Abstract
Introduction
To determine whether there is a statistically significant difference in the decrease in intraocular pressure (IOP) after selective laser trabeculoplasty (SLT) between patients receiving a 5–7 days co-administration of loteprednol versus no loteprednol over the course of 1 year.
Methods
We conducted a retrospective chart review to evaluate use of loteprednol in patients aged 30–85 years undergoing SLT for open-angle glaucoma at our center over a 3-year period.
Results
Three hundred and eighteen eyes from 313 patients who underwent a 360° SLT treatment between January 2008 and August 2011 were included in the analysis. Patients who received loteprednol showed a mean reduction of 2.5 (±SE 0.3) mmHg or 11.8% (±1.5%) in IOP versus a mean reduction of 3.2 (±0.6) mmHg or 14.9% (±2.5%) in those not treated with loteprednol. This difference showed a trend toward lower IOP without loteprednol, but this was not statistically significant (p = 0.29).
Conclusion
Postoperative use of loteprednol does not appear to significantly affect IOP in patients undergoing SLT. A randomized double-blinded study in a larger group of patients would be required to clarify this issue. Until such information is available, we recommend that individual clinical judgment be used regarding whether to use topical steroids in patients undergoing SLT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trabeculoplasty, the common term used for the treatment of the trabecular meshwork with laser to lower intraocular pressure (IOP), has a long history, beginning with Krashnov’s use of a ruby laser to treat glaucoma in 1972 [1]. Other lasers were tried, the most common procedure being the argon laser trabeculoplasty (ALT) used by Hager [2]. Today, the commercially available laser is the Q-switched, double-frequency neodymium:yttrium–aluminum–garnet (Nd:YAG) laser with a wavelength of 532 nm. This was first described by Latina in 1995 and the procedure was named selective laser trabeculoplasty (SLT) [3, 4]. This laser selectively targets pigmented trabecular meshwork cells and treatment can be repeated with no thermal damage [4, 5].
Two main theories have been suggested for ALT and have been well described [6–9]. These include the mechanical theory by Wise and Witter [6] and the cellular and biological theory by Van Buskirk [7]. These theories are based on findings from studies using electron microscopy and histopathology. Originally, it was thought that laser treatment caused a mechanical trabeculopuncture, allowing the aqueous to flow more readily through the trabecular meshwork [10–12]. In support of the mechanical theory, there has been shown to be a disruption of the trabecular beams of the uveal and corneoscleral trabecular meshwork with surrounding coagulative damage. Weeks later, fibrosis was shown to develop [10–12]. Further studies have shown shrinkage of the trabecular ring, separation of trabecular sheets, opening of aqueous channels, and an opening traction on Schlemm’s Canal [10–12]. The cellular and biological theories are based on studies that have shown an increase in a macrophage-like phagocytic activity of trabecular meshwork cells, increased turnover and synthesis of glycosaminoglycans, increased cell division of trabecular meshwork cells and an induced inflammatory response. These biologic and cellular changes generally take 4–6 weeks to develop [13]. Conversely, eyes that have undergone SLT show no alteration of the collagen structure of the trabecular beams and no evidence of coagulative damage. There was, however, a disruption of trabecular endothelial cells with subsequent cellular division and increased phagocytic activity [5].
As a result of the biologic and cellular theory, clinical studies have been performed primarily on ALT to determine the role of inflammation and its inhibition. The Fluorometholone-Laser Study Group conducted a prospective, randomized, study on the effects of corticosteroid treatment on ALT [14]. This short-term study was later extended to a follow-up period of 4.6 ± 3.4 years [14, 15]. Both the short- and long-term studies found no statistically significant difference in IOP with administration of fluorometholone between the two groups (p = 0.51).
There is a paucity of published data on the effect of corticosteroid administration and efficacy of SLT, which has a presumed biologic and cellular mechanism only [16, 17]. Realini conducted a randomized, observer-masked study of 25 participants who received bilateral 360° SLT and prednisolone acetate four times per day for 1 week in one eye only [15, 16]. This study showed no statistically significant difference in IOP decrease at 1 week, 3 weeks, and 3 months. There was also no statistically significant difference in IOP decrease over the course of 1 year for concurrent ketorolac 1% (n = 14) versus prednisolone acetate 1% (n = 60) treatment in a subset of patients participating in a multicenter, prospective, nonrandomized study that evaluated SLT as an initial and adjunctive treatment versus latanoprost [17].
There have thus far been no published studies of SLT with a follow-up greater than 3 months comparing corticosteroid versus no corticosteroid. In addition, all previous studies had small sample sizes. Thus, we conducted a retrospective chart review to evaluate use of loteprednol in a large series of patients treated with SLT at our center.
Materials and Methods
This study was a single-center, retrospective chart review of patients from Sabates Eye Centers, a multispecialty ophthalmology practice based on the Kansas City, MO, area.
We searched the Next Gen (NextGen Healthcare Information Systems, Inc. Horsham, PA, USA) database at Sabates Eye Centers (Kansas City, MO, USA) for patients aged 30–85 years with the procedure code of SLT (66984), between January 1, 2008 and August 31, 2011.
The patients were initially listed by medical record number but were subsequently assigned research identifiers to prevent patient identification. We then limited patients according to the following inclusion and exclusion criteria. Patients with a diagnosis of open-angle glaucoma (diagnosis codes of primary open-angle glaucoma, normal tension glaucoma, pigmentary glaucoma, or pseudoexfoliation glaucoma) were eligible for inclusion. Those with angle-closure, inflammatory, or secondary type of glaucoma were excluded. Also excluded were patients who received a topical corticosteroid of any type for another indication 1 month prior to SLT, or 3 months subsequent to SLT, and those with previous SLT in the same eye, cataract extraction less than 6 months prior to SLT, or any previous intraocular surgery other than cataract surgery.
We documented IOP measurements and the number of glaucoma medications at baseline, which was defined as the most recent IOP measurement prior to SLT. IOP measurements and the number of glaucoma medications at 1 month ± 2 weeks, 4 months ± 2 months, and at 1 year ± 3 months were analyzed to follow progression over the course of 1 year. Combination medications, i.e., COMBIGAN® (brimonidine tartrate/timolol maleate ophthalmic solution 0.2%/0.5% Allergan, Inc., Irvine, CA 92612, USA), were considered to be two medications. IOP was measured with Goldmann tonometry. The presence of anterior chamber reaction was not collected.
Statistical analysis was performed with support from the Department of Biomedical and Health Informatics at the University of Missouri-Kansas City (Kansas City, MO, USA). Bivariate comparisons were conducted on the age of the patient, number of medications at baseline and 1 year, and IOP at baseline and at 1 year, at which time the IOP was assumed to have stabilized. p values were determined by Chi-square comparison for the categorical variable (sex) and non-paired t tests for continuous variables. p values <0.05 were regarded as statistically significant. To control for the retrospective nature of this study and improve baseline balance between the group receiving and not receiving loteprednol, respectively, nearest-neighbor matching, as implemented by the R statistical computing (version 3.0.1) package MatchIt (version 2.4-21), was also utilized [18–20]. Each eye not receiving corticosteroid was nonexclusively matched to the three most similar eyes receiving corticosteroid. Similarity was determined based on all available baseline characteristics including IOP, age, sex, and number of medications. This statistical method has been shown to improve inferences when treatment (steroid versus no steroid) was not assigned in a randomized fashion [19].
Analyses were conducted to determine whether absolute and percentage change in IOP measurements from baseline to 1 year (i.e., 9–15 months following surgery) differed between patients receiving and those not receiving loteprednol. To test this difference, a repeated measures analysis of variance (ANOVA) was performed on the unmatched data. Additional analyses used the nearest-neighbor matching process. Mean treatment effects on the baseline to 1 year change in IOP were computed using the R statistical computing package Zelig (version 3.5.4) after controlling for baseline IOP, the baseline number of medications, and the type of glaucoma [18, 21, 22] Unless otherwise noted, results, figures, and percentages are reported for the raw unmatched data.
The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
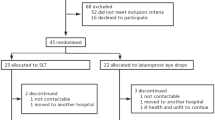
Retrospective chart reviews yielded data from 724 eyes in 642 patients. 318 eyes from 313 patients were eligible for inclusion in the analysis: 207 who received loteprednol for 5–7 days at the time of surgery, and 111 who did not. 228 of these patients were seen at or around 1 year following SLT. Bivariate and descriptive comparisons of the two groups of patients are shown in Tables 1 and 2. In these bivariate analyses, the only difference between patients receiving and those not receiving loteprednol was IOP at baseline, which was significantly higher in those not receiving loteprednol. However, following nearest-neighbor matching, baseline IOP was no longer significantly different between the treatment groups (p = 0.82).
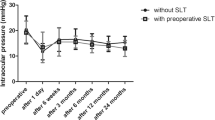
The test of the interaction effect (steroid x time) was not statistically significant (p = 0.18), as seen in Fig. 1. 195 patients (228 eyes) had IOP measurements available approximately 1 year following SLT. The tests of main effects showed that there was a statistically significant decline in IOP from baseline to 1 year (p < 0.001), and that in the raw data the loteprednol group had lower mean IOP at both time periods (p = 0.038). Absolute IOP and absolute IOP decrease can be seen in Figs. 1 and 2, respectively. After nearest-neighbor matching, the loteprednol group no longer had a significantly different IOP from the no loteprednol group at 1 year (p = 0.55). Loteprednol had no statistically significant effect on the change in IOP at 1 month (235 eyes, 95% CI −1.7 to 0.7 mmHg), 4 months (244 eyes, 95% CI −0.9 to 0.2 mmHg), or 1 year (228 eyes, 95% CI −0.5 to 0.9 mmHg).
We ran additional analyses to test whether the two groups differed in percentage change in IOP between baseline and 1 year. Patients who received loteprednol showed a mean reduction of 12% (±SE 1.5%) in IOP, while patients without loteprednol showed a mean reduction of 15% (±SD 2.5%) in IOP. However, this difference in percentage change between the groups was not statistically significant (p = 0.288). Percentage IOP decrease can be seen in Fig. 3. Following matching, this difference remained statistically insignificant (−12.0 ± 1.5% vs −12.4 ± 3.0%).
Discussion
Results of this retrospective study suggest that there is no statistically significant difference in the absolute or percentage decrease in IOP between patients receiving and those not receiving loteprednol after a 360° SLT treatment. This was somewhat surprising given that the postulated mechanism for SLT involves an immunologic reaction that would be expected to be altered by corticosteroid administration. However, our findings are consistent with previous studies that showed no effect of corticosteroid use on ALT and SLT [9, 14, 15]. The IOP-lowering effect of SLT in our study was comparable to previous studies examining laser as adjunctive therapy, supporting our findings [17].
There are a number of limitations to this study. The patients were under the care of two glaucoma specialists at the Sabates Eye Centers (KP and RK). There was a statistically significant difference in baseline IOP between the two groups (higher in the non-steroid group), which could potentially affect both mean IOP post treatment and percentage reduction in IOP. The two groups were treated by different surgeons, with differing philosophies regarding the effects of corticosteroid administration on laser trabeculoplasty. However, both surgeons were experienced, performed 360° treatment, treat patients from the same population, and have similar practice patterns. The two groups of patients were of similar age and there were no statistically significant differences in the number of glaucoma medications at any point. The recommended approach for dealing with unbalanced study designs where treatment is not randomized or independent of baseline patient characteristics is statistical matching [19]. In this study, the difference in pre-treatment IOP was eliminated following nearest-neighbor matching, thus helping to mitigate differences between physician philosophies.
Another limitation was the variability in follow-up due to the retrospective nature of the study. As patients were seen at irregular intervals following treatment over the course of the year, a range of times was used for the analysis. Despite this, some patients were still not seen or lost to follow- up, decreasing the number of time points available for analysis. Finally, we did not attempt to effectively assess post-operative inflammation or patient comfort at any time point, although a previous study of corticosteroids and SLT suggested no statistically significant difference in either of these [16].
Taking these limitations into consideration, we nevertheless believe that our study strongly suggests that corticosteroids have minimal effect on outcomes after SLT, which is supported by previous studies on SLT and ALT (Table 3). One advantage in our study is the larger sample size (228 versus 50 eyes) and longer follow-up (1 year versus 3 months compared to the only study directly looking at concurrent corticosteroids versus none with SLT [15–17]. The study by Mcllraith et al. [17] was not designed to answer the question of the effect of corticosteroids on SLT outcome. As a result, post hoc power analysis shows that with 60 eyes the study is expected to detect a 3.2 mmHg corticosteroid effect with an 80% likelihood. Due to the study design, where one eye was randomly chosen to receive corticosteroid, the study by Realini et al. [16] was powered to detect a 2.2 mmHg IOP difference at 3 months. In comparison, our study was powered to detect a 1.25 mmHg difference at 1 year.
A randomized double-blinded study with a higher power would be the best method to definitively determine whether there is any effect of corticosteroid use on the long-term outcome of SLT. A simple calculation shows that a randomized study would require more than 450 eyes to detect a significant difference of more than 1 mmHg change in IOP (α = 0.05, β = 0.80). Until such data are available, we recommend that individual clinical judgment be used regarding whether to use topical steroids in patients undergoing SLT.
References
Krasnov M. Laser puncture of the anterior chamber angle in glaucoma (a preliminary report). Vestnik Ofthalmologii. 1972;3:27–31.
Hager H. Special microsurgical interventions. 2. First experiences with the argon laser apparatus 800. Klinische Monatsblatter Fur Augenheilkunde. 1973;162:437–50.
Latina MA. C Park, Selective targeting of trabecular meshwork cells: in vitro studies of pulsed and CW interactions. Exp Eye Res. 1995;60:359–71.
Hong BK, Winer WC, Martone JF, et al. Repeat selective laser trabeculoplasty. J Glaucoma. 2009;18:3–180.
Kramer TR, Noecker RJ. Comparison of the morphologic changes after selective laser trabeculoplasty and argon laser trabeculoplasty in human eye bank eyes. Ophthalmology. 2001;108:773–9.
Wise JB. Glaucoma treatment by trabecular tightening with the argon laser. Int Ophthalmol Clin. 1981;21:69–78.
Van Buskirk EM, Pond V, Rosenquist RC, Acott TS. Argon laser trabeculoplasty: studies of mechanism of action. Ophthalmology. 1984;91:1005–10.
Bylsma SS, Samples JR, Acott TS, Van Buskirk EM. Trabecular cell division after argon laser trabeculoplasty. Arch Ophthalmol. 1988;106:544–7.
Van Buskirk EM. Pathophysiology of laser trabeculoplasty. Surv Ophthalmol. 1989;33:264–72.
Rodrigues MM, Spaeth GL, Donohoo P. Electron microscopy of argon laser therapy in phakic open angle glaucoma. Ophthalmology. 1982;89:198–210.
Van der Zypen E, Frankhauser F. Lasers in the treatment of chronic simple glaucoma. Trans Ophthalmol Soc UK. 1982;102:147–53.
Krasnov MM. Q-Switched laser goniopuncture. Arch Ophthalmol. 1974;92:37–41.
Melamed S, Epstein DL. Alterations of aqueous humour outflow following argon laser trabeculoplasty in monkeys. Brit J Ophthalmol. 1987;71:776–81.
Shin DH, Frenkel RE, David R, Cheetham JK. Effect of topical anti-inflammatory treatment on the outcome of laser trabeculoplasty. The Fluorometholone-Laser Trabeculoplasty Study Group. Am J Ophthalmol. 1996;122:349–54.
Kim YY, Glover BK, Shin DH, Lee D, Frenkel RE, Abreu MM. Effect of topical anti-inflammatory treatment on the long-term outcome of laser trabeculoplasty. Fluorometholone-Laser Trabeculoplasty Study Group. Am J Ophthalmol. 1998;126:721–3.
Realini R, Carlton J, Hettlinger M. The impact of anti-inflammatory therapy on intraocular pressure reduction following selective laser trabeculoplasty. Ophthalmic Surg Lasers Imaging. 2010;41:100–3.
Mcllraith L, Strasfeld M, Colev G, Hutnik CM. Selective laser trabeculoplasty as initial and adjunctive treatment for open-angle glaucoma. J Glaucoma. 2006;15:124–30.
R Core Team. R: a language and environment for statistical computing. 2013. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 24 July 2013.
Ho D, Imai K, King G, Stuart E. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 2007;15:199–236. http://gking.harvard.edu/files/abs/matchp-abs.shtml. Accessed 24 July 2013.
Ho D, Imai K, King G, Stuart E. Matchit: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2007b. http://gking.harvard.edu/matchit/. Accessed 24 July 2013.
Imai K, King G, Lau O. Zelig: everyone’s statistical software. 2007. http://projects.iq.harvard.edu/zelig. Accessed 24 July 2013.
Imai K, King G, Lau O. Toward a common framework for statistical analysis and development. J Comput Graph Stat. 2008;17:892–913.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article.
The authors of this manuscript have received no grant support or research funding and have no proprietary interest in the materials described in this article.
Dr. Rebenitsch is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
The information in this manuscript was presented at the following meetings:
Poster presentation at ARVO. Fort Lauderdale, FL on May 5–10, 2012
Powerpoint presentation at the Table Rock Regional Roundup. Big Cedar Lodge. Ridgedale, MO on September 28, 2012
Conflict of interest
Dr. Rebenitsch, Dr. Brown, Dr. Binder, Dr. Jani, Aaron Bonham, Dr. Krishna, and Dr. Pikey declare no conflict of interest in this article.
Compliance with Ethics Guidelines
The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Rebenitsch, R.L., Brown, E.N., Binder, N.R. et al. Effect of Topical Loteprednol on Intraocular Pressure After Selective Laser Trabeculoplasty for Open-Angle Glaucoma. Ophthalmol Ther 2, 113–120 (2013). https://doi.org/10.1007/s40123-013-0018-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-013-0018-z