Abstract
Introduction
The aim of this study is to examine the analgesic efficacy of varying doses of hydromorphone hydrochloride in conjunction with absorbable gelatin sponge for postoperative pain management in elderly individuals undergoing lumbar fusion surgery. Additionally, the study aims to assess the sustained release analgesic properties of this combination and to determine the optimal dosage of hydromorphone hydrochloride for effective pain relief.
Methods
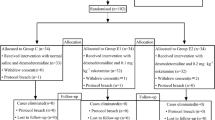
A total of 113 elderly patients (aged ≥ 65 years old) meeting the criteria for 1-2-level posterior lumbar fusion surgery at Ganzhou City People’s Hospital between July 2022 and August 2023 were randomly assigned to four groups: group A (0.2 mg hydromorphone hydrochloride 1 ml), group B (0.3 mg hydromorphone hydrochloride 1.5 ml), group C (0.4 mg hydromorphone hydrochloride 2 ml), and group D (0.9% normal saline 2 ml) for standard anesthesia induction and maintenance. Prior to suturing the incision, gelfoam was utilized to administer epidural analgesia to each group. Following the surgical procedure, an intravenous analgesia pump was utilized for pain management. The baseline infusion rate was set at 0.5 ml/h. Patient-controlled analgesia (PCA) was administered at a dose of 2 ml, with a lockout interval of 20 min, allowing the patient to self-administer as needed. Pain relief was assessed using the visual analogue scale (VAS) prior to surgery, as well as at 1 day and 3 days post-operation. The frequency of PCA requests within the initial 48-h postoperative period, the remedial analgesia with dezocine, postoperative adverse reactions, and duration of hospitalization were documented for analysis.
Results
The VAS scores of groups B and C were found to be significantly lower than those of group D 1 day after the operation. Additionally, VAS scores at 3 days post-operation, remedial rate of dezocine and PCA follow-up times at 48 h in groups A, B, and C were significantly lower compared to group D (P < 0.001). There was no statistically significant difference between group B and group C in VAS scores at 1 day and 3 days post-operation, as well as PCA follow-up times at 48 h post-operation (P < 0.001). Furthermore, the VAS scores of groups B and C were lower than those of group A at 1 day and 3 days post-operation (P < 0.05). The PCA frequency of group C was also lower than that of group A at 48 h post-operation (P < 0.05).
Conclusion
The combination of hydromorphone hydrochloride and absorbable gelatin sponge epidural analgesia has been shown to enhance postoperative pain management. A dosage of 0.4 mg of hydromorphone hydrochloride may be considered an appropriate analgesic dose, as it can provide effective pain relief without eliciting adverse reactions.
Trial Registration
ChiCTR.org.cn(ChiCTR2200064863). Registered on October 20, 2022.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There is a lack of research on the postoperative analgesic impact of varying concentrations of hydromorphone infiltrating gelatin sponge in elderly patients undergoing lumbar fusion surgery. This study aims to assess the analgesic effects of different concentrations of hydromorphone infiltrating gelatin sponge on postoperative pain management in elderly patients undergoing lumbar fusion surgery, and to determine the most effective concentration of hydromorphone. |
What was learned from this study? |
Hydromorphone hydrochloride infiltration into gelatin sponge epidural space can enhance postoperative pain, and a dose of 0.4 mg hydromorphone hydrochloride may be an appropriate analgesic dose. |
Introduction
With the global population aging, elderly patients (aged ≥ 65 years old) have become a significant demographic undergoing lumbar spine surgery. Following the procedure, patients typically experience intense pain lasting for at least 3 days. During this period, a substantial amount of analgesics is often required to manage the pain. However, the use of analgesics can lead to adverse effects such as respiratory depression, nausea, and vomiting, significantly impacting the postoperative recovery of patients [1, 2]. Furthermore, inadequate pain control can result in sleep disturbances, affect the mental well-being of patients, and even lead to postoperative restlessness and cognitive dysfunction [3,4,5]. Research indicates a positive correlation between postoperative delirium and pain [6], particularly among patients aged over 65 [7]. Therefore, pain management is crucial for the well-being of elderly patients undergoing lumbar spine surgery.
Various methods for postoperative pain relief in lumbar spine surgery are utilized both domestically and internationally. Among these, intravenous self-controlled analgesia is a convenient option that allows for tailored postoperative pain management based on individual patient requirements. It is a primary postoperative analgesic approach for lumbar spine surgery, although it comes with a relatively high cost. However, this method can lead to complications such as respiratory depression, nausea, and vomiting when used for extended periods of time [8]. On the other hand, a single injection of analgesic drugs may have limited efficacy and significant side effects. Local infiltration or nerve blocks can provide immediate pain relief but have a short duration of action, which may not be sufficient for postoperative pain management following lumbar fusion surgery for at least 3 days [9, 10]. Similarly, a single intrathecal injection of opioid drugs like morphine may not be able to sustain a 24-h analgesic effect [11]. Interestingly, several studies [12, 13] have demonstrated that postoperative continuous epidural analgesia offers effective pain relief, prolonged duration of action, and fewer adverse reactions. Nonetheless, prolonged epidural catheterization increases the risk of postoperative infection in the lumbar spine [14].
The choice of postoperative analgesia primarily involves selecting appropriate analgesic methods and drugs. In a recent study by Yang et al. [15], researchers explored the use of a sustained release hemostatic gelatin sponge during lumbar spine surgery to deliver ropivacaine, dexamethasone, and vitamin B12 for prolonged pain relief. The study found that this method provided significant and long-lasting pain relief with minimal adverse reactions, reducing the risk of infection associated with epidural catheters [14]. Furthermore, other studies [16,17,18] have highlighted the versatility of absorbable gelatin sponge in various surgical procedures, showing its ability to carry drugs, biodegrade, and sustainably release medications. This makes it an ideal carrier for epidural analgesia drugs post-lumbar spine surgery. Regarding the selection of sustained-release analgesics, research by Ummenhofer et al. [19] indicated that fentanyl and sufentanil have lower spinal cord exposure compared to morphine and hydromorphone, resulting in poorer analgesic effects in the spinal canal. Additionally, the slower onset and hydrophilic nature of morphine may lead to complications like urinary retention and skin itching [20]. Hydromorphone, which has a lipid solubility between morphine and fentanyl, has a fast onset of action and fewer adverse reactions. Multiple studies [21, 22] have shown that hydromorphone and morphine can be interchangeable. The recent research results of Meissner et al. [23] showed that, in subjects using 0.05 mg/kg hydromorphone or 0.2 mg/kg morphine, the hydromorphone group had a faster onset of postoperative pain relief, stronger pain relief under the same degree of respiratory inhibition than the morphine group, and shorter duration of respiratory inhibition. These results indicate that, for the treatment of acute pain, hydromorphone may be superior to morphine and may be more suitable for epidural sustained-release analgesia in elderly patients undergoing lumbar fusion surgery.
There is currently a lack of literature on the use of hydromorphone hydrochloride combined with absorbable gelatin sponge for epidural slow-release analgesia, as well as on the effects of varying doses of hydromorphone hydrochloride slow-release analgesia on postoperative pain relief following lumbar fusion surgery. As a result, we propose a hypothesis to investigate the postoperative analgesic impact of different doses of hydromorphone hydrochloride in combination with absorbable gelatin sponge for epidural slow-release analgesia in elderly patients undergoing lumbar fusion surgery, aiming to determine the optimal dosage of hydromorphone.
Methods
Clinical Data
This study is a prospective randomized controlled trial approved by the Medical Ethics Committee of the hospital (Ethics No.: TY-ZYK2022-018-01). Clinical trial registration (Registration No.: ChiCTR2200064863) was completed. The research plan was conducted in the operating room of Ganzhou People's Hospital from July 2022 to August 2023, in accordance with the Helsinki Declaration and its subsequent amendments. This study was approved by the Ethics Committee of the Medical Center of Ganzhou People's Hospital. All subjects obtained written informed consent before surgery. This study has been prospectively registered at the Chinese Clinical Trial Registry (ChiCTR2200064863). From July 2022 to August 2023, 120 eligible elderly patients (aged ≥ 65 years old) for 1-2-level posterior lumbar fusion at Ganzhou City People's Hospital were selected: age ≥ 65 years old, ASA grade I–II, male or female unlimited. Participants were randomly assigned in a 1:1:1:1 ratio to receive 0.2 mg hydromorphone hydrochloride 1 ml (group A = 30), 0.3 mg hydromorphone hydrochloride 1.5 ml (group B = 30), 0.4 mg hydromorphone hydrochloride 2 ml (group C = 30), or 0.9% saline 2 ml (group D = 30). The study number and group assignment were kept in sealed envelopes by a non-participating operating room nurse. The envelopes were opened before gelatin sponge placement, and the drugs were prepared and provided to the anesthesiologist in random order. Investigators, patients, and anesthesiologists were blinded to group assignment. Inclusion criteria were age ≥ 65 years old, scheduled 1-2-level posterior lumbar fusion, ASA class I~II, no history of alcohol abuse, normal communication, and normal liver and kidney function. Exclusion criteria included a history of spinal surgery, spinal tumor surgery, nonsteroidal drug or opioid allergy, long-term analgesic drug use, and dural tear. Exclusion and drop-out criteria were applied to cases not meeting the inclusion criteria or protocol requirements for 1-2-level posterior lumbar fusion. During the implementation process, patients who did not cooperate or voluntarily withdrew were considered drop-outs.
Article Preparation
The components required for the procedure included hydromorphone hydrochloride injection (2 ml: 2 mg), sodium chloride injection (10 ml: 0.09 g), 500 ml 0.9% sodium chloride injection, a 2.5-ml sterile syringe for single use, absorbent gelatin sponge (6 cm × 2 cm × 0.5 cm), an electronic infusion pump, sufentanil injection (1 ml: 50 ug), dexamethasone injection (1 ml: 2 mg), and sterile gloves.
Methods
Four groups of patients undergoing selective lumbar fusion surgery were subjected to preoperative fasting. Upon entering the operating room, standard ECG and SpO2 monitoring were conducted, along with VAS pain assessments. Peripheral venous access was established and radial artery puncture was performed under ultrasound guidance for arterial blood pressure monitoring and blood gas analysis prior to anesthesia. The maintenance concentration of sevoflurane during surgery was primarily based on information from the package insert and relevant literature [24, 25]. The Bispectral Index (BIS) was utilized to monitor the depth of anesthesia, ensuring that the inhalation concentration of sevoflurane remains within the optimal range of 0.5–1 MAC. Additionally, propofol was administered via plasma target-controlled infusion at 1.0–3.0 μg/ml, remifentanil at 0.1–0.2 μg/kg min, and intermittent intravenous cis-atracurium to sustain muscle relaxation. Sevoflurane was discontinued 30 min before the conclusion of surgery. The BIS was continuously monitored during the operation and its value maintained between 40 and 60. Anesthesia depth was adjusted based on the BIS value and vital signs. Ventilation parameters were set at VT 6–8 ml/kg, FiO2 60%, RR 10–20 breaths/min, and I/E ratio of 1:1.5. Respiratory parameters were adjusted to maintain PaCO2 levels between 35 and 45 mmHg. Fluid replacement was administered based on the individual patient's circulatory changes throughout the operation (Fig. 1).
All patients in the four groups received injections of the prepared liquid medicine evenly onto the gelatin sponge using a syringe to ensure full and even infiltration (Fig. 2). The drug preparation and gelatin sponge infiltration were carried out consistently by the same anesthetist to guarantee the effectiveness of the liquid medicine infiltration. Hydromorphone hydrochloride was diluted to a consistent concentration, with the injection (2 ml: 2 mg) being diluted to 5 ml, resulting in a concentration of 0.2 mg/ml. Specifically, 1 ml of hydromorphone hydrochloride diluent was drawn from group A, 1.5 ml from group B, 2 ml from group C, and 2 ml of normal saline from group D. The integrity of the dural sac of each patient was meticulously examined. Following confirmation of no dural tear, the gelatin sponge impregnated with hydromorphone hydrochloride was gently placed on the dural membrane, ensuring complete coverage when left in position (Fig. 3). Subsequently, all patients had drainage tubes placed in the epidural space of the corresponding surgical segment, suturing of the paravertebral muscle was performed, and the wound was sutured within 30 min. An intravenous analgesia pump was connected at the conclusion of surgery, all utilizing the same formula (sufentanil 2ug/kg + dexamethasone 0.2 mg/kg + 0.9% normal saline diluted to 100 ml). The background dose was set at 0.5 ml/h, with a PCA dose of 2 ml, a locking time of 20 min, and patients having the ability to self-administer single self-controlled analgesia based on their individual requirements. Post-surgery, the patient was transferred to the post-anesthesia care unit for anesthesia recovery and vital signs monitoring. Once the patient naturally regained consciousness and met extubation criteria, the endotracheal tube was removed, and the patient was returned to the general ward after continuous monitoring until achieving a modified Aldrete score of ≥ 9 points. After surgery, a visual analogue scale (VAS) of ≤ 3 points was maintained. If PCA intravenous analgesia was not effective,2 mg intramuscular injection of dezocine was used for remedial treatment, and additional pain treatment added every 3–6 h if necessary.
Observation Indexes
The study collected data on the general condition, operation time, bleeding volume, infusion volume, and urine volume of patients in four groups.
The primary outcome measures consisted of VAS scores assessed before surgery (T1), 1 day post-surgery (T2), and 3 days post-surgery (T3). VAS scores at each time point were categorized as per the literature [26,27,28] into mild (1–3 points), moderate (4–6 points), and severe (7–10 points) levels for comparison.
Secondary outcome indicators encompassed mean arterial pressure (MAP) and heart rate at different time points, adverse reactions like PONV, lethargy, POCD, respiratory depression, skin itching, Pittsburgh sleep quality index (PSQI) within 1 week post-surgery [29], the situation of using dezocine injection for pain relief 3 days after surgery, and total PCA times and patient satisfaction within 48 h after surgery in the four groups.
Sample Size and Statistical Analysis
We conducted preliminary experiments and found that the data in each group basically followed a normal distribution. Therefore, we used mean ± standard deviation to estimate the sample size using PASS 15.0. The mean ± standard deviation of groups A, B, C, and D from preliminary experiments were 2.33 ± 0.51, 2.17 ± 0.41, 2.00 ± 0.63, and 2.83 ± 0.41, respectively. Based on a type I error of 0.05 and a power of 0.90, the required sample size for the study was at least 100 participants. Initially, 128 patients were enrolled in the study, considering potential eliminations due to changes in clinical status. Categorical data are presented as numbers or percentages, while normally distributed quantitative data are expressed as means and standard deviations. Parametric tests were utilized for normally distributed data, while nonparametric tests were used for data without a normal distribution. The analysis of categorical data involved the Chi-square test and Fisher's exact test. A significance level of P < 0.05 was applied. Statistical analysis was conducted using SPSS 23.0.
Results
A total of 128 patients underwent posterior lumbar fusion surgery at Ganzhou People’s Hospital from July 2022 to August 2023. Among them, 120 people met the prescribed standards and were subsequently divided into 4 groups, each with 30 participants. However, 7 patients were excluded from the study as 4 patients experienced significant intraoperative bleeding and 3 patients changed their surgical approach. Therefore, this analysis included a total of 113 patients, among whom were 29 cases in group A (0.2 mg hydromorphone), 27 cases in group B (0.3 mg hydromorphone), 29 cases in group C (0.4 mg hydromorphone), and 28 cases in group D (2 ml of normal saline).
Comparison of General Data of the Four Groups of Patients Undergoing Lumbar Fusion Surgery
There were no statistically significant variances in baseline characteristics, including height, weight, and age, across the four groups (P > 0.05). Similarly, there were no statistically significant variations in intraoperative parameters, such as blood loss, urine output, autologous blood recovery, infusion volume, surgical duration and length of hospital stay, among the four groups (P > 0.05) (Table 1).
Comparison of VAS Scores at Different Time Points in the Four Groups of Patients Undergoing Lumbar Fusion Surgery
Analysis of VAS scores at various time points among four distinct patient groups based on pain severity revealed that all the data fell within the range of mild to moderate pain. Prior to surgery, there were no significant differences in VAS scores among the four patient groups (P > 0.05). However, following surgery, patients in groups B and C exhibited lower VAS scores on the 1st and 3rd postoperative days compared to those in group D (P < 0.05). Similarly, patients in group A had lower VAS scores on the 3rd postoperative day compared to group D (P < 0.05). Notably, there were no discernible variations in VAS scores between groups B and C on the first and third postoperative days (P > 0.05); however, both were elevated compared to the VAS scores of patients in group A (P < 0.05) (Table 2).
Comparison of PCA Times and PSQI Scores After Lumbar Fusion Surgery in the Four Groups
Compared with group D, PCA frequency of groups A, B, and C within 48 h after operation were significantly less (P < 0.05), PCA frequency of groups B and C within 48 h after operation showed no significant difference (P > 0.05), PCA frequency of group C within 48 h after operation were less than that of group A (P < 0.05). Compared with group D, the PSQI scores of groups B and C were lower within 1 week after operation (P < 0.05). There was no significant difference between group B and group C within 1 week after operation (P > 0.05). The PSQI scores of group C were lower than that of group A within 1 week after operation (P < 0.05) (Table 3; Fig. 4).
Comparison of MAP and Heart Rate at Different Time Points in the Four Groups of Patients
There was no statistically significant difference in preoperative mean arterial pressure and heart rate among the four groups of patients (P > 0.05).
Compared to the average arterial pressure of group D patients on the 1st and 3rd days after surgery, the average arterial pressure of groups A, B, and C was lower (P < 0.05) (Table 4; Fig. 5).
Compared to the heart rate of group D patients on the 1st and 3rd day after surgery, the average arterial pressure of groups A, B, and C was lower (P < 0.05) (Table 5 and Fig. 6).
Comparison of Incidence of Postoperative Adverse Reactions
There was no statistically significant difference in postoperative nausea and vomiting, pruritus, drowsiness, respiratory depression and POCD among the four groups of patients (P > 0.05). The incidence of drowsiness in groups B and C was higher than that in group D (P < 0.05), and there was no statistically significant difference in itching among groups A, B, and C (P > 0.05) (Table 6).
Comparison of Patient Satisfaction
Compared with group D, group B and group C had higher analgesic satisfaction (P < 0.05), and group C had higher analgesic satisfaction than group A (P < 0.05), but there was no significant difference between group B and group C (P > 0.05) (Table 7).
Comparison of Demand for Dezocine Analgesic Relief in the First 3 Days After Surgery
Compared with group D, the remedial analgesia with dezocine of group A, B, and C within 3 days after operation were significantly less (P < 0.05), but the remedial analgesia with dezocine of group B and C within 3 days after operation had no significant difference (P > 0.05), and the remedial analgesia with dezocine of group C within 3 days after operation were less than that of group A (P < 0.05) (Table 8).
Discussion
This study aimed to investigate the analgesic effect of hydromorphone hydrochloride combined with absorbable gelatin sponge following 1-2-level posterior lumbar fusion in elderly patients, as well as to determine the optimal dose of hydromorphone hydrochloride. The results indicated that the hydromorphone hydrochloride groups (A, B, C) exhibited a significant postoperative analgesic effect compared to the normal saline control group (D) (P < 0.05). Notably, group C exhibited a lower PSQI score within 1 week and PCA frequency within 48 h compared to group A (P < 0.05). These findings suggest that the combination of hydromorphone hydrochloride and absorbable gelatin sponge provides effective analgesia in elderly patients following 1-2-level posterior lumbar fusion, with 0.4 mg being identified as a suitable dose. Additionally, postoperative adverse reactions did not significantly increase with higher doses, and group C exhibited the lowest PSQI score, the rescue rate of dezocine and PCA frequency within 48 h post-operation.
Compared with group D, the mean arterial pressure and heart rate of patients in the hydromorphone group (groups A, B, C) were significantly lower at 1 day and 3 days after the operation (P < 0.05). The postoperative circulation of patients in the hydromorphone group was more stable than that of patients in group D, which is particularly beneficial for elderly patients undergoing extensive surgery. Through follow-up observation of adverse reactions during postoperative analgesia in the four groups, it was noted that the hydromorphone group did not significantly increase the incidence of nausea, vomiting, or respiratory depression (P > 0.05). However, groups B and C showed an increased incidence of skin itching (P < 0.05), consistent with previous studies [30], suggesting that hydromorphone may lead to skin itching. On the other hand, groups A, B, and C did not exhibit significant differences (P > 0.05). Additionally, the Pittsburgh Sleep Quality Index of patients in all four groups within 1 week after the operation indicated that the sleep quality of patients in the hydromorphone group (groups A, B, C) was superior to that of patients in group D. Moreover, with the increase in hydromorphone dose, group C exhibited a better sleep state than patients in group A without an increase in complications (P < 0.05). Quality sleep post-operation is conducive to the rapid recovery of patients and is a crucial factor in promoting the overall recovery quality of patients during the perioperative period [31].
The PCA times within 48 h after surgery were compared among four groups of patients. It was observed that patients in the morphinone group (groups A, B, C) had lower PCA times compared to group D patients. Additionally, group C showed fewer PCA times than group A patients with an increase in hydromorphone dose, without an increase in complications (P < 0.05). In terms of postoperative satisfaction, both group B and group C exhibited improved analgesic satisfaction compared to group D (P < 0.05). Furthermore, with an increase in hydromorphone dose, the analgesic satisfaction of group C was higher than that of group A (P < 0.05). This suggests that 0.4 mg of hydromorphone hydrochloride could significantly enhance the postoperative analgesic experience of patients and greatly improve patient satisfaction and reduce postoperative use of opioid drugs [32]. These findings confirm the effectiveness of hydromorphone epidural sustained release analgesia and support the use of 0.4 mg of hydromorphone hydrochloride as an appropriate dose, which does not significantly increase adverse reactions while ensuring analgesic effect. Although the dose of hydromorphone hydrochloride for epidural analgesia differs slightly from that of other studies [33, 34], the clinical significance of this study remains important, as it focuses on single epidural analgesic drugs without additional drug storage mediums like gelatin sponge.
Hydromorphone hydrochloride, as a derivative of morphine, has been proved to be safe and effective for postoperative analgesia by many studies at home and abroad, and provides better analgesic effect than morphine. It has been widely used in acute and chronic pain and postoperative analgesia [35, 36]. However, there have been no studies involving hydromorphone hydrochloride combined with absorbable gelatin sponge for postoperative analgesia in elderly patients undergoing 1-2-level posterior lumbar fusion, and the analgesic effect of gelatin sponge combined with some analgesic drugs has been confirmed in several studies [16, 37], with Puhto et al. [34] also actively looking for the optimal dose of hydromorphone for epidural analgesia. Therefore, this study attempts to explore the effect of different doses of hydromorphone on epidural analgesia after lumbar fusion surgery in the elderly, and to actively explore safer and more appropriate doses of hydromorphone hydrochloride for epidural analgesia. It provides a scientific basis for clinical rational drug use while ensuring good analgesic effect and long duration, reducing opioid use and less adverse reactions, reducing perioperative complications, shortening hospitalization time, and saving hospitalization costs. It helps patients recover quickly [38].
Gelatin sponge is an artificially synthesized, non-toxic, non-antigenic protein gel polymer material that exhibits excellent compressibility, water-swelling ability, and high biocompatibility. The porous structure of gelatin sponge significantly enhances its absorption capacity, enabling it to efficiently load drugs and undergo complete tissue absorption within 4–6 weeks [39]. Furthermore, studies [40] have found that the cell-interfacing surface of gelatin sponge promotes cell aggregation and proliferation, making it effective in hemostasis, tissue repair, and adhesion prevention, while providing sustained pain relief. This makes it particularly suitable for epidural retention following lumbar surgery. Therefore, in elderly patients undergoing lumbar fusion surgery, a sustained-release system comprising hydromorphone combined with an absorbable gelatin sponge can be utilized to cover the exposed dura mater, ensuring a sustained analgesic effect.
This study has identified some limitations. Firstly, the gelatin sponge delivery system used in the experiment may introduce uncertainty in drug loss, influenced by factors such as surgical trauma size and drainage volume. Future studies should delve deeper into understanding the loss ratio of the gelatin sponge delivery system. Secondly, there exists variability in the evaluation of VAS scores among patients, as individual pain thresholds for 1-2-level posterior lumbar fusion in elderly patients can differ, impacting the pain scores. Building upon the findings of this experiment, further investigations should explore the efficacy of higher doses of hydromorphone, potentially uncovering more suitable dosage levels. Our research has primarily focused on postoperative pain management, and we have not investigated the use of epidural anesthesia post-surgery. Furthermore, due to concerns regarding infection or drug leakage risks linked to postoperative epidural anesthesia, we have not delved into this area extensively.
Conclusion
Hydromorphone hydrochloride epidural analgesia can significantly improve postoperative pain and patient satisfaction, reducing the total dosage of opiates. Also, the results of administering 0.4 mg epidural gelatin sponge as sustained release analgesia showed that it is an appropriate dose providing good analgesia, while the incidence of adverse reactions did not significantly increase, so it can be used as a reference dose for clinical application.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Deyo RA, Hallvik SE, Hildebran C, Marino M, O’Kane N, Carson J, Van Otterloo J, Wright DA, Millet LM, Wakeland W. Use of prescription opioids before and after an operation for chronic pain (lumbar fusion surgery). Pain. 2018;159(6):1147–54.
Shahi P, Vaishnav AS, Melissaridou D, Sivaganesan A, Sarmiento JM, Urakawa H, Araghi K, Shinn DJ, Song J, Dalal SS, Iyer S, Sheha ED, Dowdell JE, Qureshi SA. Factors causing delay in discharge in patients eligible for ambulatory lumbar fusion surgery. Spine. 2022;47(16):1137–44.
Nadelson MR, Sanders RD, Avidan MS. Perioperative cognitive trajectory in adults. Br J Anaesth. 2014;112(3):440–51.
Skolasky RL, Riley LH 3rd, Maggard AM, Wegener ST. The relationship between pain and depressive symptoms after lumbar spine surgery. Pain. 2012;153(10):2092–6.
de Heer EW, Ten Have M, van Marwijk HWJ, Dekker J, de Graaf R, Beekman ATF, van der Feltz-Cornelis CM. Pain as a risk factor for common mental disorders. Results from the Netherlands Mental Health Survey and Incidence Study-2: a longitudinal, population-based study. Pain. 2018;159(4):712–8.
Gold C, Ray E, Christianson D, Park B, Kournoutas IA, Kahn TA, Perez EA, Berger JI, Sander K, Igram CA, Pugely A, Olinger CR, Carnahan R, Chen PF, Mueller R, Hitchon P, Howard MA, Banks M, Sanders RD, Woodroffe RW. Risk factors for delirium in elderly patients after lumbar spinal fusion. Clin Neurol Neurosurg. 2022;219: 107318.
Shi C, Yang C, Gao R, Yuan W. Risk factors for delirium after spinal surgery: a meta-analysis. World Neurosurg. 2015;84(5):1466–72.
Choi SW, Cho HK, Park S, Yoo JH, Lee JC, Baek MJ, Jang HD, Cha JS, Shin BJ. Multimodal analgesia (MMA) versus patient-controlled analgesia (PCA) for one or two-level posterior lumbar fusion surgery. J Clin Med. 2020;9(4):1087.
Li K, Ji C, Luo D, Feng H, Yang K, Xu H. Wound infiltration with ropivacaine as an adjuvant to patient controlled analgesia for transforaminal lumbar interbody fusion: a retrospective study. BMC Anesthesiol. 2020;20(1):288.
Zhang Q, Wu Y, Ren F, Zhang X, Feng Y. Bilateral ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal fusion: a randomized controlled trial. J Clin Anesth. 2021;68: 110090.
Dhaliwal P, Yavin D, Whittaker T, Hawboldt GS, Jewett GAE, Casha S, du Plessis S. Intrathecal morphine following lumbar fusion: a randomized. Placebo-Controll Trial Neurosurg. 2019;85(2):189–98.
Cohen M, Zuk J, McKay N, Erickson M, Pan Z, Galinkin J. Intrathecal morphine versus extended-release epidural morphine for postoperative pain control in pediatric patients undergoing posterior spinal fusion. Anesth Analg. 2017;124(6):2030–7.
Guilfoyle MR, Mannion RJ, Mitchell P, Thomson S. Epidural fentanyl for postoperative analgesia after lumbar canal decompression: a randomized controlled trial. Spine J. 2012;12(8):646–51.
van Samkar G, Balraadjsing PPS, Hermanns H, Hoogendijk IV, Hollmann MW, Zaat SAJ, Stevens MF. Microbiological and scanning electron microscopic evaluation of epidural catheters. Reg Anesth Pain Med. 2020;45(5):381–5.
Yang JS, Liu KX, Chu L, Chan YK, Fan H, Li XM, Liu P, Liu TJ, Hao DJ. Cocktail treatment with a gelatin sponge impregnated with ropivacaine, dexamethasone, and vitamin B12 promotes early postoperative recovery after percutaneous endoscopic lumbar discectomy: a retrospective. Case-Controll Study Pain Phys. 2020;23(2):E211–8.
Ghabach MB, Mhanna NE, Al Ezz MRA, Mezher GN, Chammas MJ, Ghabach MM. Comparison of effects of hemostatic gelatin sponge impregnated with ropivacaine versus normal saline applied on the transverse process of the operated vertebrae on postoperative pain in patients undergoing spinal instrumentation surgery: a randomized clinical trial. World Neurosurg. 2019;128:1126–30.
Sun W, Chen Y, Yuan W. Hemostatic absorbable gelatin sponge loaded with 5-fluorouracil for treatment of tumors. Int J Nanomed. 2013;8:1499–506.
Shukla A, Fang JC, Puranam S, Hammond PT. Release of vancomycin from multilayer coated absorbent gelatin sponges. J Control Release. 2012;157(1):64–71.
Ummenhofer WC, Arends RH, Shen DD, Bernards CM. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanil. Anesthesiology. 2000;92(3):739–53.
Jeon Y, Hwang J, Kang J, Han S, Rhee K, Oh Y. Effects of epidural naloxone on pruritus induced by epidural morphine: a randomized controlled trial. Int J Obstet Anesth. 2005;14(1):22–5.
Rodrigues S, Shin D, Conway M, Smulski S, Trenker E, Shanthanna H, Vanniyasingam T, Thabane L, Paul J. Hydromorphone versus morphine: a historical cohort study to evaluate the quality of postoperative analgesia. Can J Anaesth. 2021;68(2):226–34.
Shanthanna H, Paul J, Lovrics P, Vanniyasingam T, Devereaux PJ, Bhandari M, Thabane L. Satisfactory analgesia with minimal emesis in day surgeries: a randomised controlled trial of morphine versus hydromorphone. Br J Anaesth. 2019;122(6):e107–13.
Meissner K, Dahan A, Olofsen E, Göpfert C, Blood J, Wieditz J, Kharasch ED. Morphine and hydromorphone effects, side effects, and variability: a crossover study in human volunteers. Anesthesiology. 2023;139(1):16–34.
Huai X, Jiao Y, Gu X, Zhu H, Chen L, Fan Y, Yu W, Su D, Xie H. Preoperative chronic pain as a risk factor for early postoperative cognitive dysfunction in elderly patients undergoing hip joint replacement surgery: a prospective observational cohort study. Front Neurosci. 2021;17(15): 747362.
Xu C, Gu F, Liu Y, Chen R, Wang C, Lu J. The median effective analgesic concentration of ropivacaine in ultrasound-guided interscalene brachial plexus block after arthroscopic rotator cuff repair. Front Pharmacol. 2022;17(13): 928227.
He XM, He MC, Yang P, Zhang QW, Chen ZQ, He W, Wei QS. The therapeutic effect of Huo Xue Tong Luo capsules in Association Research Circulation Osseous (ARCO) stage II osteonecrosis of the femoral head: a clinical study with an average follow-up period of 7.95 years. Front Pharmacol. 2021;12:773758.
Lee M, Chu MK, Lee J, Yoo J, Song HK. Field testing primary stabbing headache criteria according to the 3rd beta edition of International Classification of Headache Disorders: a clinic-based study. J Headache Pain. 2016;17:21.
Boström A, Scheele D, Stoffel-Wagner B, Hönig F, Chaudhry SR, Muhammad S, Hurlemann R, Krauss JK, Lendvai IS, Chakravarthy KV, Kinfe TM. Saliva molecular inflammatory profiling in female migraine patients responsive to adjunctive cervical non-invasive vagus nerve stimulation: the MOXY Study. J Transl Med. 2019;17(1):53.
Niu JY, Yang N, Tao QY, He Y, Hou YB, Ning RD, Yu JM. Effect of different administration routes of dexmedetomidine on postoperative delirium in elderly patients undergoing elective spinal surgery: a prospective randomized double-blinded controlled trial. Anesth Analg. 2023;136(6):1075–83.
Chaplan SR, Duncan SR, Brodsky JB, Brose WG. Morphine and hydromorphone epidural analgesia. A prospective, randomized comparison. Anesthesiology. 1992;77(6):1090–4.
Hillman DR, Carlucci M, Charchaflieh JG, Cloward TV, Gali B, Gay PC, Lyons MM, McNeill MM, Singh M, Yilmaz M, Auckley DH. Society of anesthesia and sleep medicine position paper on patient sleep during hospitalization. Anesth Analg. 2023;136(4):814–24.
Sviggum HP, Arendt KW, Jacob AK, Niesen AD, Johnson RL, Schroeder DR, Tien M, Mantilla CB. Intrathecal hydromorphone and morphine for postcesarean delivery analgesia: determination of the ED90 using a sequential allocation biased-coin method. Anesth Analg. 2016;123(3):690–7.
Yang M, Wang L, Chen H, Tang Y, Chen X. Postoperative analgesic effects of different doses of epidural hydromorphone coadministered with ropivacaine after cesarean section: a randomized controlled trial. Pain Res Manag. 2019;3(2019):9054538.
Puhto T, Kokki M, Hakomäki H, Spalding M, Gunnar T, Alahuhta S, Vakkala M. Single dose epidural hydromorphone in labour pain: maternal pharmacokinetics and neonatal exposure. Eur J Clin Pharmacol. 2020;76(7):969–77.
Ershoff B. Intraoperative hydromorphone decreases postoperative pain: an instrumental variable analysis. Br J Anaesth. 2023;131(1):104–12.
Farid IS, Lewis JM, Kendrick EJ. The safety and efficacy of hydromorphone via patient controlled analgesia or patient controlled analgesia by proxy for pediatric postoperative pain control. J Clin Anesth. 2020;60:65–6.
Shilo-Benjamini Y, Slav SA, Kahane N, Kushnir Y, Sarfaty H, Ofri R. Analgesic effects of intraorbital insertion of an absorbable gelatin hemostatic sponge soaked with 1% ropivacaine solution following enucleation in dogs. J Am Vet Med Assoc. 2019;255(11):1255–62.
Braxton E Jr, Wohlfeld BJ, Blumenthal S, Bozzio A, Buttermann G, Guyer R, Idema J, Laich D, Morreale J, Nikolakis M, Patel A, Price JS, Witt JP, Zigler J, Martin M. Postoperative care pathways following lumbar total disc replacement: results of a modified delphi approach. Spine (Phila Pa 1976). 2019;44(24):S1–12.
Hany A, Mansour A, Sediek M, Nabil M. Role of tranexamic acid-soaked gelatin sponge in minimizing rectus sheath hematoma after cesarean section in women treated with warfarin, a simple tool for high-risk cases, a randomized controlled trial. Eur J Med Res. 2023;28(1):448.
Gonzalez-Lopez P, Harput MV, Türe H, Atalay B, Türe U. Efficacy of placing a thin layer of gelatin sponge over the subdural space during dural closure in preventing meningo-cerebral adhesion. World Neurosurg. 2015;83(1):93–101.
Acknowledgements
The authors would like to thank all the reviewers who participated in the review.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Xianwei Jin and Ruiming Deng prepared the manuscript and implemented postoperative pain evaluation. Weibo Zhong designed, interpreted the data and finally approved the version to be published. Qiaoling Weng and Qiao Yang was responsible for the implementation of anesthesia for some participants and participated in postoperative follow-up and data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare no conflicts of interest, including Xian wei Jin, Ruiming Deng, Qiaoling Weng, Qiao Yang and Weibo Zhong.
Ethical Approval
The research plan was conducted in the operating room of Ganzhou People's Hospital from July 2022 to August 2023, in accordance with the Helsinki Declaration and its subsequent amendments. This study was approved by the Ethics Committee of the Medical Center of Ganzhou People's Hospital. All subjects obtained written informed consent before surgery. This study has been prospectively registered at the Chinese Clinical Trial Registry (ChiCTR2200064863).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jin, X., Deng, R., Weng, Q. et al. Clinical Application of Different Doses of Hydromorphone Slow-Release Analgesia in Lumbar Fusion in Elderly Patients. Pain Ther 13, 1219–1233 (2024). https://doi.org/10.1007/s40122-024-00632-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-024-00632-3