Abstract
Introduction
The present study aimed to evaluate the effects of intravitreal ranibizumab (IVR) as adjunctive treatment for trabeculectomy with mitomycin C (TMC) in neovascular glaucoma (NVG).
Methods
This is a prospective study of 15 eyes from 14 consecutive patients with NVG carried out between December 2008 and December 2009. Each eye received IVR (0.5 mg/0.05 ml) 1 week before TMC. Trabeculectomy was performed with fornix-based conjunctival flap method. After TMC, additional panretinal photocoagulation (PRP), subconjunctival five fluorouracil injection, and bleb needling may be performed if indicated. The primary outcome measures were post-TMC intraocular pressure (IOP) and numbers of anti-glaucoma medication. The secondary outcome measures included of the recurrence of neovascularization at iris (NVI) and complications.
Results
Six eyes underwent adequate PRP before IVR but iris rubeosis still persisted. All eyes showed regression of NVI within 1 week after IVR. After TMC, mean IOP was significantly decreased from 37.9 mmHg preoperatively to 15.6 mmHg postoperatively (P < 0.001). Intraoperative hyphema was observed in four eyes. Thirteen eyes had controlled IOP (<21 mmHg) at last visit among which only one eye needed anti-glaucoma medication. Two eyes were considered as failure and needed further intervention. Visual acuity was maintained or improved in eight eyes. Recurrent NVI was not detected. All patients were symptom-free at last visit. Mean follow-up was 39 weeks.
Conclusion
IVR is an effective treatment adjunctive to TMC for NVG. The occurrence of intraoperative complications was low and the short-term outcomes after trabeculectomy were favorable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-vascular endothelial growth factor (anti-VEGF) has been reported as an additional treatment for neovascular glaucoma (NVG) [1–4]. Off-label use of intravitreal injection of bevacizumab (IVB; Avastin®, Genentech, Inc., San Francisco, CA, USA) effectively reduces neovascularization in ocular tissues including NVG [5]. It resulted in regression of iris rubeosis, which is theoretically beneficial as a surgical adjuvant to reduce intraoperative bleeding during trabeculectomy [3, 4]. The mechanism of action of anti-VEGF was hypothesized to improve surgical outcome after conventional trabeculectomy [3, 6, 7]. There were no severe ocular and systemic side effects found with intravitreous injection. Ranibizumab (Lucentis®; Genentech Inc., San Francisco, CA, USA) is a recombinant, humanized antibody antigen-binding fragment and is approved by the US Food and Drug Administration (FDA) for the treatment of neovascular age-related macular degeneration (AMD) as it neutralizes all active forms of VEGF-A. Intravitreal injection of ranibizumab (IVR) in NVG has been reported. It resulted in a rapid improvement of the rubeosis iridis and reduction of intraocular pressure (IOP) in radiation-induced NVG [8]. Trabeculectomy with intraoperative mitomycin C (TMC) is the gold standard for glaucoma surgical treatment [9]. Combined treatment of panretinal photocoagulation (PRP), intravitreal anti-VEGF, and subsequent TMC has been reported as a good option in the management for NVG [3, 10, 11]. So far, the benefits of IVR on surgical outcomes of trabeculectomy have not been studied.
This prospective study aimed to evaluate the safety and surgical outcomes of TMC after adjunctive treatment with IVR from 15 eyes of 14 consecutive patients with NVG.
Methods
Patients
This prospective study included 15 eyes from 14 consecutive patients with NVG who presented at the Department of Ophthalmology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand between December 2008 and December 2009. PRP was performed as soon as feasible in several sessions in eyes in which the ocular media was clear enough to perform PRP. Maximal anti-glaucoma medication including systemic acetazolamide was administered. The patients received IVR (0.5 mg/0.05 ml) injection through the pars plana in the operating room as soon as possible in eyes with any evidence of previous PRP, or after first session of PRP, or in eyes for which PRP could not be carried out. A fornix-based conjunctival flap technique TMC was performed within 2 weeks after IVR.
Surgical Technique
All surgery was performed by Naris Kitnarong or one of co-authors (under the supervision of Naris Kitnarong). After retrobulbar anesthesia, the eye was prepared with sterile drapes. A wire lid speculum was inserted and a corneal traction suture placed adjacent to the inferior limbus for ocular stability. A fornix-based conjunctival flap was created by limbal peritomy at the superonasal or superotemporal quadrant for 3–4 clock hours. Disinsertion of the conjunctiva and Tenon’s capsule was achieved by blunt posterior dissection using westcott scissors. Oblique relaxing incisions at one or both sides allowed for conjunctival flap retraction superiorly away from the surgical limbus, as well as adequate scleral exposure. Hemostasis of the sclera was controlled using a diathermy.
A partial thickness 3.5 mm × 3.5 mm (base × height) triangular or rectangular scleral flap was then created using a surgical blade no. 15, dissecting anteriorly towards the clear cornea by lamellar dissection. Mitomycin C (MMC; 0.4 mg/ml) was applied by thin cellulose sponge to the scleral flap below the conjunctiva and Tenon’s capsule for 1–3 min. The duration of MMC application based upon the preoperative evaluation of each patient’s risk factors for surgical failure including the preoperative quality of the subconjunctiva and Tenon’s capsule. In general, thicker Tenon’s capsule and active inflammation of conjunctivae received a longer application. The surgical area was then vigorously irrigated with balanced saline solution.
Paracentesis was performed through the temporal clear cornea with a 20-gage needle. The anterior chamber was then entered via sclerotomy, using a 15° blade for sharp dissection. The sclerotomy was widened with a Kelly Descemet punch. Peripheral iridectomy was performed to decrease the risk of sclerostomy occlusion and pupillary block. The scleral flap was sutured with 10-0 nylon sutures anchored at its corners. The tension and number of sutures were individualized for each case to allow for adequate flow of aqueous humor and adjustments were made in the case of leakage or flattening of the anterior chamber. The fornix-based flap was closed securely with round needle 10-0 nylon sutures anchored at the limbus and closed relaxing incisions with a continuing suture. The bleb and wound closure were checked postoperatively with 2% fluorescein dye.
Postoperative Procedure
In the early postoperative period, topical antibiotics and 1% prednisolone were applied six times a day for 7 days. A combination of antibiotics and dexamethasone was then administered four times a day for the following 1 month or until the signs of inflammation disappeared.
The visual acuity, IOP, blood pressure and pulse rate, number of anti-glaucoma medications, and the appearance of neovascularization at the iris (NVI) by ophthalmoscopy were compared pre- and post-IVR and before and after trabeculectomy. The intraoperative and postoperative complications were also recorded. A follow-up was scheduled at 1 day, 1 week, and every 4 weeks period thereafter. The IOP, blood pressure, and pulse rate pre- and post-IVR and before and after TMC were compared using paired Student’s t test (SPSS, version 18.0.3; IBM Corporation, Armonk, NY, USA). Statistical significance was set at P ≤ 0.05.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Results
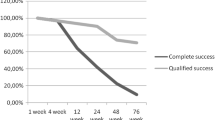
Fifteen eyes from 14 patients were enrolled in this study. Eight patients were male and six were female with a mean age of 65 years. The demographic and preoperative data are presented in Table 1. The underlying causes of NVG included proliferative diabetic retinopathy (seven eyes), central retinal vein occlusion (six eyes), central retinal artery occlusion (one eye), and branch retinal vein occlusion (one eye). Six eyes underwent adequate PRP before IVR but NVI still persisted. Five eyes received partial PRP before or within 1 week after IVR whether or not NVI regression occurred. There were four eyes in which PRP could not be applied because of ocular media opacity. Mean IOP was 37.9 mmHg before IVR and 36.1 mmHg after IVR (P = 0.62). The absolute regression of NVI was observed within 1 week after IVR in all eyes. Mean systolic blood pressure, diastolic blood pressure, and pulse rate did not significantly change before IVR (144/72 mmHg, 76 beats per minute, respectively) and after IVR (153/76 mmHg, 68 beats per minute, respectively; P = 0.38, 0.08, and 0.24, respectively).After TMC, mean IOP was significantly reduced from 36.1 mmHg (range 17–60 mmHg) preoperatively to 15.6 mmHg (range 3–32 mmHg) on the first postoperative day (P < 0.01). The sequential IOP change is demonstrated in Fig. 1. Four eyes had intraoperative hyphema, which resolved spontaneously within 1 week postoperatively. The postoperative data and surgical outcome are shown in Table 2. After a mean follow-up of 39 weeks (range 16–62 weeks), 13 eyes had controlled IOP (mean 12.1 mmHg, range 6–20 mmHg), among which there was only 1 eye which needed 1 anti-glaucoma medication. Two eyes were considered as failure. One eye underwent glaucoma drainage device implantation and the other eye needed cyclophotocoagulation. Nine eyes received additional PRP. Visual acuity was improved in three eyes, unchanged in five eyes, and worse in seven eyes, of which one eye lost light perception. There were no observed complications related to IVR. Recurrent NVI was not detected in any patient. There were no significant symptoms in all eyes. The detail of each eye is presented in Table 3.
Discussion
The present study demonstrates that IVR administered before conventional TMC results in rapid regression of NVI and subsequently reduces the chance of intraoperative bleeding. Ranibizumab is an anti-VEGF which inhibits angiogenic activity and fibroblast proliferation. Both effects are critical for ocular neovascularization and wound modulation. The key success for NVG management is to eliminate the ischemic process of ocular tissue, leading to decrease in VEGF production. Conventionally, NVG treatments comprise PRP, retinal cryotherapy, or endolaser to reduce oxygen consumption by ischemic retina, but the effect is compromising and slow. The application of intravitreal anti-VEGF has been introduced as an adjunctive treatment for NVG. The off-label use of IVB has been reported to dramatically and rapidly reduce retinal and anterior segment neovascularization, as well as improve probable outcomes in NVG management [1, 2, 8]. Bevacizumab has been approved as an anti-angiogenic agent for the treatment of metastatic colorectal cancer in combination with chemotherapy, but not for intraocular application [12, 13]. There were several reports on the benefits of bevacizumab on Ahmed glaucoma implantation and trabeculectomy in NVG [3, 11, 14, 15]. Ranibizumab is approved by the US FDA for intraocular use as a treatment of neovascular AMD [5, 16]. To our knowledge, ranibizumab is not yet widely applied for a treatment of NVG [17]. The combination of intravitreous anti-VEGF and retinal treatment are ideal and theoretically more effective in controlling retinal ischemia. Anti-VEGF transiently reduces neovascularization in the early treatment period, whereas PRP permanently controls retinal ischemia in the later period. We considered administering ranibizumab as soon as possible to limit ocular tissue damage, especially in eyes which PRP could not apply. After the critical period, we performed PRP in eyes which had not received PRP before or in eyes in which PRP was not adequate. This procedure may account for the success of neovascular control in our series. Some case series have demonstrated that intravitreous anti-VEGF alone provided transient IOP reduction [1, 2], but there was no IOP-lowering effect found in the current study. The IOP reduction effect probably depended upon the disease stage and the short drug’s life. For most NVG in the advanced stage or with extensive peripheral synechia of anterior chamber angle, the prognosis is usually poor in terms of IOP or disease control. The patients with sustained uncontrolled IOP required further interventions such as cyclophotocoagulation or glaucoma drainage implantation [1, 18]. Despite maximal medication therapy, surgery was indicated. Trabeculectomy in patients with NVG usually shows frequent intraoperative complications and poor surgical outcomes [19]. Kobayashi et al. [11] reported good long-term outcomes after combined preoperative IVB and PRP before trabeculectomy in NVG. The present study, using ranibizumab, demonstrates excellent outcome in 13 out of 15 eyes. Anti-VEGF probably improved the surgical outcome by providing ischemia and wound healing control, which are among the important factors for surgical success. Compared to PRP or retinal cryotherapy alone, they can eliminate retinal ischemia only. All eyes participating in this study showed persistent NVI even in six eyes with adequate PRP. After IVR, all eyes demonstrated rapid regression of NVI. With the absence of preoperative NVI, we found a minimal intraoperative hyphema in 4 out of 15 eyes, which resolved spontaneously without specific treatment or complication. The surgical outcome was favorable in terms of IOP control. Twelve eyes had IOP under 21 mmHg without medication and only 1 eye with medication. There were only 2 eyes which were considered as failures (IOP more than 21 mmHg with maximal medication treatment). A patient with bilateral NGV had one successful eye and one eye which failed. The failed eye was the eye for which the surgeon could not apply PRP before or after TMC due to a corneal scar and vitreous hemorrhage. This eye was considered as more severely affected and having a poorer prognosis when compared to the first operated eye. Cyclodestruction was then applied. The other eye which failed was an eye with history of proliferative diabetic retinopathy and already showed the evidence of adequate PRP before IVR. This eye finally developed a new vitreous hemorrhage and underwent additional PRP with glaucoma drainage device implantation. Although IVR-augmented TMC could reserve vision at least equal to that at presentation in only 8 eyes, all 15 eyes were symptom-free at the last visit (no pain, irritation, or redness). There was no eye which experienced recurrence of neovascularization during the follow-up period (range 16–62 weeks). Once trabeculectomy was performed, the VEGF had a new drainage channel. We hypothesized that ranibizumab, PRP, and a new VEGF-draining channel may account for the prevention of the recurrence of neovascularization and the excellent surgical outcome. There is no need for repeating anti-VEGF application in this series. However, given the short-term follow-up time of this study, long-term recurrent neovascularization may still occur. At the last follow-up, all eyes showed benefits from IVR and filtration, including being symptom-free. The majority of eyes preserved visual acuity and had good IOP control. Neither local nor systemic side effects were noted.
This study has some limitations including the small numbers of participants, a short follow-up period, and the variable stages of disease. The eyes with advanced NVG or opaque ocular media did not allow adequate PRP on retina. Because we tried to eliminate neovascularization as soon as possible to prevent permanent ocular tissue damage, we did not wait for PRP to show its effect before filtration surgery. These factors may be associated with differences in surgical outcome. Further study is needed to investigate the optimal time to apply IVR, the suitable period in which to perform trabeculectomy, and the factors related to positive visual and surgical outcomes.
Conclusions
Preoperative IVR can be used as an adjunctive therapy in eyes with NVG. It is an effective treatment which resulted in rapid NVI regression, subsequently minimizing intraoperative complications during trabeculectomy. Applying IVR and PRP can improve the short-term surgical outcome after TMC. All eyes were symptom-free and most eyes retained their visual acuity with good IOP control. A long-term study should be conducted to determine the long-term outcomes, the safety, and the duration of the effect of IVR.
References
Iliev ME, Domig D, Wolf-Schnurrbursch U, Wolf S, Sarra G-M. Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol. 2006;142(6):1054–6.
Vatavuk Z, Bencic G, Mandic Z. Intravitreal bevacizumab for neovascular glaucoma following central retinal artery occlusion. Eur J Ophthalmol. 2007;17(2):269–71.
Kitnarong N, Chindasub P, Metheetrairut A. Surgical outcome of intravitreal bevacizumab and filtration surgery in neovascular glaucoma. Adv Ther. 2008;25(5):438–43.
Yoshida N, Hisatomi T, Ikeda Y, et al. Intravitreal bevacizumab treatment for neovascular glaucoma: histopathological analysis of trabeculectomy specimens. Graefes Arch Clin Exp Ophthalmol. 2011;249(10):1547–52.
Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr Opin Ophthalmol. 2007;18(6):502–8.
Horsley MB, Kahook MY. Anti-VEGF therapy for glaucoma. Curr Opin Ophthalmol. 2010;21(2):112–7.
Cornish KS, Ramamurthi S, Saidkasimova S, Ramaesh K. Intravitreal bevacizumab and augmented trabeculectomy for neovascular glaucoma in young diabetic patients. Eye (Lond). 2009;23(4):979–81.
Dunavoelgyi R, Zehetmayer M, Simader C, Schmidt-Erfurth U. Rapid improvement of radiation-induced neovascular glaucoma and exudative retinal detachment after a single intravitreal ranibizumab injection. Clin Exp Ophthalmol. 2007;35(9):878–80.
Rulli E, Biagioli E, Riva I, et al. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmol. 2013;131(12):1573–82.
Alkawas AA, Shahien EA, Hussein AM. Management of neovascular glaucoma with panretinal photocoagulation, intravitreal bevacizumab, and subsequent trabeculectomy with mitomycin C. J Glaucoma. 2010;19(9):622–6.
Kobayashi S, Inoue M, Yamane S, Sakamaki K, Arakawa A, Kadonosono K. Long-term outcomes after preoperative intravitreal injection of bevacizumab before trabeculectomy for neovascular glaucoma. J Glaucoma. 2015;. doi:10.1097/IJG.0000000000000211.
Marshall JL. Bevacizumab in the treatment of colorectal cancer. Clin Adv Hematol Oncol. 2007;5(1 Suppl 1):8–9.
Caprioni F, Fornarini G. Bevacizumab in the treatment of metastatic colorectal cancer. Future Oncol. 2007;3(2):141–8.
Zhou M, Xu X, Zhang X, Sun X. Clinical outcomes of ahmed glaucoma valve implantation with or without intravitreal bevacizumab pretreatment for neovascular glaucoma: a systematic review and meta-analysis. J Glaucoma. 2015. doi:10.1097/IJG.0000000000000241.
Sothornwit N. Intravitreal bevacizumab for ahmed glaucoma valve implantation in neovascular glaucoma: a case report. J Med Assoc Thai. 2008;91(Suppl 1):S162–5.
Gillies MC, Campain A, Walton R, et al. Time to initial clinician-reported inactivation of neovascular age-related macular degeneration treated primarily with ranibizumab. Ophthalmology. 2015;122(3):589 e1–594 e1.
Luke J, Nassar K, Luke M, Grisanti S. Ranibizumab as adjuvant in the treatment of rubeosis iridis and neovascular glaucoma—results from a prospective interventional case series. Graefes Arch Clin Exp Ophthalmol. 2013;251(10):2403–13.
Mason JO 3rd, Albert MA Jr, Mays A, Vail RC. Regression of neovascular iris vessels by intravitreal injection of bevacizumab. Retina. 2006;26(7):839–41.
Sivak-Callcott JA, O’Day DM, Gass JDM, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001;108(10):1767–76.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
Naris Kitnarong, Chuenjanok Sriyakul, and Siriwan Chinwattanakul declare no conflict of interest.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kitnarong, N., Sriyakul, C. & Chinwattanakul, S. A Prospective Study to Evaluate Intravitreous Ranibizumab as Adjunctive Treatment for Trabeculectomy in Neovascular Glaucoma. Ophthalmol Ther 4, 33–41 (2015). https://doi.org/10.1007/s40123-015-0033-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-015-0033-3