were converted to logarithm of the minimal angle of resolution (logMAR) equivalents for the purpose of the data analysis. A p value of 0.05 or less was considered statistically significant in our analyses. Failed eyes were excluded from further analyses of IOP, VA, or glaucoma medications beyond the time-point of failure.
Compliance with Ethics Guidelines
The Institutional Review Board of the University of Illinois at Chicago Human Subject Research Committee approved this study. Informed consent was obtained from all patients for being included in the study.
Results
Baseline characteristics of study patients are presented in Table 1. A total of 37 patients met the eligibility criteria for the study. The majority of patients were male (62%) and most patients were African American (51%). The most common diagnosis was primary open-angle glaucoma (POAG, 73%).
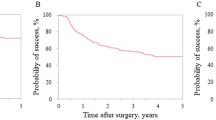
Baseline and follow-up IOP measurements are shown in Table 2 and Fig. 1. The number of patients who were censored at 3 months, 6 months, and 1 year was 2 at each time point. Additional reduction in the number of patients at follow-up time points was the result of missed visits. IOP was consistently and statistically significantly lower at each follow-up time point compared to IOP at baseline.
The number of glaucoma medications at baseline and follow-up is reported in Table 2 and Fig. 2. Fewer glaucoma medications were used at every follow-up time point compared to baseline.
A total of six cases (16%) had failure. The most common cause of failure was inadequate IOP reduction in four patients (10%), followed by persistent hypotony in one patient (2%) and reoperation for glaucoma in another patient (2%). One patient underwent shunt revision for hypotony at postoperative month 3 with successful outcome. Another patient underwent cyclophotocoagulation at postoperative year 1 for inadequate IOP reduction with successful outcome. The remaining 31 cases of successful outcome included three cases (7%) of complete success and 28 cases (75%) of qualified success.
Postoperative complications and interventions are shown in Table 3. A total of five patients (15%) experienced postoperative complications. One patient required reoperation to treat the complication. One additional patient required reforming of anterior chamber with viscoelastic due to shallow anterior chamber noted postoperatively. The treatment outcome for this patient was considered a qualified success.
Mean Snellen VA ± standard deviation at baseline, 6 months postoperatively, and 1 year postoperatively were 0.5 ± 0.6, 0.5 ± 0.6, and 0.4 ± 0.4, respectively. Mean Snellen VA was not statistically different at 6 months or 1 year postoperatively from baseline (p = 0.88 and p = 0.57, respectively). A total of four patients (10%) had progression of cataract.
Discussion
This retrospective case series investigated the outcomes of primary Baerveldt glaucoma implant surgery in patients with medically uncontrolled glaucoma without prior cataract or incisional glaucoma surgery and yielded three key findings. First, primary Baerveldt glaucoma implant surgery resulted in a significant IOP reduction by 6 months and that reduction persisted at 1 year following surgery. Second, patients required significantly fewer glaucoma medications at 3 months following shunt implantation. Third, primary Baerveldt shunt implantation is safe with respect to visual acuity and postoperative complications.
For decades, trabeculectomy has been the incisional surgery of choice for the modern management of uncontrolled glaucoma [5–7]. Trabeculectomy has shown to be effective at reducing IOP and reducing the risk of progressive glaucomatous visual field loss [8, 9]. Antimetabolites such as MMC or 5-FU augment that IOP reduction and can enhance the success rate of trabeculectomy surgery; [10, 11] indeed, antimetabolite therapy is routinely combined with trabeculectomy among most glaucoma practitioners [12]. However, adjunctive antimetabolite therapy is associated with significant complications, including bleb leaks, hypotony, and cataract [10]. The rate of wound leaks after trabeculectomy with an adjunctive antifibrotic agent has been reported as high as 32% when Seidel testing is rigorously performed at each clinic, leading some practitioners to investigate other surgical options.
Historically, aqueous shunt implant surgery has been reserved for patients with advanced glaucoma who previously had failed trabeculectomy or in cases with a poor prognosis with filtering surgery, such as active neovascular glaucoma, aphakic/pseudophakic glaucoma with severe uveitis, or in patients with extensive perilimbal conjunctival scarring [13–16]. With the known complications of trabeculectomy with adjuvant antimetabolite therapy and the growing improvement in surgical techniques and physician comfort with aqueous shunt implants, there has been renewed interest in expanding the role of aqueous shunt implantation [3, 17, 18]. The Tube Versus Trabeculectomy (TVT) study compared the safety and efficacy of non-valved tube shunt surgery with trabeculectomy with MMC in patients who had previously undergone cataract extraction with IOL implantation and/or failed filtering surgery. In this randomized prospective study, tube shunt surgery was more likely to maintain IOP control and avoid persistent hypotony or reoperation for glaucoma than trabeculectomy with MMC during 1 year of follow-up [18]. Although there was less need for supplemental medical therapy after trabeculectomy at 1 year, vision loss occurred at a similar rate after both procedures [18]. At 5 years, the rates of late postoperative complications and reoperation for complications were similar. Overall rate of complications was significantly higher in the trabeculectomy group compared with the tube group: in particular, bleb leaks and dysethesia occurred with significantly greater frequency in the trabeculectomy group. In contrast, no postoperative complications were significantly more common in the tube group [16]. While a prospective study is underway comparing primary tube vs. trabeculectomy, the study suggests a favorable role in expanding the role of BGI.
Recently, outcomes were reported comparing the safety and efficacy of Baerveldt glaucoma implant surgery and trabeculectomy with MMC in patients who had not undergone prior incisional surgery [4]. At 6 months of follow-up, Baerveldt glaucoma implant surgery produced a 50% decrease in IOP whereas trabeculectomy with MMC produced a 62% reduction, a result similar to earlier reports in the literature [4, 19–21]. Those IOP reductions were stable at 5 years in both groups, suggesting that the IOP at 6 months may be a good predictor of long-term IOP reduction. In the current study examining the efficacy of BGI as a primary glaucoma surgery, IOP was reduced from baseline by 49.5% at 6 months and 55.6% at 1 year. As in the study by Panarelli et al. [4], IOP was reduced by 6 months and that reduction was stable at 1 year. These results are similar to those seen at similar postoperative time points in the tube group of the TVT study, where IOP was reduced from baseline by 50.1% at 1 year following surgery in patients with prior incisional ocular surgery [16].
Patients in the current study required significantly fewer glaucoma medications at all time points following surgery. The mean number of glaucoma medications was 2.6 ± 1.2 at 6 months and 2.7 ± 1.7 at 1 year, somewhat higher than the mean number (1.8 ± 1.5 and 1.9 ± 1.5, respectively) reported in the Baerveldt group of the study by Panarelli et al. [4], as well as that of the tube group of TVT at 1.3 ± 1.3 (at 1 year) [16]. Possibly medication non-adherence, a well-known challenge in glaucoma management [22, 23], may have accounted for this difference.
There were a total of five postoperative complications in this study, including anterior chamber shallowing, hyphema, chronic iritis, persistent choroidal effusion, and rhegmatogenous retinal detachment. The overall rate of postoperative complications was 15%, similar to that seen in a recent study of patients undergoing primary BGI [4]. While that study included persistent corneal edema, bleb leak, and persistent diplopia as postoperative complications in the BGI group, in this study there were no patients with those complications. Although variability among practitioners may account for this difference, the current study was also not adequately powered to detect relatively rare complications of BGI surgery [15, 24]. Interestingly, in both the current study and that by Panarelli et al. [4]., the overall complication rates (15% and 20%, respectively) were lower than the 34% rate observed in the tube group of the TVT study [25].
Prior studies have indicated that postoperative visual acuity significantly decreased following BGI [4, 25]. In patients with no prior incisional ocular surgery, Snellen visual acuity decreased by more than two lines in 33% of patients following BGI at 3 years [4], similar to that reported for the 39% of patients without complications and the 51% of patients with complications who experienced decreased visual acuity in the tube group of TVT at 5 years [25]. In the current study, mean visual acuity was not statistically different at 6 months or 1 year postoperatively from baseline, although a total of four patients did have progression of cataract. Given that results were analyzed at 1 year following BGI in the current study, further follow-up will be required to assess the consistency of these visual acuity outcomes with other reports. Another factor that potentially contributed to unchanged visual acuity was exclusion of failure cases from visual acuity analysis beyond the time point of failure.
This study has several limitations. Like all retrospective studies, no standard protocol was used to collect data at the time of follow-up visits, which may have led to an underestimation of adverse events. Postoperative interventions and additional medications were left to the discretion of the surgeon, and no standardized protocols were used for postoperative management. The study did not have adequate power to detect rare complications of BGI surgery such as corneal edema or diplopia [4]. Post-operative follow-up was limited to 1 year following surgery, so understanding the long-term effectiveness and complications of BGI requires further inquiry. This study also had specific inclusion criteria and only examined outcomes in patients who had undergone surgery with the 350 mm2 Baerveldt glaucoma implant, so it may be difficult to generalize the results to valved tube shunts (e.g. Ahmed valved implant) or other non-valved devices. Finally, the ideal measure of success in any glaucoma therapy is the prevention of further optic nerve damage and visual field loss. While this study focused on lowering IOP as the primary goal of therapy given its well-known role in delaying visual field loss and optic disc progression [26], further investigation is needed to assess the particular effectiveness of BGI surgery on preventing glaucomatous nerve damage compared to other surgical procedures [9].
In summary, this study will help provide evidence-based information that will assist in surgical decision making for patients requiring a primary procedure to manage glaucoma. Primary BGI is effective at reducing IOP at 6 months, significantly decreases the medication burden, and is associated with relatively similar post-operative and visual acuity complications at 1 year compared to results seen in other studies, regardless of prior incisional ocular surgery [4, 25]. The current study adds substantial evidence that primary aqueous shunt implants in general can significantly lower IOP in patients with uncontrolled glaucoma and no prior incisional ocular surgery [27, 28]. While further randomized prospective studies are needed to establish definitively the comparative effectiveness of various procedures in the primary surgical management of patients with uncontrolled glaucoma, the current study supports an ever-expanding role for BGI. While the risks and benefits of any surgical option must be weighed with respect to the individual characteristics of the patient’s disease, this study underscores that BGI is a viable alternative for the primary surgical management of uncontrolled glaucoma.
Conclusions
Primary BGI significantly reduces IOP and the medication burden in patients suffering glaucomatous optic neuropathy. Further randomized prospective studies are needed to compare various procedures in the primary surgical management of patients with uncontrolled glaucoma.
References
Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL, Tube Versus Trabeculectomy Study Group. Three-year follow-up of the tube versus trabeculectomy study. Am J Ophthalmol. 2009;148(5):670–84.
Joshi AB, Parrish RK, Feuer WF. 2002 Survey of the American Glaucoma Society: practice preferences for glaucoma surgery and antifibrotic use. J Glaucoma. 2005;14:172–4.
Ramulu PY, Corcoran KJ, Corcoran SL, Robin AL. Utilization of various glaucoma surgeries and procedures in Medicare beneficiaries from 1995 to 2004. Ophthalmology. 2007;114(12):2265–70.
Panarelli JF, Banitt MR, Gedde SJ, Shi W, Schiffman JC, Feuer WJ. A retrospective comparison of primary Baerveldt implantation versus trabeculectomy with mitomycin C. Ophthalmology. 2016;123(4):789–95.
Cairns DE. Trabeculectomy: preliminary report of a new method. Am J Ophthalmol. 1968;66:673–9.
Boland MV, Ervin AM, Friedman DS, et al. Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158(4):271–9.
Musch DC, Lichter PR, Guire KE, Standardi CL. The Collaborative Initial Glaucoma Treatment Study: study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999;106(4):653–62.
Jay J, Murray SB. Early trabeculectomy versus conventional management in primary open angle glaucoma. Br J Ophthalmol. 1988;72(12):881–9.
Musch DC, Gillespie BW, Niziol LM, Lichter PR. Varma R; CIGTS Study Group. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011;118(9):1766–73.
Wilkins M, Indar A, Wormald R. Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev. 2005:CD002897.
Wormald R, Wilkins MR, Bunce C. Post-operative 5-fluorouracil for glaucoma surgery. Cochrane Database Syst Rev. 2001;3(3):CD001132. doi:10.1002/14651858.CD001132.
Spaeth GL, Mutlukan E. The use of antimetabolites with trabeculectomy: a critical appraisal. J Glaucoma. 2001;10:145–51.
Melamed S, Fiore PM. Molteno implant surgery in refractory glaucoma. Surv Ophthalmol. 1990;34(6):441–8.
Minckler DS, Vedula SS, Li TJ, Mathew MC, Ayyala RS, Francis BA. Aqueous shunts for glaucoma. Cochrane Database Syst Rev. 2006;(2).
Aref AA, Gedde SJ, Budenz DL. Glaucoma drainage implant surgery. Dev Ophthalmol. 2012;50:37–47.
Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL, Tube Versus Trabeculectomy Study Group. Treatment Outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789–803.
Desai MA, Gedde SJ, Feuer WJ, et al. Practice preferences for glaucoma surgery: a survey of the American Glaucoma Society in 2008. Ophthalmic Surg Lasers Imaging. 2011;42:202–8.
Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC, Tube Versus Trabeculectomy Study Group. Surgical complications in the tube versus trabeculectomy study during the first year of follow-up. Am J Ophthalmol. 2007;143:23–31.
Freedman J, Rubin B. Molteno implants as a treatment for refractory glaucoma in black patients. Arch Ophthalmol. 1991;109:1417–20.
Palmer SS. Mitomycin as adjunct chemotherapy with trabeculectomy. Ophthalmology. 1991;98:317–21.
Broadway DC, Lester M, Schulzer M, Douglas GR. Survival analysis for success of Molteno tube implants. Br J Ophthalmol. 2001;85:689–95.
Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid Study. Ophthalmology. 2009;116:191–9.
Friedman DS, Okeke CO, Jampel HD, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology. 2009;116:1097–105.
Budenz DL, Feuer WJ, Barton K, Ahmed Baerveldt Comparison Study Group, et al. Postoperative complications in the Ahmed Baerveldt comparison study during five years of follow-up. Am J Ophthalmol. 2016;163:75–82.
Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–14.
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–79.
Molteno AC, Bevin TH, Herbison P, Husni MA. Long-term results of primary trabeculectomies and Molteno implants for primary open-angle glaucoma. Arch Ophthalmol. 2011;129:1444–50.
Välimäki JO, Ylilehto AP. Molteno implantation as primary glaucoma surgery. J Ophthalmol. 2014;2014:167564.
Acknowledgments
This study was funded by an unrestricted Grant from Research to Prevent Blindness. No support was received for the publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosure
Abed Namavari, Robert A. Hyde, Daniel Wang, Thasarat S. Vajaranant, and Ahmad A. Aref have nothing to disclose.
Compliance with Ethics Guidelines
The Institutional Review Board of the University of Illinois at Chicago Human Subject Research Committee approved this study. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/C4E4F06011FF9225.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Namavari, A., Hyde, R.A., Wang, D. et al. Primary Baerveldt Shunt Implantation: Outcomes and Complications. Ophthalmol Ther 5, 253–262 (2016). https://doi.org/10.1007/s40123-016-0056-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-016-0056-4