Abstract
Introduction
Up to 50% of diabetic patients with neuropathy suffer from chronic pain, namely painful diabetic neuropathy (PDN), an unmet medical need with significant impact on quality of life. Gabapentin is widely used for PDN, albeit with frequent dose-limiting effects. Trazodone, an antidepressant with multi-modal action, has shown promising results when given at low doses as an add-on to gabapentin. Upon previous clinical trials and experimental evidence, a fixed-dose combination (FDC) of both compounds, at low doses, was developed for neuropathic pain.
Methods
This was a phase II, randomized, double-blind, placebo and reference controlled, dose-finding, multicenter, international, prospective study. Male and female diabetic patients aged 18–75 years and affected by PDN were eligible for enrolment. Patients were randomized (1:1:1:1:2 ratio) to trazodone and gabapentin (Trazo/Gaba) 2.5/25 mg t.i.d. for 8 weeks, Trazo/Gaba 5/50 mg t.i.d. for 8 weeks, Trazo/Gaba 10/100 mg t.i.d. for 8 weeks, gabapentin (Gaba), or placebo (PLB). The aim of the study was to collect preliminary information on the effect of the 3 different FDCs of Trazo/Gaba on pain intensity based on the 11-point numeric rating score (NRS) after 8 weeks of treatment. The secondary objectives were the evaluation of the percentage of responders, neuropathic pain symptoms, anxiety, sleep, quality of life, safety, and tolerability. The primary efficacy endpoint was evaluated with last observation carried out forward (LOCF), using an analysis of covariance (ANCOVA), including treatment and centers as factors and baseline as covariate and applying linear contrast test, excluding the active treatment. Only if the linear contrast test was significant (p < 0.05), the step-down Dunnett test would be used to determine the minimum effective dose significantly different from PLB. If linearity was not verified, an adjusted ANCOVA model and comparisons with Dunnett test were performed. Before the application of the ANCOVA model, the non-significance of interaction treatment per baseline was verified.
Results
A total of 240 patients were included in the modified intention-to-treat (m-ITT) population: 39 in Trazo/Gaba 2.5/25 mg, 38 in Trazo/Gaba 5/50 mg, 37 in Trazo/Gaba 10/100 mg, 83 in PLB, and 43 in Gaba. After 8 weeks of treatment, changes of the average daily pain score based on the 11-point NRS from baseline were − 2.52 ± 2.31 in Trazo/Gaba 2.5/25 mg group, − 2.24 ± 1.96 in Trazo/Gaba 5/50 mg group, − 2.46 ± 2.12 in Trazo/Gaba 10/100 mg group, − 1.92 ± 2.21 in Gaba group, and − 2.02 ± 1.95 in the PLB group. The linear contrast test did not result in significant differences (p > 0.05) among treatment groups. Consequently, the minimum effective dose against PLB was not determined. The multiple comparison with Dunnett adjustment did not show any statistically significant differences vs. PLB after 8 weeks of treatment: Trazo/Gaba 2.5/25 mg (95% confidence interval (CI) − 1.2739, 0.2026; p = 0.1539); Trazo/Gaba 5/50 mg (95% CI − 0.9401, 0.5390; p = 0.5931); Trazo/Gaba 10/100 mg (95% CI − 1.0342, 0.4582; p = 0.4471). However, patients receiving the lowest dose of Trazo/Gaba 2.5/25 mg showed a statistically significant difference to PLB after 6 weeks of treatment (95% CI − 1.6648, − 0.2126; p = 0.0116).
Positive results were also found for responder patients, other items related to the pain, anxiety, depression, sleep, and quality of life, consistently in favor to the lowest Trazo/Gaba FDC.
Two serious adverse events (SAEs) occurred but were judged unrelated to the study treatment. Treatment-emergent adverse events (TEAEs) were mainly mild-to-moderate in intensity and involved primarily nervous system, gastrointestinal disorders, and investigations.
Conclusions
The primary end point of the study was the change from baseline of the average daily pain score based on the 11-point NRS after 8 weeks of treatment. While the primary endpoint was not reached, patients treated with Trazo/Gaba 2.5/25 mg t.i.d. showed statistically significant improvement of pain and other scores after 6 weeks and reported consistent better results in comparison to PLB on primary and secondary endpoints for the overall study duration. According to these results, the lowest dose of Trazo/Gaba FDC may be the best candidate for further clinical development to confirm the potential benefits of the FDC drug for this condition.
Clinical Trial Registration
NCT03749642.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The treatment of painful diabetic neuropathy (PDN) still remains an important unmet medical need. |
Low doses of trazodone added to full gabapentin dose recently demonstrated improvement in pain and sleep outcomes while being well tolerated in patients with PDN. |
This is a phase II, randomized, placebo-controlled, dose-finding study which aimed to assess the efficacy and safety of three different dosages of Trazo/Gaba fixed-dose combinations (FDCs) in PDN. |
In this phase II randomized controlled trial, the lowest tested dose of Trazo/Gaba FDC was able to improve pain and quality of life compared to placebo (PLB) in patients with PDN. |
Introduction
Neuropathic pain (NP) is one of the most frequent and persistent forms of chronic pain worldwide [1, 2]. Epidemiological studies across specialty and primary care settings indicate that up to 10% of the global population suffer from pain of neuropathic features [1, 3]. The International Association for the Study of Pain (IASP) defines NP as a subjective pain experience reflecting a somatosensory nervous system lesion or disease [4].
Pain associated with diabetic neuropathy represents one of the most common forms of chronic NP, being the underlying disease of high prevalence and incidence; current figures report around 415 million diabetic patients worldwide and point out an alarming epidemic growth [5].
It is estimated that 30–50% of diabetic patients develop a high-morbidity peripheral polyneuropathy, mostly of the mixed large- and small-fiber type [6]. Up to 50% of these individuals suffer from NP, namely painful diabetic neuropathy (PDN), mostly characterized by spontaneous pain that may be associated with an allodynic component and fluctuating unpleasant sensory discomfort [5, 7]. PDN (2024 ICD-10-CM Diagnosis Code E11.40) significantly contributes to the dramatic impact of diabetes, and related multi-organ complications, on patients’ phyco-physical health. Because of its high prevalence and detrimental effect on quality of life, PDN can be conceived as a disease in itself, which calls for dedicated management in routine medical practice [5, 6].
Systematic reviews and meta-analyses indicate that over 50% of PDN patients report an inadequate response to currently approved drugs (e.g., duloxetine, gabapentinoids, amitriptyline). In addition, poor tolerability and dose-limiting side effects are frequently reported [5, 6]. Therefore, PDN represents a major medical unmet need that deserves consistent clinical research to inform current management approaches, develop better therapeutic solutions, and ultimately raise the quality of standards of care [5, 6].
Owing to previous clinical reports about trazodone’s antinociceptive effects in PDN [8, 9], considering the widespread utilization of gabapentin for PDN [10], and given the lack of known negative drug–drug interactions between the two compounds, a new fixed-dose combination (FDC) with trazodone and gabapentin (Trazo/Gaba) was developed by Angelini Pharma S.p.A.
Trazodone is an antidepressant with a multimodal, multifunctional, and dose-dependent pharmacological activity; it primarily blocks the post-synaptic serotonin (5-HT) receptors 5-HT2A and 5-HT2C and inhibits the presynaptic 5-HT reuptake transporter [11]. Although gabapentinoids are widely used for pain, the exact mode of action is not fully elucidated. Direct inhibition of voltage-gated calcium channels by binding to its a2d-1 subunit with downstream reduction of presynaptic glutamate release is considered the critical mechanism [10].
A first phase II randomized, double-blind, placebo-controlled, multi-center, international, prospective trial was run to investigate whether low doses of trazodone administered as add-on therapy to gabapentin are safe and effective in PDN [12]. Albeit not statistically significant, 8-week treatment with trazodone 10 mg t.i.d. added on to gabapentin showed better efficacy outcomes when compared with a higher dosage or gabapentin alone.
Upon the hypothesis of shared neural substrates between trazodone and gabapentin for the antinociceptive effect [12,13,14,15,16], preclinical studies investigating synergy of a FDC of the two compounds were carried out [16]. A writhing test coupled with isobolographic analysis supports the existence of a synergistic effect that has also been characterized behaviorally in the chronic constriction injury rodent model [16]. In particular, the study showed that combined administration of trazodone and gabapentin is associated with significant antinociceptive activity at subtherapeutic doses of 0.1–3 and 1–100 mg/kg, respectively.
Consequently, a phase I trial was performed to assess dose proportionality, drug–drug interactions and safety of different Trazo/Gaba FDCs in healthy volunteers [17]. The proportionality for trazodone at doses of 2.5–30 mg and for gabapentin at doses of 25–300 mg was investigated for the first time. Absence of a pharmacokinetic interaction was shown as well as the good safety and tolerability profile of the drug.
In the present article, we report the results of a phase II, randomized, placebo-controlled, dose-finding study which aimed to assess the efficacy and safety of three different dosages of Trazo/Gaba FDCs in PDN.
Methods
Study Design
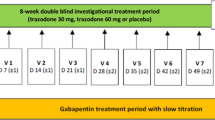
This was a randomized, double-blind, placebo and reference controlled, double-dummy, dose-finding, parallel-group, multicenter, international, prospective study that was performed in 30 investigational sites in four countries (Czech Republic, France, Poland, and the United Kingdom). The EudraCT trial number was 2018-000133-12, while the Clinical Trial registration is NCT03749642. The study was approved by the Competent Authorities and applicable Ethic Committees of Czech Republic (State Institute for Drug Control, Thomayerova nemocnice a IKEM, Ethics Committee Nemocnice Pardubického kraje a.s., Axon Clinical, Etická komise CLINTRIAL s.r.o.), France (ANSM Agence Nationale de Sécurité du Médicament et des produits de santé and Comité de Protection des Personnes (CPP) désigné par le système CNRIPH), Poland (Departament Badań Klinicznych Produktów Leczniczych Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych and Komisja Bioetyczna przy Śląskiej Izbie Lekarskiej w Katowicach), and United Kingdom (Medicines and Healthcare products Regulatory Agency and East of England—Cambridgeshire and Hertfordshire Research Ethics Committee) and was conducted in accordance with Good Clinical Practice guideline and ethical standards as laid down in the 1964 Declaration of Helsinki. Written informed consent (including personal data processing) was obtained from all individual participants included in the study prior starting any study-related procedure. The study consisted of three main study periods: the screening and wash-out, the 8-week double-blind investigational treatment period, and the 8-day tapering period, for a maximum of ten visits (Fig. 1).
Participants
The patients were eligible to enter the study if the following main inclusion criteria were met: male or female patient between 18 and 75 years of age (limits included); neuropathic pain at feet/legs confirmed by DN4 score ≥ 4 at screening visit; bilateral distal symmetrical polyneuropathy confirmed by Toronto Clinical Neuropathy Scoring System (TCNSS) score > 5 at screening visit; pain persisting or taking pain medication for neuropathic pain for at least 3 months; diabetic patient (type 1 or 2 diabetes mellitus) with value of HbA1c ≤ 11% at screening visit and stable antidiabetic medication regimen for ≥ 30 days; patient who was currently not receiving treatment for diabetic neuropathic pain or patient who was receiving treatment, with drug/s other than gabapentin, and had completed the required washout; average daily pain score ≥ 4 based on the 11-point NRS at visit 0, calculated from a minimum of four pain ratings in daily electronic device entries during the baseline period; women of childbearing potential with a negative pregnancy test at screening visit; patients legally capable to give their consent to participate in the study and available to sign and date the written informed consent (including personal data processing). Main exclusion criteria included: known hypersensitivity to trazodone or gabapentin or any excipients of the test drugs, other form of non-diabetic distal symmetric polyneuropathy or any other pain condition that could impair the study endpoints; concomitant treatment with other medications for pain management; use of trazodone or gabapentin in the previous 3 months; active foot ulcer or previous major limb amputation; glomerular filtration rate (GFR) value < 50 ml/min calculated with Modification of Diet in Renal Disease (MDRD) formula; significant mental disorders.
At each clinical site, investigators, who were mainly neurologists with experience in managing patients suffering from PDN, were responsible for assessing the eligibility of the patients including diagnosis and all related criteria.
A total of 240 evaluable patients were planned to be enrolled in the study and randomized to one of the following treatment groups in double-blind condition:
-
Group 1: Trazo/Gaba 2.5/25 mg, capsules, t.i.d. for 8 weeks.
-
Group 2: Trazo/Gaba 5/50 mg, capsules, t.i.d. for 8 weeks.
-
Group 3: Trazo/Gaba 10/100 mg, capsules, t.i.d. for 8 weeks.
-
Group 4: Gaba, capsules, administered as per Summary Product Characteristics (SPC) up to the maximum dosage of 1800 mg daily.
-
Group 5: PLB, capsules, t.i.d. for 8 weeks.
After the 8-week treatment period, patients allocated into the Gaba group started 8 days of tapering off in double-blind conditions. Thus, patients allocated in the other groups received an 8-day tapering period with PLB in order to maintain the double-blind conditions.
During the study, medications for pain management, including but not limited to tricyclic antidepressants, serotonin, and norepinephrine reuptake inhibitors, other GABA analogue different from gabapentin, opioids, mexiletine hydrochloride, carbamazepine, phenytoin, valproate sodium, dextromethorphan, capsaicin, corticosteroids, nonsteroidal anti-inflammatory drugs, skeletal muscle relaxants, benzodiazepines, and over-the-counter medications with centrally acting properties were prohibited and had to be discontinued for a period specific to the taper schedule (based on five elimination half-lives of the used medication) before randomization. In case of need, only paracetamol as analgesic and aspirin for prophylaxis of myocardial infarction or transient ischemic attack were allowed.
Randomization and blinding
At visit 0 (baseline, day 0) patients fulfilling eligibility criteria were randomly assigned to receive Trazo/Gaba 2.5/25 mg, or Trazo/Gaba 5/50 mg, or Trazo/Gaba 10/100 mg, or Gaba, or PLB, in a 1:1:1:1:2 ratio. To randomize patients, investigators were properly directed to assign the product following the sequential order of the randomization number reported on the label of each patient’s drug package. The randomization scheme was a computer-generated sequence. Before supplying the investigational drug to clinical sites, all the test drugs were covered in anonymous DB AA-EL capsules in order to maintain the double-blind condition of the study. In case of a medical emergency, the investigator was able to unblind the treatment code through the blinded labels provided by the sponsor.
Study Outcome Measures
The primary end point of the study was the change from baseline of the average daily pain score based on the 11-point NRS (“from 0 = no pain to 10 = worst possible pain”) after 8 weeks of treatment (visit 6, day 56 ± 2). At baseline and each subsequent time point, the scores were averaged from the last seven entries done by patients in the electronic device, calculated from a minimum of four pain ratings.
As secondary endpoints, this study assessed the following parameters: change from baseline of 11-point NRS at each other visits; % of responders (defined as ≥ 30% and ≥ 50% reduction from baseline of the average daily 11-point NRS; the neuropathic pain symptoms using the Neuropathic Pain Symptom Inventory (NPSI) and BPI-SF; anxiety and depression using the Hospital Anxiety and Depression Scale (HADS) and Beck Depression Inventory—Second Edition (BDI-II); sleep using the Insomnia Severity Index (ISI); quality of life using the Euroqol-5D-5L (EQ-5D-5L); safety and tolerability.
Among the pain rating scales, the 11-point NRS has been extensively used and validated as pain is always subjective and self-assessment scale provides the most valid measure of the experience [18]. BPI-SF has been specifically validated for PDN [19], while NPSI is a validated self-questionnaire designed to evaluate the different symptoms of neuropathic pain [20]. BDI-II consists of 21 items to assess the intensity of depression in clinical and normal patients [21], while HADS is commonly used to assess the level of anxiety and depression that a patient is experiencing [22]. ISI is a seven-item self-reported instrument measuring the patient's perception of his/her insomnia targeting the severity of symptoms, the consequences of insomnia, as well as the degree of distress caused by those difficulties [23, 24]. EQ-5D-5L is based on self-report of five domains: mobility, self-care, usual activities, pain and discomfort, anxiety and depression, plus a visual analogue scale (VAS) that records the patient’s self-rated health on a vertical scale [25]. CGI-C provides a global rating of patient’s improvement [26].
As safety variables, adverse events were monitored throughout the whole study from the signature of the informed consent up to the last study visit. A complete physical examination (including the measurement of vital signs) was performed at all visits, while laboratory analysis (hematology, serum chemistry, and urinalysis) were done at the screening visit, visit 4, and visit 6. Blood and urine samples were analyzed by the Central Laboratory ACM located in UK and lab reports were assessed by the investigator. The patients were also monitored for the cardiac safety at the screening visit, visit 1, visit 4, visit 5, and visit 6: 12-Lead ECGs were evaluated by the Central Cardiac Laboratory Bioclinica located in US.
Statistical Analysis
The sample size of this study was calculated supposing a linear relationship between dose and response, using the linear contrast method, with an unequal allocation, duplicating the PLB arm to increase the power of the study [27]. A total of 200 patients with a ratio 2:1:1:1 (80 patients for the PLB group and 40 for the other Trazo/Gaba groups) was required to detect a difference in the primary endpoint, supposing an effect of − 2 for PLB, − 2.25 for Trazo/Gaba 2.5/25 mg, − 3 for Trazo/Gaba 5/50 mg, and – 3.5 for Trazo/Gaba 10/100 mg, with a two-tailed confidence level of 95% a power > 85%, assuming a standard deviation of 2.3 and 20% of withdrawals. Forty patients were also added for the Gaba group for assay sensitivity, reaching a total sample size of 240 patients.
For statistical purposes, the safety population was defined as all randomized patients who took at least one dose of the study medication, while the m-ITT population was defined as all randomized patients who took at least one dose of the study medication and having a baseline and at least one post-baseline NRS evaluation. The LOCF method was implemented as imputation scheme for missing data in the m-ITT population.
The primary efficacy endpoint between Trazo/Gaba and PLB was evaluated in the m-ITT with LOCF, using an analysis of covariance (ANCOVA), including treatment and centers as factors and baseline as covariate and applying linear contrast test, excluding the active treatment (Gaba). Only if the linear contrast test was significant (p < 0.05), would the step-down Dunnett test be used to determine the minimum effective dose significantly different from PLB. If linearity was not verified (p > 0.05), an ANCOVA model, including treatment and center as factors and baseline as covariate, and comparisons with Dunnett test would be performed for the primary endpoint in the LOCF m-ITT and PP populations. If statistical assumptions underlying the ANCOVA were not satisfied, an ANOVA model would be applied. Before the application of the ANCOVA model, the non-significance of interaction treatment per baseline was verified.
Furthermore, mixed models for repeated measures (MMRM) in the m-ITT population were conducted as supportive analysis on the primary endpoint.
All secondary efficacy endpoints were reported descriptively in the m-ITT population. An ANCOVA or Cochran–Mantel–Haenszel test was applied, if appropriate. All secondary endpoints and secondary analyses were of exploratory nature and therefore no adjustment for multiplicity was planned.
Results
Patient Disposition and Baseline Characteristics
The trial was carried out from November 2018 (first patient in) to June 2020 (last patient out), and 369 patients were evaluated for eligibility, 129 patients were excluded for different reasons (111 screening failure, 15 requested to be excluded, three other reasons), while 240 patients were randomized and received the allocated treatment: 39 in Trazo/Gaba 2.5/25 mg, 38 in Trazo/Gaba 5/50 mg, 37 in Trazo/Gaba 10/100 mg, 83 in PLB, and 43 in Gaba group (safety population). Three patients in the PLB group and one patient in the Gaba group were excluded from the m-ITT population due to the lack of post-baseline NRS evaluation. Consequently, the m-ITT population included 236 patients (39 in Trazo/Gaba 2.5/25 mg, 38 in Trazo/Gaba 5/50 mg, 37 in Trazo/Gaba 10/100 mg, 80 in PLB, and 42 in Gaba).
Compliance with the study medication was generally high: 92.3% in Trazo/Gaba 2.5/25 mg, 94.7% in Trazo/Gaba 5/50 mg, 86.5% in Trazo/Gaba 10/100 mg group, 78.3% in PLB group, and 81.4% in Gaba group, respectively. Four patients discontinued in the Trazo/Gaba 2.5/25 mg (10.3%), three in Trazo/Gaba 5/50 mg (7.9%), five in Trazo/Gaba 10/100 mg (13.5%), 21 in PLB (25.3%), and eight in Gaba (18.6%), respectively. Reasons for discontinuation as well as the number of patients completing the study are reported in Fig. 2. Patient characteristics (Table 1), neuropathic pain efficacy measures, and other assessments (Table 2) at baseline were similar in each treatment group and no statistically significant differences were seen.
Primary Efficacy Result
After 8 weeks (56 ± 2 days) of treatment, the mean ± SD average daily pain score based on the 11-point NRS was reduced in the m-ITT population from 6.89 ± 1.03 (baseline) to 4.37 ± 2.14 (V6) in Trazo/Gaba 2.5/25 mg group; 6.96 ± 1.04 (baseline) to 4.72 ± 2.03 (V6) in Trazo/Gaba 5/50 mg group; 6.91 ± 1.47 (baseline) to 4.45 ± 2.10 (V6) in Trazo/Gaba 10/100 mg group; and from 7.08 ± 1.20 (baseline) to 5.06 ± 2.20 (V6) in PLB group. Mean changes of the average daily pain score based on the 11-point NRS from baseline to visit 6 were − 2.52 ± 2.31 in Trazo/Gaba 2.5/25 mg group; − 2.24 ± 1.96 in Trazo/Gaba 5/50 mg group, − 2.46 ± 2.12 in Trazo/Gaba 10/100 mg group, and − 2.02 ± 1.95 in PLB group (Fig. 3).
The linear contrast test did not result in statistically significant difference (p > 0.05), consequently the step-down Dunnett test was not performed to estimate the minimum effective dose in comparison to PLB.
Although no statistically significant differences vs. PLB were reached at visit 6 by the multiple comparisons with Dunnett adjustment (Trazo/Gaba 2.5/25 mg (95% CI − 1.2739, 0.2026; p = 0.1539); Trazo/Gaba 5/50 mg (95% CI − 0.9401, 0.5390; p = 0.5931); Trazo/Gaba 10/100 mg (95% CI − 1.0342, 0.4582; p = 0.4471), a greater pain reduction was observed starting from visit 3 in patients receiving Trazo/Gaba 2.5/25 mg in comparison to the other treatment groups, with a clear separation from the effect shown in PLB and Gaba groups. In addition, at visit 5 (week 6) a statistically significant difference was reported in favor of the lowest dosage of Trazo/Gaba 2.5/25 mg in comparison to PLB (95% CI − 1.6648, − 0.2126; p = 0.0116).
For primary endpoint, the measures of effect size are calculated as Cohen's d, and they resulted in: 0.25 for the Trazo/Gaba 2.5/25 mg, 0.11 for Trazo/Gaba 5/50 mg, and 0.23 for Trazo/Gaba 10/100 mg dose, respectively.
Interestingly, a similar trend between PLB and Gaba group was also observed during the treatment period.
Furthermore, a MMRM was conducted as supportive analysis on the primary endpoint in the m-ITT population to evaluate changes of the average NRS daily pain score in time and across treatments. A statistically significant difference was detected for treatment vs. time (p < 0.05) with promising trends observed at visit 1 and visit 5 (Table 3).
Similarly, a numerically but non-significantly larger proportion of patients in Trazo/Gaba 2.5/25 mg compared to the other groups achieved ≥ 30% and ≥ 50% reduction from baseline in 11-point NRS at visit 6 (Table 4).
Secondary Efficacy Results
Results from the analysis of items of the BPI-SF confirmed a promising trend for Trazo/Gaba FDC, especially for the lowest dose (2.5/25 mg). Interestingly, after 4 weeks of treatment, the mean changes ± SD were: − 2.35 ± 2.45 in Trazo/Gaba 2.5/25 vs. − 1.27 ± 2.65 in PLB for the item 9C (“how much the pain interfered, during the past 24 h, with the patient’s walking ability”) (95% CI − 2.0166, − 0.2867; p = 0.0093); − 2.76 ± 2.24 in Trazo/Gaba 2.5/25 vs. − 1.35 ± 2.62 in PLB for item 9D (“how much the pain interfered with the patient’s normal work”) (95% CI − 2.1395, − 0.5077; p = 0.0016); and − 2.81 ± 2.51) in Trazo/Gaba 2.5/25 vs. − 1.53 ± 3.17 in PLB for item 9F (“how much the pain interfered with the patient’s sleep”) (95% CI − 0.9815, 0.4638; p = 0.0354). All these differences were statistically significant (p < 0.05).
A reduction in the NPSI total score as compared to baseline was observed at scheduled visits in all treatment groups, with consistent better trend observed in Trazo/Gaba 2.5/25 mg group (Fig. 4). In addition, the analysis of each single item of NPSI revealed significant p value in favor of Trazo/Gaba 2.5/25 vs. PLB for the item Q9 (pain provoked or increased by pressure) after 8 weeks of treatment (95% CI − 1.9488, − 0.09653; p = 0.0306) (Fig. 5).
Also, the analysis of BDI-II, HADS, and ISI showed a reduction of the scores in all treatment groups from baseline to 8 weeks of treatment. Although no statistically significant differences were observed, the most promising trends were in favor of the lowest dose of Trazo/Gaba 2.5/25 mg in comparison to PLB, except for HADS scores for whom the higher dose of Trazo/Gaba (10/100 mg) showed the best one.
In a consistent way, the patient’s self-rated health of the quality-of-life EQ-5D-5L highlighted a statistically significant improvement in patients treated with Trazo/Gaba 2.5/25 mg in comparison to PLB after 8 weeks as reported in Fig. 6 (95% CI 1.2213, 14.3421; p = 0.0203).
Safety and Tolerability Results
A total of 240 patients were included in the safety analysis. Two SAEs judged not related to the study treatment occurred during the trial. A total of 165 TEAEs were recorded during the study, as displayed in Table 5.
The most frequent TEAEs involved principally the investigations (21 events: two in Trazo/Gaba 2.5/25 mg, three in Trazo/Gaba 5/50 mg, four in Trazo/Gaba 10/100 mg, ten in PLB, and two in the Gaba group) followed by gastrointestinal disorders (13 events: four in Trazo/Gaba 2.5/25 mg, two in Trazo/Gaba 5/50 mg, one in Trazo/Gaba 10/100 mg, three in PLB, and three in the Gaba group) and nervous system disorders (12 events: four in Trazo/Gaba 2.5/25 mg, four in Trazo/Gaba 5/50 mg, one in the Trazo/Gaba 10/100 mg, two in PLB, and two in the Gaba group), as summarized in Table 6.
The safety review of laboratory (blood and urine) analysis, vital signs, ECG, and physical findings did not show any significant clinical effect of the study treatments. Some clinically significant alterations were mainly related to the underlying or concomitant diseases.
Overall, a favorable safety profile of the FDCs containing trazodone and gabapentin was demonstrated in this study.
Discussion
We conducted a phase II multi-centric RCT to investigate safety and efficacy of a novel FDC with trazodone and gabapentin developed by Angelini Pharma S.p.A. Trazodone is a well-known antidepressant drug with a multi-modal mechanism of action, which was suggested as an alternative treatment for pain relief by NICE clinical guideline Neuropathic pain—pharmacological management, while gabapentin is a licensed drug widely used for the treatment of neuropathic pain [28]. Low doses of trazodone have already been used in previous studies in the neuropathic pain setting [8, 9]. In addition, the result of a recent RCT suggested the potential benefit of a low dose of trazodone administered on top of gabapentin as background therapy in patients affected by PDN [12]. Eventually, experimental studies point out a synergistic interaction between low-dose Trazo/Gaba FDC [13,14,15,16].
The treatment group Trazo/Gaba 2.5/25 mg shows the greatest pain reduction magnitude in the time frame between Visit 3 and 5 when compared to all other study arms, especially Gaba alone and PLB, with such a difference being statistically significant against PLB at Visit 5 (p < 0.05). This result is consistent with the analysis of responders, defined as patients who achieved at least 30% or 50% of reduction in the 11-point NRS pain score from baseline to visit 6. Furthermore, patients treated with Trazo/Gaba 2.5/25 mg consistently reported favorable trends on several items of BPI-SF (especially those describing how the pain can impair different aspects of the patient’s daily life), depression (BDI-II), neuropathic pain symptoms (NPSI), sleep (ISI), and quality of life (EQ-5D-5L).
A favorable safety profile of Trazo/Gaba FDC was demonstrated in this study, with a frequency of AEs – including laboratory tests, vital signs, ECG parameters, and physical evaluation – similar across treatment and PLB groups. The lowest dose of 2.5/25 mg t.i.d. showed lower incidence of AEs than expected by using Gaba alone and it was well tolerated even in patients with a high number of comorbidities and concomitant medications with no signal of detrimental drug–drug interactions.
Safety and tolerability data found in the present RCT suggest that all the three dosages of Trazo/Gaba FDCs are not associated with clinically meaningful risk of QT prolongation or other cardiovascular AEs. This observation holds considerable clinical value given the high rates of cardiovascular comorbidity in diabetic patients as well as the risk of dose-dependent QT interval prolongation with antidepressants used for chronic pain, either approved or off-label. Such an observation is of higher relevance if considering the pathological condition in question, i.e., diabetic chronic pain, which has a high prevalence among older adults – a population in which QT interval prolongation is more likely to occur [29].
Overall, although the primary efficacy endpoint was not reached in the present RCT, statistically significant results alongside various and consistent trends encourage further investigation of the Trazo/Gaba FDC for NP. Eventually, the results of this RCT are backed up by previous experimental findings, suggesting that synergy between trazodone and gabapentin is likely to occur at the least doses while higher dosages do not bring about additional benefit [16, 17, 30]. Moreover, we argue that the positive trend toward a mood and sleep quality improvement effect (as indicated by specific validated tools utilized in the present RCT) suggests that the Trazo/Gaba FDC may help improve multidimensional aspects of diabetes such as pain, insomnia, and depressive-like disturbances.
In summary, the drug investigated in this trial for PDN matches all the key “requirements” behind the rationale of a FDC formulation, including unique pharmacodynamic advantages due to complementary mode of action and synergistic effect. Moreover, fewer side effects and harmful drug–drug interaction are expected with the usage FDC formulation. In particular, better safety represents one the cornerstones of FDC strategies – especially when targeting patients with comorbidities and polytherapy.
As already mentioned, it is estimated that up to 50% of diabetic patients suffer from PDN and that, despite the fact that several drugs are approved for this indication, the efficacy results and tolerability are still unsatisfactory for almost half of the patients. For this reason, PDN is still considered a major medical and patients’ unmet need.
Results of this phase dose-finding II study, thus not designed to demonstrate efficacy, are intriguing because they suggest that very low doses of trazodone and gabapentin, administered as FDC, might be effective in reducing pain and increase quality of life and other associated symptoms in patients with PDN. Obtaining such promising results, with doses that are one-tenth of the regular dose of the SOC gabapentin (active control), might represent an improvement in the benefit/risk ratio of available treatment options.
The present RCT is not free from caveats and issues typically affecting the clinical development of drugs for chronic neuropathic pain – including concomitant psychological factors and lack of biomarker-based: eligibility criteria, phenotypic profiling and outcome measures (to name a few) – which are all likely to influence treatment outcomes and may account for the moderate trial outcomes and dilute results through large placebo responses [31, 32]. In line with this concern, we carried out the analysis of assay sensitivity, which did not reveal differences between gabapentin and placebo on primary endpoint and the majority of secondary endpoints, confirming how relevant the placebo effect in this setting can be [33]. Because of the underpowered total sample size, we could not control comparison analysis by psychological factors such as mood, anxiety, and sleep disorders, which may impact subjective response to pharmacological treatments [34].
Last but not least, the fragmented understanding of the pathophysiological mechanisms and related neural substrates of NP and PDN represent one of the greater hurdles toward the development of drugs with predictable benefit; it is currently hypothesized that the initial somatosensory nervous system lesion or disease sets off and sustains disordered nociceptive signal transmission coupled with maladaptive central processing and multi-level integration [4, 7]. Along this conceptual framework lies the opportunity of Trazo/Gaba FDC and its putative innovative mode of action for NP. Although a full mechanistic interpretation of the synergy calls for extensive studies, preliminary experimental data suggest a potential role of 5-HT2A and the mGLU2/3 heterodimer since both receptors co-localize in rodent spinal cord synaptosomes (and brain areas) and reciprocally interact in antagonistic fashion [13, 14]. In the spinal cord, the mGlu2/3-5-HT2A receptors crosstalk was impaired in conditions recapitulating neuropathic pain dynamics, which recovered by antagonizing the 5-HT2A counterpart of the receptor–receptor complex [13, 14]. Finally, a strong synergic effect was observed when suboptimal doses of trazodone were associated with suboptimal doses of gabapentin [16].
Limitations of the Study
This is the first trial assessing the efficacy and safety of Trazo/Gaba FDC in patients with PDN. The small sample size of this phase II, five-arm study was probably insufficient to demonstrate the primary endpoint. Further investigation is needed to confirm the preliminary observation of this study. Following studies can be designed capitalizing these results in both selecting the most promising dosage of Trazo/Gaba FDC and in calculating the most appropriate sample size.
In this trial, we also applied stringent ECG safety criteria, thus probably causing the high frequency of discontinuation for the QT prolongation. At each study visit, the QT interval was calculated and corrected according to Fridericia’s correction formula. Any Fridericia’s correction formula value > 450 ms (male) or 470 ms (female) resulted in patient discontinuation, though no patient reported any cardiac symptoms or signs. This aspect should be taken into account in designing further investigation without comprising the safety of the patients.
Conclusions
This was the first RCT investigating the efficacy and safety of three different doses of Trazo/Gaba FDCs compared with Gaba alone and PLB in patients with PDN. While the primary endpoint was not reached, the results seem to support the choice of the lowest dose of Trazo/Gaba FDC as the dose with the better benefit/risk ratio. Results observed in both primary and secondary endpoints as well as on spanning neuropathic symptoms, sleep issues, mood disturbances, and quality of life are in fact consistent and coherent. Taken together, and in light of experimental data in animal models of neuropathic pain, efficacy and safety profile shown in this trial support further clinical investigation of Trazo/Gaba 2.5/25 mg as a promising candidate drug for diabetes patients with neuropathy as comorbidity.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Smith BH, Hébert HL, Veluchamy A. Neuropathic pain in the community: prevalence, impact, and risk factors. Pain. 2020;161(Suppl 1):S127–37. https://doi.org/10.1097/j.pain.0000000000001824.
Blyth FM. Global burden of neuropathic pain. Pain. 2018;159(3):614–7. https://doi.org/10.1097/j.pain.0000000000001127.
van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–62. https://doi.org/10.1016/j.pain.2013.11.013. (Epub 2013 Nov 26. Erratum in: Pain. 2014 Sep;155(9):1907).
Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–73. https://doi.org/10.1016/S1474-4422(14)70251-0. (Epub 2015 Jan 7).
Tesfaye S, Brill S, Eerdekens M, Labrador MM, Petersen G, de Rooij Peek A, Reta A, Ryan D, Schaper N, Tölle T, Truini A, Ziegler D. Diagnosis, management and impact of painful diabetic peripheral neuropathy: a patient survey in four European countries. J Diabetes Complications. 2023;37(4):108417. https://doi.org/10.1016/j.jdiacomp.2023.108417. (Epub 2023 Feb 10).
Shillo P, Sloan G, Greig M, Hunt L, Selvarajah D, Elliott J, Gandhi R, Wilkinson ID, Tesfaye S. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep. 2019;19(6):32. https://doi.org/10.1007/s11892-019-1150-5.
Selvarajah D, Sloan G, Teh K, Wilkinson ID, Heiberg-Gibbons F, Awadh M, Kelsall A, Grieg M, Pallai S, Tesfaye S. Structural brain alterations in key somatosensory and nociceptive regions in diabetic peripheral neuropathy. Diabetes Care. 2023;46(4):777–85. https://doi.org/10.2337/dc22-1123.
Khurana RC. Treatment of painful diabetic neuropathy with trazodone. JAMA. 1983;250(11):1392. https://doi.org/10.1001/jama.1983.03340110016015.
Wilson RC. The use of low-dose trazodone in the treatment of painful diabetic neuropathy. J Am Podiatr Med Assoc. 1999;89(9):468–71. https://doi.org/10.7547/87507315-89-9-468.
Wiffen PJ, Derry S, Bell RF, Rice AS, Tölle TR, Phillips T, Moore RA. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD0007938. https://doi.org/10.1002/14651858.CD007938.pub4.
Stahl SM. Mechanism of action of trazodone: a multifunctional drug. CNS Spectr. 2009;14(10):536–46. https://doi.org/10.1017/s1092852900024020.
Lipone P, Ehler E, Nastaj M, Palka-Kisielowska I, Cruccu G, Truini A, Di Loreto G, Del Vecchio A, Pochiero I, Comandini A, Calisti F, Cattaneo A. Efficacy and safety of low doses of trazodone in patients affected by painful diabetic neuropathy and treated with gabapentin: a randomized controlled pilot study. CNS Drugs. 2020;34(11):1177–89. https://doi.org/10.1007/s40263-020-00760-2.
Olivero G, Grilli M, Vergassola M, Bonfiglio T, Padolecchia C, Garrone B, Di Giorgio FP, Tongiani S, Usai C, Marchi M, Pittaluga A. 5-HT2A-mGlu2/3 receptor complex in rat spinal cord glutamatergic nerve endings: a 5-HT2A to mGlu2/3 signalling to amplify presynaptic mechanism of auto-control of glutamate exocytosis. Neuropharmacology. 2018;1(133):429–39. https://doi.org/10.1016/j.neuropharm.2018.02.030. (Epub 2018 Feb 27).
Cisani F, Roggeri A, Olivero G, Garrone B, Tongiani S, Di Giorgio FP, Pittaluga A. Acute low dose of trazodone recovers glutamate release efficiency and mGlu2/3 autoreceptor impairments in the spinal cord of rats suffering from chronic sciatic ligation. Front Pharmacol. 2020;17(11):1108. https://doi.org/10.3389/fphar.2020.01108.
Quintero JE, Dooley DJ, Pomerleau F, Huettl P, Gerhardt GA. Amperometric measurement of glutamate release modulation by gabapentin and pregabalin in rat neocortical slices: role of voltage-sensitive Ca2+ α2δ-1 subunit. J Pharmacol Exp Ther. 2011;338(1):240–5. https://doi.org/10.1124/jpet.110.178384. (Epub 2011 Apr 4).
Garrone B, di Matteo A, Amato A, Pistillo L, Durando L, Milanese C, Di Giorgio FP, Tongiani S. Synergistic interaction between trazodone and gabapentin in rodent models of neuropathic pain. PLoS ONE. 2021;16(1): e0244649. https://doi.org/10.1371/journal.pone.0244649.
Ruggieri A, Picollo R, Del Vecchio A, Calisti F, Dragone P, Comandini A, Rosignoli MT, Cattaneo A, Donath F, Wedemeyer RS, Todorova-Sanjari M, Warnke A, Blume HH. Investigations on dose proportionality and drug–drug interaction for a fixed-dose combination of trazodone and gabapentin. Int J Clin Pharmacol Ther. 2021;59(1):71–86. https://doi.org/10.5414/CP203845.
Koltzenburg M, McMahon S, Tracey I, Turk DC, editors. Wall and Melzack’s textbook of pain. 6th ed. London: Saunders, imprint of Elsevier Ltd.; 2013.
Zelman DC, Gore M, Dukes E, Tai KS, Brandenburg N. Validation of a modified version of the brief pain inventory for painful diabetic peripheral neuropathy. J Pain Symptom Manag. 2005;29(4):401–10.
Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, Rostaing S, Lanteri-Minet M, Collin E, Grisart J, Boureau F. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108(3):248–57.
Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-II. San Antonio: Psychological Corporation; 1996.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Morin CM. Insomnia: psychological assessment and management. New York: Guilford Press; 1993.
Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307.
Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, Swinburn P, Busschbach J. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013;22(7):1717–27. https://doi.org/10.1007/s11136-012-0322-4. (Epub 2012 Nov 25).
Guy W. ECDEU assessment manual for psychopharmacology. Rockville: National Institute of Mental Health, Psychopharmacology Research Branch; 1976. revised, pp. 217–22, 313–31, 534–7.
Mallinckrodt CH, Tamura RN, Tanaka Y. Recent developments in improving signal detection and reducing placebo response in psychiatric clinical trials. J Psychiatr Res. 2011;45(9):1202–7. https://doi.org/10.1016/j.jpsychires.2011.03.001. (Epub 2011 Mar 31).
Neuropathic pain—pharmacological management. NICE clinical guideline 173. https://www.nice.org.uk/guidance/cg173/evidence/full-guideline-pdf-4840898221.
Guettler N, Rajappan K, Nicol E. The impact of age on long QT syndrome. Aging (Albany NY). 2019;11(24):11795–6. https://doi.org/10.18632/aging.102623. (Epub 2019 Dec 28).
Oggianu L, Garrone B, Fiorentini F, Del Bene F, Rosignoli MT, Di Giorgio FP, Kaminski RM. PK/PD analysis of trazodone and gabapentin in neuropathic pain rodent models: translational PK-PD modeling from nonclinical to clinical development. Clin Transl Sci. 2023;16(4):606–17. https://doi.org/10.1111/cts.13472. (Epub 2023 Feb 13).
Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DLH, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice ASC, Serra J, Smith BH, Treede RD, Jensen TS. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–606. https://doi.org/10.1097/j.pain.0000000000000492.
Boussageon R, Howick J, Baron R, Naudet F, Falissard B, Harika-Germaneau G, Wassouf I, Gueyffier F, Jaafari N, Blanchard C. How do they add up? The interaction between the placebo and treatment effect: a systematic review. Br J Clin Pharmacol. 2022;88(8):3638–56. https://doi.org/10.1111/bcp.15345. (Epub 2022 May 2).
Finnerup NB, Haroutounian S, Baron R, Dworkin RH, Gilron I, Haanpaa M, Jensen TS, Kamerman PR, McNicol E, Moore A, Raja SN, Andersen NT, Sena ES, Smith BH, Rice ASC, Attal N. Neuropathic pain clinical trials: factors associated with decreases in estimated drug efficacy. Pain. 2018;159(11):2339–46. https://doi.org/10.1097/j.pain.0000000000001340.
Marchettini P, Wilhelm S, Petto H, Tesfaye S, Tölle T, Bouhassira D, Freynhagen R, Cruccu G, Lledó A, Choy E, Kosek E, Micó JA, Späth M, Skljarevski V, Lenox-Smith A, Perrot S. Are there different predictors of analgesic response between antidepressants and anticonvulsants in painful diabetic neuropathy? Eur J Pain. 2016;20(3):472–82. https://doi.org/10.1002/ejp.763. (Epub 2015 Aug 27).
Acknowledgements
We would like to thank the participants of the study, the investigators and site staff involved in the conduction of this clinical trial. We would like to also mention Prof. Giorgio Cruccu and Dr. Andrea Truini from University La Sapienza in Rome for the fruitful insight in the clinical study design and protocol. Special thanks to Raffaella Fallone and Sara Fioravanti from the Data Management department of Angelini Pharma S.p.A. for their contribution in developing the eCRF, performing the study database setup, and validation and data cleaning.
Funding
This study was sponsored by Angelini Pharma S.p.A. (Rome, Italy). The study sponsor is also funding the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Solomon Tesfaye, Paola Lipone, Fabrizio Calisti, and Alessandro Comandini contributed to the study conception and design. Elisa Quarchioni carried out the statistical analysis of the study. Solomon Tesfaye, Ponnusamy Saravanan, Edvard Ehler, Karel Zinek, Ilona Palka-Kisielowska, Marcin Nastaj, and Pierre Serusclat contributed to the study management and data collection. Andrea Vergallo and Agnese Cattaneo reviewed and commented on the study results. The first draft of the manuscript was written by Paola Lipone and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Solomon Tesfaye received: honoraria from Procter & Gamble, Viatris, Grunenthal, Novo Nordisk, Pfizer, Merck, Eva Pharma, Hikma, Astellas Pharma, Abbott, AstraZeneca, Berlin-Chemie, Worwag Pharma, Nevro, Haisco Pharmaceutical Group, for educational meetings; consultancy fees for advisor board membership from Angelini Pharma, Bayer, GSK, Worwag Phrama and Nevro; research equipment donated to Sheffield Teaching Hospitals from Impeto Medical and Neurometrix; and unrestricted, competitive research grants from Viatris and Procter and Gamble. Ponnusamy Saravanan was the Principal Investigator of the experimental center located in Nuneaton (United Kingdom) and has no conflict of interest. Edvard Ehler was the Principal Investigator of the experimental center located in Pardubice (Czech Republic) and has no conflict of interest. Karel Zinek was the Principal Investigator of the experimental center located in Litomyšl (Czech Republic) and has no conflict of interest. Ilona Palka-Kisielowska was the Principal Investigator of the experimental center located in Katowice (Poland) and has no conflict of interest. Marcin Nastaj was the Principal Investigator of the experimental center located in Lublin (Poland) and has no conflict of interest. Pierre Serusclat was the Principal Investigator of the experimental center located in Venissieux (France) and has no conflict of interest. Paola Lipone, Elisa Quarchioni, Fabrizio Calisti, Alessandro Comandini, Agnese Cattaneo are full-time employees in Angelini Pharma S.p.A. Andrea Vergallo was a full-time employee of Angelini Pharma S.p.A. at the time of the completion of the manuscript.
Ethical Approval
The study was approved by the Competent Authorities and applicable Ethic Committees of Czech Republic (State Institute for Drug Control, Thomayerova nemocnice a IKEM, Ethics Committee Nemocnice Pardubického kraje a.s., Axon Clinical, Etická komise CLINTRIAL s.r.o.), France (ANSM Agence Nationale de Sécurité du Médicament et des produits de santé and Comité de Protection des Personnes (CPP) désigné par le système CNRIPH), Poland (Departament Badań Klinicznych Produktów Leczniczych Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych and Komisja Bioetyczna przy Śląskiej Izbie Lekarskiej w Katowicach), and United Kingdom (Medicines and Healthcare products Regulatory Agency and East of England—Cambridgeshire and Hertfordshire Research Ethics Committee) and was conducted in accordance with Good Clinical Practice guideline and ethical standards as laid down in the 1964 Declaration of Helsinki. Written informed consent (including personal data processing) was obtained from all individual participants included in the study prior to starting any study-related procedure.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tesfaye, S., Saravanan, P., Ehler, E. et al. Efficacy and Safety of Trazodone and Gabapentin Fixed-Dose Combination in Patients Affected by Painful Diabetic Neuropathy: Randomized, Controlled, Dose-Finding Study. Pain Ther 13, 987–1006 (2024). https://doi.org/10.1007/s40122-024-00624-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-024-00624-3