Abstract
Introduction
Central post-stroke pain (CPSP) is a common type of central neuropathic pain (CNeP) that can occur following the onset of stroke. The oral gabapentinoid mirogabalin besylate (mirogabalin) is a selective α2δ ligand that is effective for the treatment of CNeP, including CPSP. However, it is unknown whether the analgesic effect of mirogabalin on CPSP varies in patients with different background factors.
Methods
This was a post hoc subgroup analysis of a multinational, open-label, long-term phase 3 study of mirogabalin for the treatment of CNeP conducted between March 2019 and December 2020. Data from patients with CPSP were stratified by type of stroke (ischemic or hemorrhagic), stroke location (thalamus, putamen, brainstem, or other), presence/absence of motor weakness, median time since stroke (≥ 59 or < 59 months), and median duration of CPSP (≥ 55.5 or < 55.5 months). Efficacy was assessed with the short-form McGill Pain Questionnaire (SF-MPQ), and treatment-emergent adverse events (TEAEs) and adverse drug reactions (ADRs) were recorded.
Results
This subanalysis included all 94 patients with CPSP from the phase 3 study; all were Japanese, and the mean age was 65.3 years. The least squares mean change [95% confidence interval] in SF-MPQ visual analog scale (VAS) score from baseline at week 52 (last observation carried forward) was − 17.0 [− 22.1, − 11.9] mm. Among the subgroups, least squares mean changes in SF-MPQ VAS scores were not different. Most TEAEs were mild or moderate; severe TEAEs occurred in six patients (6.4%). Somnolence (25.5%), peripheral edema (13.8%), dizziness (11.7%), and weight gain (6.4%) were the most common ADRs, and the types and frequencies of ADRs were similar among subgroups.
Conclusion
Mirogabalin was generally effective and well tolerated in patients with CPSP, regardless of background factors such as stroke type or location, presence/absence of motor weakness, time since stroke, and duration of CPSP.
Trial Registration
Trial registration number NCT03901352.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There are limited treatment options for patients with central post-stroke pain (CPSP). |
Mirogabalin, a selective α2δ ligand drug, is a new treatment option for patients with CPSP, but it is unknown if the analgesic effect and the safety are affected by patient background factors. |
This post hoc subgroup analysis of a long-term, open-label, phase 3 study examined the efficacy and safety of mirogabalin in patients with CPSP by type of stroke, stroke location, presence or absence of motor weakness, time since stroke, and duration of CPSP. |
What was learned from this study? |
No clinically meaningful differences in efficacy or safety of mirogabalin were observed in any subgroup of patients with CPSP. |
Mirogabalin may be a useful treatment option for patients with CPSP, regardless of the type of stroke, stroke location, presence or absence of motor weakness, time since stroke, or duration of CPSP. |
Introduction
Stroke is one of the leading causes of mortality and causes long-term physical, psychological, and social disabilities that require long-term care [1]. These disabilities and the associated care requirements lead to a worsened quality of life (QOL) [2] and an increased socioeconomic burden [3] for patients who have experienced stroke and require long-term care and/or rehabilitation, and thus the patients may continue to have unmet needs [4,5,6]. Pain relief is one of the most frequently reported unmet needs following stroke and is associated with a reduction in QOL [5]. Therefore, there has been an increasing global interest in the management of post-stroke pain over the past 10 years [7].
Different types of pain can occur after stroke, including shoulder pain, painful spasticity, other types of musculoskeletal pain as well as central post-stroke pain (CPSP) [8]. Unique clinical features of CPSP, which help distinguish it from other types of pain, are sensory loss and signs of hypersensitivity in the body parts that correspond to the somatosensory pathway damaged by stroke [8, 9]. Patients with CPSP report persistent or intermittent burning, aching, pricking, freezing, and squeezing pain, and the location can range from a small area to the entire side of the patient’s body [8].
The prevalence of CPSP among patients who have experienced stroke has been reported in more than 10 papers with ranges between 1% and 12% [8, 10]. A large study with 15,754 patients with ischemic stroke observed that 2.7% of patients developed CPSP within 1 year following a stroke [11], whereas a more recent systematic review reported that the pooled prevalence of CPSP after stroke was 11%, with 31% of patients developing CPSP within 1 month of experiencing the stroke [12]. Although the exact prevalence of CPSP differs among studies, CPSP is understood to be a common condition, and there is a need for the development of effective treatment options for CPSP.
In 2010, the European Federation of the Neurological Societies recommended the gabapentinoid pregabalin as a first-line agent for the treatment of central neuropathic pain (CNeP) [13]. In 2015, the International Association for the Study of Pain also stated that there was strong evidence for the use of pregabalin as a first-line agent for the treatment of neuropathic pain, including CPSP [14]. In clinical practice, pregabalin is often used for the treatment of CPSP, either alone or in combination with non-steroidal anti-inflammatory medications [15,16,17], and previous reviews reported that pregabalin has variable efficacy in patients with CPSP and is associated with adverse reactions [10, 18].
Mirogabalin besylate (mirogabalin) is another oral gabapentinoid, which is a selective α2δ ligand that has been approved in Japan for the treatment of neuropathic pain, including both peripheral neuropathic pain and CNeP [19]. Mirogabalin has been reported to be effective for the treatment of CNeP including CPSP in previous phase 3 study, both during the double-blind 14-week and long-term 52-week periods [20, 21]. However, it is currently unknown whether the effects of mirogabalin on CPSP vary among patients with different clinical characteristics, such as stroke type, stroke location, motor weakness, time since stroke, and duration of CPSP.
Therefore, we conducted this post hoc subgroup analysis to examine the efficacy and safety of mirogabalin by selected background factors, using the dataset of a previous long-term (52-week), open-label, phase 3 study [21].
Methods
Study Design and Patients
This study was a post hoc subgroup analysis of a multinational, open-label, long-term, phase 3 study of mirogabalin for the treatment of CNeP (ClinicalTrials.gov identifier NCT03901352) [21]. The open-label study was the long-term extension of a randomized, double-blind, placebo-controlled, phase 3 trial [20], and was conducted between March 2019 and December 2020 at 121 sites in Japan, Korea, and Taiwan. The study was conducted in compliance with the ethical principles that have their origins in the Declaration of Helsinki, the International Council for Harmonisation Good Clinical Practice guidelines, and the Japanese Ministry of Health, Labour and Welfare. The study protocol, protocol amendments, informed consent forms, and information sheets were approved by the relevant independent ethics committees or institutional review boards at each study center [21], and all patients provided written informed consent prior to participating in the study.
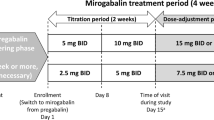
Full details of the long-term part of the phase 3 study design have been reported previously [21]. In brief, the study duration was 54 weeks, including a 1-week observation period prior to initiating treatment, 52 weeks of treatment (a 4-week titration period, a 47-week maintenance period, and a 1-week taper period), and a 1-week follow-up period after the final dose.
Mirogabalin was administered orally at 5 mg twice daily (BID) for the first 2 weeks, titrated to 10 mg BID for the next 2 weeks, and then increased to the maintenance dose of 15 mg BID. During the maintenance dose period, the dose could be reduced to 10 mg BID if safety concerns arose. During the taper period (week 51), the dose was reduced: patients receiving a 10-mg BID maintenance dose tapered to 10 mg once daily, and patients who received a 15-mg BID maintenance dose tapered to 15 mg once daily. Patients who had reduced renal function (creatinine clearance [CrCL] of 30 to < 60 mL/min) received a 50% lower dose of mirogabalin than patients with normal renal function or mild renal impairment (CrCL ≥ 60 mL/min). The use of pregabalin, gabapentin, and other investigational agents was prohibited throughout the study, including during the post-treatment follow-up period. Patients who had previously received a gabapentinoid were required to undergo a 4-week washout period prior to the 1-week observation period.
This post hoc subgroup analysis included patients with CPSP from the open-label phase 3 study, for which the full eligibility criteria have been published [21]. For the present analysis, the criteria for patients with CPSP were stroke occurring at least 6 months prior to screening with stable CPSP for at least 3 months prior to screening, damage to the somatosensory pathways caused by stroke (confirmed by computed tomography or magnetic resonance imaging), location of pain neurologically matched with somatosensory pathways, and a pain score of ≥ 40 mm on the short-form McGill Pain Questionnaire (SF-MPQ) visual analog scale (VAS) at screening and enrollment [22]. The key exclusion criteria were grade ≥ 5 on the Modified Rankin Scale and bleeding at screening.
Study Assessments
Patients completed a self-assessment of their pain using the SF-MPQ at each study visit (the first 2 weeks and once every 4 weeks during the treatment period). The SF-MPQ comprised three parts: 15 pain descriptors (11 sensory and 4 affective) ranging from 0 (none) to 3 (severe); a pain VAS ranging from 0 (no pain) to 100 (worst possible pain); and a present pain intensity index from 0 to 5. SF-MPQ data and a time course of SF-MPQ VAS scores were analyzed.
The safety outcomes were treatment-emergent adverse events (TEAEs) and adverse drug reactions (ADRs), which were coded using the Medical Dictionary for Regulatory Activities, version 23.0.
Statistical Analysis
As this study was a post hoc subgroup analysis, the target sample size was not pre-specified. The same analysis set as for the long-term part of the previous phase 3 study [21] was used in this study: the efficacy and safety analysis sets were identical and included all patients who provided informed consent and received at least one dose of mirogabalin. Patients were stratified by type of stroke (ischemic or hemorrhagic), stroke location (thalamus, putamen, brainstem, or other), presence/absence of motor weakness (as assessed by the attending physician), median time since stroke (≥ 59 or < 59 months), and median duration of CPSP (≥ 55.5 or < 55.5 months). Patients were also stratified by time since stroke (≥ 12 or < 12 months); however, these results were not reported because a large bias was evident between the patient groups.
Continuous variables were summarized by number, mean, and standard deviation (SD), and categorical variables were summarized by percentage. The least squares (LS) means and their 95% confidence intervals (CIs) for SF-MPQ VAS scores, total scores, and subscale scores were calculated using an analysis of covariance model with baseline value as a covariate; statistical comparisons between subgroups were not conducted. Missing data for efficacy endpoints were handled according to the standard scoring instructions of the SF-MPQ using the last observation carried forward (LOCF) method, and missing safety data were not imputed.
All statistical analyses were performed using SAS version 9.3 or higher (SAS Institute Inc., Cary, NC, USA).
Results
Patients
The patient disposition is shown in Fig. 1. Among the 114 patients with CPSP in the overall study who were assessed for eligibility, 94 were enrolled and included in the current analysis. Of the 20 patients excluded, the most common reason for exclusion was screening failure (n = 13). Seventy-nine patients (84.0%) completed the 52-week study, and the main reason for discontinuation was TEAEs (n = 12, 12.8%).
The patient baseline characteristics are shown in Table 1 and Table S1 in the electronic supplementary material. All patients were Japanese, the mean (SD) age was 65.3 (11.1) years, and 54/94 (57.4%) patients were ≥ 65 years old. The mean (SD) VAS at baseline was 71.0 (15.1) mm and the mean CrCL was 72.7 (26.0) mL/min. The proportion of patients with motor weakness was 55.3%. The thalamus was the most common stroke location (n = 43, 45.7%), followed by the putamen (n = 20, 21.3%), brainstem (n = 16, 17.0%), and other (n = 15, 16.0%). Forty-six patients had an ischemic stroke (48.9%), and 48 had a hemorrhagic stroke (51.1%). There were some differences in characteristics between patients with ischemic stroke and those with hemorrhagic stroke, e.g., male ratio (84.8% and 56.3%) and motor weakness (32.6% and 77.1%), respectively. A higher proportion of patients with the putamen as the stroke location had motor weakness compared with the brainstem (75.0% and 37.5%). The baseline SF-MPQ VAS values (SD) were slightly higher in patients with ischemic vs hemorrhagic stroke (65.6 [13.0] mm vs 76.2 [15.1] mm) and in patients with motor weakness vs those without (75.5 [14.7] mm vs 65.5 [13.7] mm). Baseline SF-MPQ VAS scores were similar among patients with different stroke locations, post-stroke duration, and CPSP duration.
Efficacy
The SF-MPQ VAS scores are shown in Table 2 and Fig. 2, and further details are provided in Table S2 and Fig. S1 in the electronic supplementary material. Overall and in each subgroup, the mean SF-MPQ VAS scores initially decreased after mirogabalin treatment until week 8 (Fig. 2 and Fig. S1 in the electronic supplementary material). The scores then remained stable until week 48, whereafter an increase occurred between weeks 48 and 52, which included the 1-week taper period (weeks 51–52). The LS mean [95% CI] in SF-MPQ VAS score from baseline at week 52 (LOCF) was − 17.0 [− 22.1, − 11.9] mm (Fig. 2d). The LS mean [95% CI] changes from baseline at week 52 (LOCF) for the subgroups were − 17.8 [− 25.7, − 10.0] (ischemic stroke), − 16.1 [− 23.0, − 9.3] (hemorrhagic stroke), − 16.0 [− 23.4, − 8.5] (stroke location: thalamus), − 25.2 [− 39.2, − 11.2] (stroke location, putamen), − 15.5 [− 25.4, − 5.6] (stroke location, brainstem), − 10.5 [− 24.6, 3.6] (stroke location, other), − 16.8 [− 23.6, − 10.0] (with motor weakness), and − 17.2 [− 25.3, − 9.1] (without motor weakness). Patients with ≥ 59 and < 59 months since stroke onset had LS mean [95% CI] changes from baseline at week 52 (LOCF) of − 20.4 [− 27.4, − 13.3] and − 13.0 [− 20.5, − 5.4], respectively, and patients with a CPSP duration of ≥ 55.5 and < 55.5 months had LS mean [95% CI] changes from baseline at week 52 (LOCF) of − 20.6 [− 28.0, − 13.1] and − 13.4 [− 20.5, − 6.2], respectively. Among all subgroups, SF-MPQ VAS scores improved from baseline throughout the 52-week study period or at week 52 (LOCF) (Fig. 2, Fig. S1a, b).
SF-MPQ VAS scores by patient group. Data are arithmetic mean (SD), except where otherwise stated. SF-MPQ VAS scores by a type of stroke, b stroke location, c presence or absence of motor weakness, and d LS mean change [95% CI] from baseline at week 52 (LOCF). CI confidence interval, CPSP central post-stroke pain, LOCF last observation carried forward, LS least squares, SD standard deviation, SF-MPQ short-form McGill Pain Questionnaire, VAS visual analog scale
The SF-MPQ total scores and subscale scores are shown in Table 3, Fig. 3, and Table S3 in the electronic supplementary material. The overall LS mean [95% CI] changes from baseline at week 52 (LOCF) in the sensory score, affective score, total score, and present pain intensity score were − 3.6 [− 4.7, − 2.4], − 1.5 [− 2.0, − 1.0], − 5.1 [− 6.6, − 3.5], and − 0.8 [− 1.1, − 0.6], respectively (Fig. 3). Regarding the sensory score, affective score, total score, and present pain intensity score of the SF-MPQ, the LS mean changes improved from baseline at week 52 (LOCF) among all subgroups (Fig. 3).
Change from baseline in SF-MPQ subscale and total scores at week 52 (LOCF). Data are LS mean [95% CI]. The panels show a SF-MPQ sensory scores, b SF-MPQ affective scores, c SF-MPQ total scores, and d SF-MPQ present pain intensity scores. CI confidence interval, CPSP central post-stroke pain, LOCF last observation carried forward, LS least squares, SF-MPQ short-form McGill Pain Questionnaire
The SF-MPQ subscale scores were not notably different between ischemic and hemorrhagic stroke groups (LS mean [95% CI] change from baseline at week 52 (LOCF): sensory score, − 2.7 [− 4.4, − 0.9] vs − 4.4 [− 6.1, − 2.8]; affective score, − 1.3 [− 1.9, − 0.7] vs − 1.6 [− 2.4, − 0.9]; total score, − 4.0 [− 6.3, − 1.7] vs − 6.1 [− 8.3, − 3.8]; and present pain intensity score, − 0.7 [− 1.1, 0.4] vs − 0.9 [− 1.2, − 0.6]).
Regarding stroke location, the SF-MPQ subscale scores in the stroke locations of thalamus, putamen, and brainstem were as follows: sensory score, − 3.7 [− 5.1, − 2.2], − 5.0 [− 7.8, − 2.2], − 2.3 [− 5.5, 0.9], respectively; affective score, − 1.7 [− 2.3, − 1.1], − 1.4 [− 2.6, − 0.2], − 1.5 [− 2.8, − 0.2], respectively; total score, − 5.4 [− 7.2, − 3.5], − 6.4 [− 10.2, − 2.6], − 3.8 [− 8.2, 0.6], respectively; and present pain intensity score, − 0.9 [− 1.3, − 0.6], − 0.9 [− 1.2, − 0.5], − 0.4 [− 1.1, 0.2], respectively.
The SF-MPQ subscale scores in patients with and without motor weakness were similar: sensory score, − 4.3 [− 5.8, − 2.8] vs − 2.7 [− 4.6, − 0.7]; affective score, − 1.7 [− 2.4, − 1.0] vs − 1.3 [− 1.9, − 0.6]; total score, − 6.0 [− 8.0, − 3.9] vs − 3.9 [− 6.5, − 1.4]; and present pain intensity score − 1.0 [− 1.3, − 0.7] vs − 0.6 [− 1.0, − 0.3].
Safety
The incidence of TEAEs was 87.2%, with a similar incidence observed among subgroups (Tables 4 and S4). The most common TEAEs were somnolence, peripheral edema, nasopharyngitis, and dizziness, and most TEAEs were mild or moderate. Peripheral edema was more common in patients with ischemic stroke than patients with hemorrhagic stroke, and less common in patients with the putamen as the stroke location than patients with stroke occurring in the thalamus or brainstem, and less common in patients with motor weakness than those without motor weakness.
Severe TEAEs occurred in 6 patients (6.4%), including cerebral hemorrhage (n = 2, 2.1%), cerebral infarction (n = 1, 1.1%), spinal compression fracture (n = 1, 1.1%), cholecystitis acute (n = 1, 1.1%), and hyperkalemia (n = 1, 1.1%); none of these events were considered related to mirogabalin treatment.
ADRs are shown in Table 5 and Table S5 in the electronic supplementary material. Among all patients, the proportion with at least one ADR was 54.3%, and the most common ADRs were somnolence (25.5%), peripheral edema (13.8%), dizziness (11.7%), and weight gain (6.4%). No severe ADRs were reported, and similar types and frequencies of ADRs were observed among the subgroups.
Discussion
The present study evaluated the efficacy of mirogabalin specifically in patients with CPSP, excluding patients with other types of post-stroke pain (e.g., shoulder pain, spasticity, or headache). To our knowledge, there have been no prior reports detailing the analgesic effect of mirogabalin on CPSP according to patient background factors. Moreover, few trials have investigated the long-term outcomes of patients with CPSP. Therefore, this post hoc subgroup analysis is the first to examine the long-term efficacy and safety of oral pharmacotherapy for the treatment of CPSP by stroke type, stroke location, and presence of motor weakness. We found that mirogabalin had a consistent therapeutic effect across all subgroups evaluated, as shown by similar changes from baseline at 52 weeks in SF-MPQ VAS scores and subscale scores. Furthermore, mirogabalin was generally tolerable in patients with CPSP regardless of background factors. These findings show that mirogabalin is both effective and has an acceptable safety profile in patients with CPSP, regardless of background factors such as stroke type, stroke location, presence/absence of motor weakness, time since stroke, or duration of CPSP.
Pregabalin has been reported to be an effective treatment for CPSP [10, 14,15,16,17,18] and is recommended by the European Federation of Neurological Societies treatment guidelines [13]. However, the accessible published studies often have differences in study design, such as different assessment measurements or treatment periods, which limits the suitability for historical comparison with the current study. One pregabalin study showed a significant analgesic effect and had a small sample size and patients with mixed CNeP, yet the article did not report results specifically from those with CPSP only [23]. Another previous pregabalin study found no significant improvement compared with placebo for patients with CPSP [17]. One open-label, long-term study of pregabalin in patients with CNeP (CPSP, n = 60; spinal cord injury, n = 38; and multiple sclerosis, n = 5) [16] had a similar study design to ours, with both being single-arm studies lacking a placebo control. Results from the pregabalin study demonstrated a mean (SD) change from baseline of − 20.1 (25.2) mm in the SF-MPQ VAS [16]; this was similar to the observed mean (SD) change in SF-MPQ VAS score from baseline at week 52 (LOCF) in our analysis (− 17.0 [25.0] mm). Furthermore, the pregabalin study reported mean (SD) changes from baseline at 52 weeks of − 4.4 (6.5) for the sensory pain score; − 1.3 (2.8) for the affective pain score; − 5.7 (8.6) for the total score; and − 0.8 (1.0) for present pain intensity score [16], which are similar to the findings in this analysis (mean [SD] change in sensory score, affective score, total score, and present pain intensity score − 3.6 [6.2], − 1.5 [2.7], − 5.1 [8.4], and − 0.8 [1.2], respectively). Some subgroups in our analysis, such as those with a stroke location of the thalamus or the brainstem, had an increase in VAS score between weeks 48 and 52. This may be partly explained by the reduction in mirogabalin dose between weeks 51 and 52 according to the study protocol. Our data indicate that mirogabalin was generally effective for the treatment of a broad range of patients with CPSP, which may underscore the importance of mirogabalin in addressing the unmet needs of post-stroke pain management.
The open-label, long-term study of pregabalin reported a TEAE rate of 87.4%, with the most commonly reported TEAEs being somnolence, weight gain, dizziness, and peripheral edema [16]. The types of TEAEs in our analysis were similar to those in the pregabalin study. Furthermore, most TEAEs and ADRs observed in this analysis were mild or moderate, and no severe ADRs occurred. Overall, our results indicate that mirogabalin had a consistent safety profile for patients with CPSP, regardless of the type of stroke, stroke location, presence or absence of motor weakness, time since stroke, or duration of CPSP.
Limitations
As this study had an open-label design, there was no control group. This study was a post hoc analysis and had a relatively small number of cases in each subgroup; therefore, no statistical comparisons between subgroups were conducted. Motor weakness was determined objectively by the attending physician, but was not graded in severity. Further study is needed to determine whether the effects of mirogabalin on CPSP are related to the severity of motor deficits. CPSP manifests as various pain symptoms, such as hyperpathia and allodynia. Therefore, future studies may clarify whether the effects of mirogabalin on CPSP differ depending on specific pain symptoms. We did not evaluate QOL or activities of daily living in this study. Specific conditions under which patients completed self-assessment of pain via the SF-MPQ were not pre-specified, which may have introduced some variability into the efficacy results. However, the fact that the SF-MPQ was measured at multiple time points over the 52-week study period suggests that such variation would not significantly change the interpretation of the efficacy results. Patients with severe renal impairment (CrCL < 30 mL/min), acute CPSP, or severe stroke were excluded; thus, the safety and efficacy of mirogabalin in such patients was not confirmed. Furthermore, all patients were Japanese, which may limit the generalizability of the findings to other racial groups.
Conclusions
This post hoc subgroup analysis found that the efficacy and safety of mirogabalin for the treatment of CPSP remained consistent within patient subgroups with different background factors such as type of stroke, stroke location, presence/absence of motor weakness, time since stroke, and duration of CPSP. Despite the lack of a control group and the relatively small sample size, our results provide valuable insight into the potential of mirogabalin for management of CPSP. As CPSP is a common yet intractable condition affecting patients with stroke, mirogabalin represents a promising treatment option.
Data Availability
Deidentified individual participant data and applicable supporting clinical study documents are available upon reasonable request at https://vivli.org/. In cases where clinical study data and supporting documents are provided pursuant to company policies and procedures, Daiichi Sankyo Co., Ltd. will continue to protect the privacy of clinical study participants. Details on data sharing criteria and the procedure for requesting access are available at https://vivli.org/ourmember/daiichi-sankyo/.
References
Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17:18–29.
Ramos-Lima MJM, Brasileiro IC, Lima TL, Braga-Neto P. Quality of life after stroke: impact of clinical and sociodemographic factors. Clinics (Sao Paulo). 2018;73:e418.
Strilciuc S, Grad DA, Radu C, et al. The economic burden of stroke: a systematic review of cost of illness studies. J Med Life. 2021;14:606–19.
Andrew NE, Kilkenny M, Naylor R, et al. Understanding long-term unmet needs in Australian survivors of stroke. Int J Stroke. 2014;9:106–12.
Kim KT, Chang WK, Jung YS, et al. Unmet needs for rehabilitative management in common health-related problems negatively impact the quality of life of community-dwelling stroke survivors. Front Neurol. 2021;12:758536.
Chen T, Zhang B, Deng Y, Fan JC, Zhang L, Song F. Long-term unmet needs after stroke: systematic review of evidence from survey studies. BMJ Open. 2019;9:e028137.
Xiong F, Shen P, Li Z, et al. Bibliometric analysis of post-stroke pain research published from 2012 to 2021. J Pain Res. 2023;16:1–20.
Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009;8:857–68.
Watson JC, Sandroni P. Central neuropathic pain syndromes. Mayo Clin Proc. 2016;91:372–85.
Hosomi K, Seymour B, Saitoh Y. Modulating the pain network–neurostimulation for central poststroke pain. Nat Rev Neurol. 2015;11:290–9.
O’Donnell MJ, Diener HC, Sacco RL, Panju AA, Vinisko R, Yusuf S. Chronic pain syndromes after ischemic stroke: PRoFESS trial. Stroke. 2013;44:1238–43.
Liampas A, Velidakis N, Georgiou T, et al. Prevalence and management challenges in central post-stroke neuropathic pain: a systematic review and meta-analysis. Adv Ther. 2020;37:3278–91.
Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113.
Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–73.
Kalita J, Chandra S, Misra UK. Pregabalin and lamotrigine in central poststroke pain: a pilot study. Neurol India. 2017;65:506–11.
Onouchi K, Koga H, Yokoyama K, Yoshiyama T. An open-label, long-term study examining the safety and tolerability of pregabalin in Japanese patients with central neuropathic pain. J Pain Res. 2014;7:439–47.
Kim JS, Bashford G, Murphy TK, Martin A, Dror V, Cheung R. Safety and efficacy of pregabalin in patients with central post-stroke pain. Pain. 2011;152:1018–23.
Bo Z, Jian Y, Yan L, et al. Pharmacotherapies for Central Post-Stroke Pain: a systematic review and network meta-analysis. Oxid Med Cell Longev. 2022;2022(18):3511385.
Mirogabalin besylate (TarligeⓇ) tablets [package insert, version 7]. Daichii-Sankyo Co., Ltd.; Tokyo, Japan: 2023. https://pins.japic.or.jp/pdf/newPINS/00070625.pdf. [In Japanese]. Accessed 13 Mar 2024.
Ushida T, Katayama Y, Hiasa Y, et al. Mirogabalin for central neuropathic pain after spinal cord injury: a randomized, double-blind, placebo-controlled, phase 3 study in Asia. Neurology. 2023;100:e1193–206.
Ushida T, Katayama Y, Hiasa Y, et al. Long-term safety and efficacy of mirogabalin for central neuropathic pain: a multinational, phase 3, 52-week, open-label study in Asia. Pain Ther. 2023;12:963–78.
Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7.
Vranken JH, Dijkgraaf MG, Kruis MR, van der Vegt MH, Hollmann MW, Heesen M. Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain. 2008;136:150–7.
Acknowledgements
The authors thank the staff of all participating institutions for their support of this research, and the patients themselves.
Medical Writing, Editorial, and Other Assistance
The authors thank Hannah Read, PhD, of Edanz (http://www.edanz.com), for providing medical writing support, which was funded by Daiichi Sankyo Co., Ltd., in accordance with Good Publication Practice guidelines (https://www.ismpp.org/gpp-2022).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This research was funded by Daiichi Sankyo Co., Ltd., Tokyo, Japan, which also funded the Rapid Service Fees for publication of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualization of the study, the methodology, review and editing of the manuscript, and visualization of the published work. Koichi Hosomi helped write the original draft and supervised the study. Yoichi Katayama and Takahiro Ushida supervised the study. Hiroshi Sakoda helped write the original draft, and managed the entire project. Kunika Kikumori verified the data, performed the formal analysis, helped write the original draft, and contributed to project administration and funding acquisition. Masanori Kuroha verified the data, helped write the original draft, supervised the study, and contributed to project administration and funding acquisition. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Conflict of Interest
Koichi Hosomi received lecture fees, consulting fees, and contract research funds from Daiichi Sankyo Co., Ltd., lecture fees outside the submitted work from Medtronic Japan Co., Ltd., Boston Scientific Japan K.K., and Nippon Zoki Pharmaceutical Co., Ltd., consulting fees outside the submitted work from Teijin Pharma Ltd., Araya Inc., and the Japanese Society of Psychiatry and Neurology, travel expenses outside the submitted work from Integra Japan Co., Ltd., and grants outside the submitted work from the Japan Agency for Medical Research and Development, Japan Society for the Promotion of Science, and Taiju Life Social Welfare Foundation. Takahiro Ushida received lecture fees and scholarship fees from Daiichi Sankyo Co., Ltd., and lecture fees outside the submitted work from Asahi Kasei Pharma Corp., AbbVie G.K., Viatris Inc., Eisai Co., Ltd., Suzuken Co., Ltd., Shionogi & Co., Ltd., Taisho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Teijin Pharma Ltd., Nipro Corp., Eli Lilly Japan K.K., Nippon Zoki Pharmaceutical Co., Ltd., Hisamitsu Pharmaceutical Co., Inc., Pfizer Japan Inc., Boston Scientific Japan K.K., Mundipharma K.K., Medical QOL Co., Ltd., Janssen Pharmaceutical K.K., Tsumura & Co., Taisho Toyama Pharmaceutical Co., Ltd., and Mitsubishi Tanabe Pharma Corp. The institution to which Takahiro Ushida is affiliated (Aichi Medical University) received research funding outside the submitted work from Nippon Zoki Pharmaceutical Co., Ltd., Kyoto University, Pascal Universe Co., Ltd., and BonBon Inc., and scholarship fees outside the submitted work from Astellas Pharma Inc., AbbVie G.K., Osuga Clinic, Zenki Clinic, Clinics of Youteikai, Kowa Medical Society, Otsuka Pharmaceutical Co., Ltd., Tsumura & Co., Shionogi & Co., Ltd., Daiichi Sankyo Co., Ltd., Taisho Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Nipro Corp., Eli Lilly Japan K.K., Pfizer Japan Inc., Boston Scientific Japan K.K., and Mochida Pharmaceutical Co., Ltd. Yoichi Katayama has no conflicts of interest to declare. Hiroshi Sakoda, Kunika Kikumori, and Masanori Kuroha are employees of Daiichi Sankyo Co., Ltd., which funded this study.
Ethical Approval
The study was conducted in compliance with the ethical principles that have their origins in the Declaration of Helsinki, the International Council for Harmonisation Good Clinical Practice guidelines, and the Japanese Ministry of Health, Labour and Welfare. The study protocol, protocol amendments, informed consent forms, and information sheets were approved by the relevant independent ethics committees or institutional review boards at each study center [21], and all patients provided written informed consent prior to participating in the study.
Additional information
Prior Publication: Data from the double-blind, placebo-controlled phase of this study were previously published in Neurology, 2023;100:e1193–206. Data from the long-term, open-label extension study were previously published in Pain Therapy, 2023;12:963–78.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hosomi, K., Katayama, Y., Sakoda, H. et al. Usefulness of Mirogabalin in Central Neuropathic Pain After Stroke: Post Hoc Analysis of a Phase 3 Study by Stroke Type and Location. Pain Ther (2024). https://doi.org/10.1007/s40122-024-00616-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40122-024-00616-3