Abstract
Aim
The main treatment for meibomian gland dysfunction (MGD), a major cause of dry eye, is eyelid warming. Lack of compliance is the main reason for treatment failure. This has led to the development of eyelid-warming devices that are safe, effective and convenient. To obtain robust evidence demonstrating their efficacy, the authors conducted a 3-arm randomized clinical study.
Methods
The authors conducted a 3-month assessor-blinded, randomized, controlled trial of patients from the Singapore National Eye Centre experiencing at least one of eight dry eye symptoms ‘often’ or ‘all the time’. Patients who wore contact lenses, had an active infection or known diagnosis of thyroid dysfunction and rheumatoid arthritis were excluded from the study. MGD participants were randomly assigned to warm towel (n = 25), EyeGiene® (Eyedetec Medical Inc., Danville, CA, USA) (n = 25) and Blephasteam® (Spectrum Thea Pharmaceuticals LTD, Macclesfield, UK) (n = 25) treatments. The primary efficacy and safety outcomes included the proportions of participants with improved symptoms and changes in best corrected visual acuity (BCVA), respectively. Other outcomes included tear break up time (TBUT), Schirmer test, corneal fluorescein dye staining and number of visibly occluded meibomian gland (MG) orifices.
Results
The study population was 53.5 ± 11.1 years old and predominantly Chinese. For severity of symptom after 3 months of treatment, 78.3% Blephasteam® participants reported improvement compared to 45.5% warm towel participants (p = 0.023). The corresponding proportions for improvement in the frequency of symptoms were 82.6% and 50.0%, respectively (p = 0.020). The proportions of improvement of symptoms in EyeGiene® patients were not significantly different from warm towel intervention. At 1 month of treatment, the crude odds ratio of improvement of severity of irritation for Blephasteam® compared to control was 3.0 (95% CI 0.88–10.18). However, the odds ratio adjusted by age was 5.67 (1.30–24.66). The lid-warming treatments did not significantly change the TBUT, Schirmer test results or number of visibly occluded MGs in the study period. All treatment modalities did not worsen BCVA after 3 months.
Conclusion
Blephasteam® is more effective than warm towel for MGD treatment, with warm towel and EyeGiene® being comparable effective. Older age might predict for treatment efficacy. All studied therapies were safe for visual acuity (VA) for 3 months of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dry eye is a common condition with symptoms that impact patients’ quality of life [1, 2]. It is perceived to be as distressing as chest pain [3] and imposes considerable healthcare costs, up to US$1.1 million per 1,000 persons annually [4], and productivity costs [1]. Meibomian gland dysfunction (MGD) is a chronic abnormality of the meibomian glands (MGs) [5]. It may cause eye discomfort [6] and affect tear film stability [5] which leads to poorer visual function, faster tear evaporation and ocular surface damage [7]. MGD is thought to be a major cause of dry eye that affects 46.2–69.3% Asians and 3.5–19.9% Caucasians [8–10].
The cornerstone therapy for MGD is warm compress [11]. Various forms of eyelid-warming therapy have been shown to improve patients’ symptoms [12–19], tear film stability [12–18], slow down tear evaporation [14] and reduce ocular surface damage [18, 20]. Other additional treatments such as lubricating drops, antibiotics, anti-inflammatory cyclosporine and topical azithromycin are prescribed for more severe MGD [11].
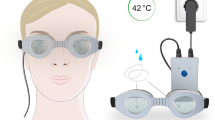
The recommended regimen for warm compress is daily treatment [11]. This is prone to poor compliance and difficulty in delivering therapeutic temperatures [21]. Several eyelid-warming devices with features that improve convenience and deliver heat at safe, calibrated temperatures to the eyelids have been developed recently [15, 22]. Two examples are EyeGiene® (Eyedetec Medical Inc., Danville, CA, USA) and Blephasteam® (Spectrum Thea Pharmaceuticals LTD, Macclesfield, UK). EyeGiene® is a compact system composed of an eye mask and sachets containing heat-generating chemicals. Blephasteam® is a portable pair of goggles which uses moist heat instead of physical contact for heat transfer to the eyelids.
However, little evidence exists on the effectiveness, especially in long-term use, of these eyelid-warming devices. Current reports on EyeGiene® [14, 16] and Blephasteam® [17–20, 22] do not have control groups for comparison and all except one abstract [16] show effects of short-term (up to 3 weeks) studies, or after just one application of the device. Two articles [20, 22] also reported effects in healthy as opposed to MGD participants.
The aim of this study was to evaluate the effectiveness and safety of EyeGiene® and Blephasteam® against the conventional warm towel compress in a randomized controlled trial involving MGD patients.
Methods
Study Design and Participants
This is a 3-month randomized, controlled trial comparing two eyelid-warming devices, EyeGiene® and Blephasteam®, and warm towel compress in MGD participants.
From February 2012 to October 2013, all patients at the Singapore National Eye Centre dry eye clinic who met the eligibility criteria were briefed about this study and invited for screening. The MGD in this study is not classified into severity levels but as long as the morphological eligibility criterion below and specified symptoms are present, they can be included. Eligible participants were then enrolled with written informed consent by the clinical trial coordinator.
Compliance with Ethics Guidelines
The study has been approved by the SingHealth Centralized Institutional Review Board and adhered to the tenets of the Declaration of Helsinki of 1975, as revised in 2000 and 2008.
Eligibility
These patients were included for the study:
-
At least one of eight dry eye symptoms (Supplementary Fig. 1—questionnaire modified after Schein et al. [23]) was experienced “often” or “all the time.”
-
At least one MG opening with pouting and a visible plug above the eyelid margin that cannot be removed by gentle wiping with a cotton tip.
-
No ocular pathology requiring treatment other than eye lubricant and conventional eyelid hygiene within the last month and during the study.
-
Participants with data at baseline and 1 month after treatment.
These patients were excluded from the study:
-
Known diagnosis of thyroid dysfunction and rheumatoid arthritis.
-
Ocular surgery within the previous 6 months and laser-assisted in situ keratomileusis (LASIK) within the previous year.
-
Central nervous system and hormonal drugs required within the last month and during the study.
-
Active ocular infection or presence of pterygium.
-
Necessity to wear contact lens during the study.
-
Living in the same household as another participant of the study.
Randomization and Blinding
The nursing manager and two witnesses drew lots to determine the random allocation sequence of participants to the three treatment methods in a 1:1:1 ratio. The assessor of the participants’ clinical signs was blinded to the treatment method of each participant.
Interventions
All participants were required to self-administer the eyelid-warming therapies twice daily, for 10 min each time, then briefly massage their eyes and clean their eyelids with Blephagel® (Spectrum Thea Pharmaceuticals LTD, Macclesfield, UK) and cotton pads.
Control Arm
Participants were given a towel to warm in warm water before placing it over their eyes. They were instructed to re-warm the towel when they feel it get cooler.
EyeGiene®
The manufacturer claims that when the chemicals in the disposable sachets are mixed together and placed in the pockets of the eye mask, they deliver 40 °C heat to the eyelids for 8–10 min. The clinical trial coordinator would demonstrate how to activate the warming unit and use the eye mask as well as watch the participant activating one warming unit.
Blephasteam®
The Blephasteam® goggles are electrically powered and have to be preheated for 15 min. New Blephasteam® rings were moistened and fitted in the goggles before each 10-min session. Printed instructions from Blephasteam® were provided to each Blephasteam® participant.
Compliance Measures and Concurrent Medications
The participants were given a diary to record details of each treatment session (time and duration), eyelid cleaning with Blephagel® and use of eye lubricant. Participants were allowed to use eye lubricants but not antibiotics, steroid and anti-inflammatory eye drops such as cyclosporine as stipulated in the inclusion criteria.
Efficacy Outcome Measures
The primary efficacy outcome was the proportion of participants with improvement in severity and frequency of eye discomfort after 1 month of treatment. The secondary efficacy outcomes were the proportions of participants with improvement in symptom severity and frequency between 1 and 3 months of treatment. The symptom severity and frequency were performed on a visual analog scale as previously described [24]. The details of all the study procedures are provided in the supplementary file.
Safety Outcome
Adverse events (AEs) and serious adverse events (SAEs) were monitored but the pre-specified safety outcome was changed in VA after 3 months of treatment. This is because a previous study found VA to be affected by lid warming [25].
Exploratory Efficacy Outcomes
Our exploratory outcomes were the changes in TBUT, number of plugged MG openings, corneal fluorescein staining and Schirmer’s test after 1 and 3 months of treatment.
Statistical Analyses
Sample Size Calculation
There was little literature on the magnitude of symptom change on this scale that would be clinically significant. Based on clinical assumption, and taking into account the cost of the eyelid-warming devices, the authors endeavored to detect a 40% difference in proportion of participants with the primary outcome of improved symptom severity/frequency between the EyeGiene®/Blephasteam® and the warm towel groups after 1 month of treatment. Conservatively, the proportion of warm towel participants (the current practice) with improved severity/frequency was assumed to be 40%. Hence, 22 participants in each study arm were required for 80% power and a two-sided significance level of 5%. Therefore, the aim was to recruit 25 participants per arm to allow for three losses to follow-up or withdrawals per arm.
Statistical Analyses
Data were checked for normality to determine the appropriate parametric or non-parametric test. To test for differences among groups, relevant Chi square test, one-way analysis of variance (ANOVA) and Kruskal–Wallis equality-of-populations rank test were used. Where there was difference among groups, the authors performed the relevant Student’s t test or Wilcoxon rank sum test. Chi square test was used to evaluate the differences in the primary and secondary outcomes between groups. Logistic regression was used to adjust for baseline differences. The analysis was by intention to treat. Statistically significant difference was based on 0.05 level of significance. All analyses were performed with Stata software, version 12.1 (StataCorp. College Station, TX, USA).
Results
Of 102 patients who were assessed for eligibility, 75 were enrolled. Subsequently three patients were found to have a history of thyroid disease and were therefore considered not eligible. The other 72 patients were randomized into three equal groups (Supplementary Fig. 2). Table 1 shows the patients (n = 65) who were successfully followed up and had data at 1 month (primary outcome analysis). The ages of these participants were 53.5 ± 11.1 years, with 67.7% female (Table 1) and predominantly Chinese (exceptions are 2 Malay patients, 2 Indian patients and 1 Burmese patient). There were no comorbidities in any group.
Then MGD grading was similar in all the groups studied. The mean number of blocked glands in each of the groups was not significantly different (Table 1). Baseline severity of eye discomfort was significantly less in EyeGiene® (23.6 ± 14.9) compared to the other two groups (p = 0.0008) (Table 1). Corneal staining was significantly less severe in the Blephasteam® group (p = 0.030; Table 1). All other baseline characteristics were not significantly different among the three groups. Overall, the mean TBUT ± SD was 2.4 s ± 1.4 and the Schirmer I was 11.7 mm ± 10.4 at baseline.
There were eight withdrawals from the EyeGiene® group before the assessment at the 1-month time point. These cases had more severe and frequent eye discomfort and higher Schirmer I readings compared to the remaining EyeGiene® participants. The reason for withdrawal was the inability to activate the EyeGiene® warming units. The problem was not resolved with additional counseling; the manufacturers would improve the device in future batches (personal communication). Because the number of patients on EyeGiene® for analysis was less than the calculated number required, we recruited another seven patients (non-randomized) who were treated with EyeGiene® for an additional analysis and performed a two group analysis comparing this treatment with warm towels (Supplementary Tables 2, 3). Essentially the conclusions concerning the efficacy outcomes comparing the augmented EyeGiene® group versus the original towel groups are the same as that presented below.
The proportions of participants with reduced frequency of eye discomfort after 1 month of treatment (primary efficacy outcome) were 54.2% for warm towel, 52.9% EyeGiene® and 79.2% for Blephasteam® (Table 2). The proportions with reduced severity at 1 month were 50.0%, 52.9% and 75.0%, respectively. There were no significant differences at this 1-month treatment time point.
At the last follow-up (3 months) in the study, the proportions in each study arm with improvement in the frequency of irritation were; warm towel 50.0%, EyeGiene® 41.2% and Blephasteam® 82.6%. The Blephasteam® arm had a significantly greater proportion with improvement in the frequency compared to the warm towel group (p = 0.020). The proportions of patients with reduced severity of symptoms were 45.5%, 41.2% and 78.3%, respectively. Again the improvement was significantly greater in the Blephasteam® compared to the towel group (p = 0.023).
Since EyeGiene® gave similar results as warm towel (Table 2), this report focused on the logistic regression comparing Blephasteam® against warm towel (Table 3). The final logistic regression model for improvement of symptom severity after 1 month of treatment included the variables of treatment (Blephasteam® versus warm towel) and age (Table 3). In this model, Blephasteam® is 5.67 (95% Confidence interval [CI]: 1.30–24.66) times more efficacious than warm towel. Older participants were slightly more likely to have reduced symptom severity after 1 month of treatment.
There was no significant difference among the three groups for change in TBUT at 1 month (p = 0.669; Table 4). After 3 months of treatment, there was also no significant difference in the change in TBUT (p = 0.612). There was no significant change in Schirmers I, Marx’s line score and meibum viscosity among the participants at 3 months (data not shown). Although there was a significant decrease in the total number of plugged MG orifices (p < 0.001) over 12 weeks, there was no significant difference among the three treatment groups after 1 month (p = 0.656) and 3 months of treatment (p = 0.926) (Table 5).
All three eyelid-warming methods did not worsen participants’ VA after 3 months of twice-daily treatment (p = 0.672, 0.769 and 0.900; Supplementary Table 4). There were two reports of unexpected AEs that were not related to study treatment.
Discussion
At 1 month, all types of lid warming improved symptoms by 50% or more. Blephasteam® relieved symptoms of eye discomfort for a significantly greater proportion of participants than warm towel up to 3 months of treatment. In this study, EyeGiene® did not show any advantage over the warm towel but this could be due to either a lower symptom severity at baseline or reduced participation related to technical difficulty of using the device. All treatment modalities did not harm vision.
While the methods of assessing symptomatic improvement and duration of study differ, three previous studies are essentially in agreement with our results that demonstrate Blephasteam® improved patients’ symptoms [17–19]. EyeGiene®, which has also been marketed as iHeat, relieved ocular symptoms in 56% participants in a previous 2-week study [15]. This study showed similar results, with ~50% who improved after 1 month (Table 2) followed by a slightly lower 41.2% between 1 and 3 months of treatment. This, however, was not different than warm towel. Significant improvement in VA after a one-time Blephasteam® treatment in healthy participants [20] and dry eye patients has been reported. This study, however, was interested in the longer-term effect of heat therapy on the eyes and showed no significant change of BCVA in MGD participants after 3 months of therapy. Villani et al. [17] reported significant improvement in TBUT after 3 weeks of Blephasteam® treatment but Doan et al. [19] disagreed. The majority of the Blephasteam® participants had increased TBUT after 1 and 3 months of treatment but this change in TBUT was not significantly different compared to warm towel or EyeGiene® participants (Table 4). There was no significant change in TBUT in a 2-week study of EyeGiene® treatment [15]. Instead of looking at just the change in TBUT with EyeGiene® treatment, the authors wanted to see if the change was different among the three treatment groups but it was not (Table 4). There were no reports on effect of eyelid-warming therapy on plugging of MG openings for comparison. Besides these studies participants’ complaints of inability to use EyeGiene®, 2 cases (1.4%) of moderate ocular burning and minor discomfort or pain were also previously reported [15]. Four EyeGiene® participants also gave feedback of short (only about 6 min) and inconsistent heat therapy. It may be that tighter quality control is necessary for EyeGiene® before widespread use in MGD patients.
The possible reason for the advantage of Blephasteam® over conventional warm towel is the more consistent delivery of heat over the time of application of the device. It is not clear if the use of moist heat in the Blephasteam® device is advantageous over dry heat.
The strengths of this study are randomization, its duration and the presence of warm towel therapy for comparison. This is also the first report on efficacy of Blephasteam® and EyeGiene® in a predominantly Chinese population and the results concur with those in previous studies.
This study has several limitations. The patients were recruited largely from a referral clinic and may not always reflect the general patients with MGD. The authors had an unexpected number of withdrawals from the EyeGiene® group. The eight withdrawals from the EyeGiene® group had more severe symptoms at baseline. To compensate for the reduced participants, the authors included more EyeGiene® subjects in a non-randomized way using exactly the same eligibility criteria, and compared this group to the towel group. In this additional analysis, the baseline global irritation score was still significantly lesser in the EyeGiene® group compared to the control group (Supplementary Table 2). The safety assessment of the Eyegiene® device may not be robust enough given reduced patient numbers analyzed.
Temperature for the towel was not specified and thus might defer from previously published studies. The study might not be generalizable to all MGD patients because our patients were predominantly Chinese. As participants could not be blinded, there might be some placebo-like effect affecting the results of the study. Factors not covered in this report, possibly inflammation, meibum composition and MG atrophy can be associated with symptom improvement.
Besides understanding the factors predicting for treatment success, it would also be valuable to conduct pathological or imaging studies to understand the physiological or anatomical mechanism behind the recovery of MGD after eyelid-warming therapies. This trial also included the measurement of tear evaporation as well as meibography and laboratory investigations such as tear and meibum lipidomics analyses which will be presented in upcoming reports.
Conclusion
A high proportion of participants respond symptomatically to lid warming. Blephasteam® is more efficacious than conventional warm towel compress for at least 3 months of MGD therapy. Studies to understand the predictors of treatment success such as older age would be beneficial for treatment recommendations.
References
Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care. 2008;14(3 Suppl):S102–6 (Epub 2008/05/09).
The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):93–107 (Epub 18/05/2007).
Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412–9 (Epub 18/07/2003).
Clegg JP, Guest JF, Lehman A, Smith AF. The annual cost of dry eye syndrome in France, Germany, Italy, Spain, Sweden and the United Kingdom among patients managed by ophthalmologists. Ophthalmic Epidemiol. 2006;13(4):263–74 (Epub 01/08/2006).
Nelson JD, Shimazaki J, Benitez-del-Castillo JM, Craig JP, McCulley JP, Den S, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):1930–7 (Epub 01/04/2011).
Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113(10):1266–70 (Epub 01/10/1995).
Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78(3):347–60 (Epub 27/04/2004).
Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52(4):1994–2005 (Epub 01/04/2011).
Siak JJ, Tong L, Wong WL, Cajucom-Uy H, Rosman M, Saw SM, et al. Prevalence and risk factors of meibomian gland dysfunction: the Singapore Malay eye study. Cornea. 2012;31(11):1223–8 (Epub 13/01/2012).
Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–8 (Epub 02/03/2012).
Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O’Brien T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050–64 (Epub 01/04/2011).
Olson MC, Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact Lens. 2003;29(2):96–9 (Epub 16/04/2003).
Mori A, Shimazaki J, Shimmura S, Fujishima H, Oguchi Y, Tsubota K. Disposable eyelid-warming device for the treatment of meibomian gland dysfunction. Jpn J Ophthalmol. 2003;47(6):578–86 (Epub 26/11/2003).
Goto E, Monden Y, Takano Y, Mori A, Shimmura S, Shimazaki J, et al. Treatment of non-inflamed obstructive meibomian gland dysfunction by an infrared warm compression device. Br J Ophthalmol. 2002;86(12):1403–7 (Epub 26/11/2002).
Lane SS, DuBiner HB, Epstein RJ, Ernest PH, Greiner JV, Hardten DR, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31(4):396–404 (Epub 10/01/2012).
Guillon M, Maissa C, Wong S, et al. Effects of controlled heat delivery to the eyelid margin and eyelid hygiene on symptomatology and the tear film in MGD. ARVO Meeting Abstracts. 2013;6044–A0107.
Villani E, Garoli E, Canton V, et al. Heating wet chamber goggles (Blephasteam®) in meibomian gland dysfunction unresponsive to warm compress treatment. ARVO Meeting Abstracts. 2012;53:6.
Matsumoto Y, Dogru M, Goto E, Ishida R, Kojima T, Onguchi T, et al. Efficacy of a new warm moist air device on tear functions of patients with simple meibomian gland dysfunction. Cornea. 2006;25(6):644–50 (Epub 02/11/2006).
Doan S, Gabison EE, et al. Efficacy of heating wet chamber goggles (Blephsteam®) in adult blepharitis. ARVO Meeting Abstracts. 2011;52:3833.
Pult H, Riede-Pult BH, Purslow C. A comparison of an eyelid-warming device to traditional compress therapy. Opto Vis Sci Off Publ Am Acad Opto. 2012;89(7):E1035–41 (Epub 26/06/2012).
Blackie CA, Solomon JD, Greiner JV, Holmes M, Korb DR. Inner eyelid surface temperature as a function of warm compress methodology. Opto Vis Sci Off Publ Am Acad Opto. 2008;85(8):675–83 (Epub 05/08/2005).
Purslow C. Evaluation of the ocular tolerance of a novel eyelid-warming device used for meibomian gland dysfunction. Contact Lens Anterior Eye J Br Contact Lens Assoc. 2013;36(5):226–31 (Epub 10/04/2013).
Schein OD, Tielsch JM, Munoz B, et al. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology. 1997;104(9):1395–401.
Schaumberg DA, Gulati A, Mathers WD, Clinch T, Lemp MA, Nelson JD, et al. Development and validation of a short global dry eye symptom index. Ocul Surf. 2007;5(1):50–7 (Epub 26/01/2007).
Solomon JD, Case CL, Greiner JV, Blackie CA, Herman JP, Korb DR. Warm compress induced visual degradation and Fischer–Schweitzer polygonal reflex. Opto Vis Sci Off Publ Am Acad Opto. 2007;84(7):580–7 (Epub 17/07/2007).
Acknowledgments
All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. Sponsorship and article processing charges for this study were funded by grants from the National Medical Research Council (Helios, Singapore) and the Biomedical Research Council (Centros, Singapore). The authors belong to the Collaborative Research Initiative for Meibomian gland dysfunction (CORIM). This group also consists of Markus Wenk from National University of Singapore; Lee Hwee Kuan from Singapore Bioinformatics Institute; Guanghou Shui and Sin Man Lam from Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, China. We would like to thank Barry J. Linder from Eyedetec Medical (Eyedetec Medical Inc., Danville, California, USA) for contributing the EyeGiene® devices and Arul Earnest and Wong Nan Soon for their advice.
Conflict of interest
H. S. Sim, A. Petznick, S. Barbier, J. H. Tan, U. R. Acharya, S. Yeo and L. Tong declare no conflicts of interest.
Compliance with ethics guidelines
The study has been approved by the SingHealth Centralized Institutional Review Board and adhered to the Tenets of the Declaration of Helsinki of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study.
Funding
National Medical Research Council, Singapore grant NMRC/CSA/045/2012, Biomedical Research Council, Singapore BMRC(TCRP)10/1/35/19/670. ARVO/AFER Vistakon Dry Eye Fellowship 2012.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Trial Registration: ClinicalTrials.gov #NCT 01448369.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sim, H.S., Petznick, A., Barbier, S. et al. A Randomized, Controlled Treatment Trial of Eyelid-Warming Therapies in Meibomian Gland Dysfunction. Ophthalmol Ther 3, 37–48 (2014). https://doi.org/10.1007/s40123-014-0025-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-014-0025-8