Abstract
Introduction
To determine risk factors associated with postoperative cerebrospinal fluid leaks (CSFLs) after intrathecal drug delivery system (IDDS) and external pump implantation.
Methods
The clinical data of 248 patients with advanced cancer who underwent IDDS implantation from January 2021 to December 2022 at the Department of Pain Medicine at the Hunan Cancer Hospital were retrospectively reviewed. Information regarding age, gender, height, weight, body mass index (BMI), tumour type, albumin levels, haemoglobin levels, history of diabetes and pre- and postoperative anti-tumour therapy was collected and analysed.
Results
Postoperative CSFLs occurred in 7 of 231 patients (3.30%). Statistical analysis indicated that gender, age, height, weight, BMI, tumour type, albumin levels, haemoglobin levels, history of diabetes, pre- and postoperative chemotherapy, pre- and postoperative radiotherapy, preoperative immunotherapy and postoperative targeted therapy were not independent factors for CSFLs. Preoperative targeted therapy [odds ratio (OR): 16.64; 95% confidence interval (CI): 1.42, 195.56; P = 0.01] and postoperative immunotherapy (OR: 13.38; 95% CI: 1.60, 111.65; P = 0.017) were factors associated with an increased postoperative CSFL rate. Of the two locations where CSFLs can occur, the back (puncture site of catheter, n = 4) and the hypochondriac region (location of infusion port implanted, n = 3), back CSFLs occurred earlier than in the hypochondriac region (18.25 ± 6.45 vs 115 ± 62.02 days, P = 0.032).
Conclusion
Based on the data from our study, the timing of preoperative targeted therapy and postoperative immunotherapy should be considered to prevent the occurrence of CSFLs in cancer pain patients who have an IDDS and external pump.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Postoperative cerebrospinal fluid leaks (CSFLs) are among the severe complications for patients who have had intrathecal drug-delivery system (IDDS) with external pump implantation. |
Determining the risk factors associated with CSFLs will reduce the incidence of this potentially preventable complication. |
Preoperative targeted therapy and postoperative immunotherapy were associated with an increased postoperative CSFL rate in patients receiving IDDS and external pump implantation. |
CSFLs occurred earlier at the catheter's puncture site compared to the infusion port's location. |
Introduction
Cancer cases are predicted to reach 28.4 million by 2040 worldwide, a 47% increase from 2020 [1]. However, there is an increasing survival rate due to modern cancer treatments. Pain is among the most common and difficult to control symptoms in cancer patients and is associated with substantial healthcare costs. More than one-third of cancer patients suffer moderate and severe pain during oncological treatment and palliative care [1]. Most cancer pain can be managed following the three-step analgesic ladder guideline, but 10–15% of patients with refractory cancer pain suffer considerably despite standardized drug treatment [2]. Severe pain can cause cancer patients to refuse follow-up treatments and can cause symptoms such as anxiety and depression that significantly affect their prognosis and overall quality of life. Therefore, satisfactory pain control is necessary for all patients with cancer pain.
Intrathecal therapy is an alternative to standard medical management for cancer patients with refractory pain [3]. Studies and reviews have shown that intrathecal drug-delivery systems (IDDS), as an invasive management strategy, can provide better analgesia while reducing the consumption of opioids and possible side effects [4, 5]. Intrathecal therapy delivers medication directly into the intrathecal space of the spinal column via an indwelling catheter connected to an implanted reservoir controlled by a programmable pump that can be implanted or attached externally (Fig. 1). This therapy has been widely utilized in patients with chronic pain and spasticity [6, 7].
Initially, IDDS was only recommended for patients with a life expectancy > 3 months because of the costs [8]. However, a recent study from the Cleveland Clinic has found intrathecal patient-controlled analgesia (PCA) to be cost-effective and resulted in better patient satisfaction [9]. Stearns et al. [10] suggest that IDDS with an external pump is the best and most cost-effective choice for patients with a short life expectancy [11]. For several reasons, including economics, IDDS devices with external pumps are widely used in China.
The first intrathecal therapy with morphine for cancer pain analgesia was reported in 1979 [12]. During the past 30 years, the complications of IDDS have been well studied. Complications include device failures, problems related to medication administration, infection and catheter kinking and fracture [13, 14]. However, studies focusing on complications of IDDS with external pumps are limited.
The Pain Department at the Hunan Cancer Hospital has a large volume of patients whose pain is managed by IDDS with external pumps. One significant complication reported in patients who have had implanted IDDS with external pumps for an extended period is postoperative cerebrospinal fluid leaks (CSFLs). CSFLs can cause postural headaches and even life-threatening meningitis. Patients with CSFLs must undergo surgery to remove the implanted device to stop the CSFL, and the patients also lose the benefit of this type of pain control.
At this time, risk factors for developing CSFLs in patients with IDDS and external pumps are unknown. Currently, there are rare published reports of CSFL-related complications following the implantation of intrathecal catheters and external pumps in cancer patients with pain. Based on our internal data, there were two locations where CSFLs occur in patients with IDDS with external pumps. One site is located on the back of patients at the puncture site of the catheter. The other site is located in the hypochondriac region where the infusion port is implanted.
This study aims to determine the locations and how often CSFLs occur, emphasizing the risk factors associated with CSFLs after placement of an IDDS and external pump. Furthermore, this study is intended to understand CSFLs better, hopefully allowing clinicians to reduce the incidence of this potentially preventable complication.
Methods
Study Design
The study is designed to determine potential risk factors for the development of postoperative CSFLs after IDDS implantation and external pump use in patients with advanced cancer pain. This is a single-center retrospective observational study, and the study protocol was reviewed and approved by the Academic Committee of Hunan Cancer Hospital (2023 Scientific Research Quick Review No. 59). As this is a retrospective study and most patients have passed away or lost contact, informed consent was exempted.
Participants
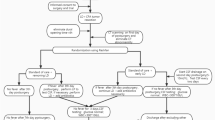
This study was a monocentric retrospective study performed at the Hunan Cancer Hospital. Data from patients who underwent IDDS implantation and external pump from January 1, 2021, to December 31, 2022, were utilized. All patients were treated by the same team. All patients’ medical records and operative notes were reviewed in detail, and all patients were followed up for at least 3 months. Patients who did not use IDDS for pain relief or had incomplete data were excluded from the study set (17 patients were excluded).
Surgical Procedure
A (SOPHYSA Soph-A-Port®-20210 Implantable access ports and accessories) needle was inserted into the subarachnoid space at the L2–L3 or L3–L4 level using a direct approach. A silicone catheter was inserted through the puncture needle, and a small incision was made next to the puncture site. An incision in the hypochondrium was made, and the subcutaneous tissue was locally expanded until the infusion port could be easily placed. A guide needle was then used to establish a subcutaneous tunnel between two incisions, and the silicone tube was guided to the rib incision and connected to the infusion port. The two incisions were then sutured. Next, a 22G non-coring needle (BBraun Winged Surecan®-04448383) was inserted through the skin into the infusion port, and the other end of the needle was connected to the external infusion pump (REHN(M01) Ai-PCA®).
Variables
The following information about the patients was recorded: gender, age, height, weight, BMI, tumour type, albumin levels, haemoglobin levels, type of cancer, history of diabetes, preoperative chemotherapy, postoperative chemotherapy, preoperative radiotherapy, postoperative radiotherapy, preoperative immunotherapy, postoperative immunotherapy, preoperative targeted therapy, and postoperative targeted therapy. Targeted therapy includes: epidermal growth factor receptor (EGFR)-Tyrosine kinase inhibitors (TKI) and/or Anti-vascular endothelial growth factor (VEGF) inhibitors. Immunotherapy includes: programmed death-1 (PD-1), programmed death-ligand (PD-L1), and cytotoxic T lymphocyte associated proteins (CTLA)-4. Diagnostic criterion for CSFLs was that clear fluid continued to flow from the dorsal incision or the puncture needle hole in the hypochondriac region (Fig. 2).
Statistical Analysis
Continuous data are described by mean ± standard deviation (SD), and categorical data are described by counts and percentage. As CSFLs rarely occurred, the categorical and continuous variables were compared between the subjects with and without CSFLs using a Fisher’s exact test and a Student’s t-test, respectively. Univariate binary logistic regression analysis assessed the association between sociodemographic and clinical factors and CSFLs. The model categorized BMI into three ranges: ≤ 18.4, 18.5–23.9, and ≥ 24. Albumin < 40 was classified as below normal. Haemoglobin < 120 for males and < 110 for females was considered below normal. Integrating relevant research findings, data-driven results and the perspectives of clinical expert teams, seven variables (BMI, albumin, diabetes, postoperative chemotherapy, preoperative targeted therapy, postoperative targeted therapy, postoperative immunotherapy) were included in a multivariable Firth's penalized-likelihood logistic regression model to explore independent factors affecting CSFLs. A Mann-Whitney U test was used to assess time differences between the occurrence of CSFLs in the hypochondrium and back. The statistical analyses were performed using the SAS v.9.4 (SAS Institue Inc, Cary, NC, USA) and R software (version 4.1.2, R Foundation for Statistical Computing, Vienna, Austria). Significance tests were two-tailed, and P values < 0.05 were statistically significant.
Results
A total of 248 advanced cancer patients underwent IDDS implantation and were screened for inclusion in the study. Seven patients experienced CSFLs. Four patients were excluded because the IDDS implantation was not for pain relief; 13 patients were excluded because of missing data. None of the excluded patients experienced CSFLs. Ultimately, 231 patients were included in the analysis. The proportion of CSFLs was 3.30% overall. Table 1 summarizes selected demographic and clinical characteristics of the study patients. There were 109 female patients and 122 male patients. The average patient age at the time of IDDS insertion was 57.49 (57.49 ± 12.03) years old. The average patient BMI was 20.32 (20.32 ± 3.04) kg/m2, and they had an average height of 161.81 (161.81 ± 7.33) cm. The average patient weight was 53.20 (53.20 ± 8.57) kg. The average laboratory values were albumin level 34.29 (34.29 ± 4.43) g/l and haemoglobin level 104.25 (104.25 ± 20.39) g/l. The three most common cancer types were lung, colorectal and gynaecological cancers, accounting for 31.6%, 12.6% and 12.1% of cases, respectively. Of the patients, 9.1% (21) had a history of diabetes, 51.1% (118) had received preoperative chemotherapy, 11.3% (26) had received postoperative chemotherapy, 33.3% (77) had received preoperative targeted therapy, 17.7% (41) had received postoperative targeted therapy, 23.4% (54) had received preoperative immunotherapy, 11.3% (26) had received postoperative immunotherapy, 16.9% (39) had received preoperative radiotherapy and 2.6% (6) had received postoperative radiotherapy. Patients who had postoperative chemotherapy, preoperative targeted therapy, postoperative targeted therapy or postoperative immunotherapy had significantly more CSFLs compared to those patients who did not have any of these therapies (all P < 0.05) (Table 1).
As shown in Table 2, the univariate binary logistic regression suggested postoperative chemotherapy (OR: 6.55; 95% CI: 1.38, 31.13), preoperative targeted therapy (OR: 12.93; 95% CI: 1.53, 109.42), postoperative targeted therapy (OR: 6.74; 95% CI: 1.45, 31.37) and postoperative immunotherapy (OR: 12.24; 95% CI: 2.57, 58.28) were all positively associated with the risk of CSFLs. No association was found for BMI, albumin levels, haemoglobin levels, type of cancer, history of diabetes, preoperative chemotherapy, preoperative immunotherapy, preoperative radiotherapy and postoperative radiotherapy (Table 2).
The factors were analysed in the multivariable logistic model. According to the clinical experience and previous studies, we added BMI, albumin and diabetes as new variables, which is highly relevant to wound healing in clinic [15,16,17]. Hence, we conducted a cross-sectional study to collect data and explore the factors influencing CFSL, without conducting repeated measurements. The results suggested patients with a history of preoperative targeted therapy (OR: 7.46; 95% CI: 1. 39, 71.77) or postoperative immunotherapy (OR: 9.23; 95% CI: 1.41,81.86) were more likely to develop postoperative CSFLs. These two factors were independent factors affecting CSFLs (Fig. 3).
Among patients with CSFLs, 42.90% (3/7) occurred in the hypochondriac region, and 57.14% (4/7) occurred at the back incision. CSFLs at the back incision appeared earlier than those in the hypochondriac region (18.25 ± 6.45 vs. 115 ± 62.02 days; P = 0.032) (Fig. 4).
Discussion
Tumour progression and unrelieved refractory cancer pain affected patients’ physical and psychological states, destroying their confidence in their overall treatment. Intrathecal therapy is widely used among complicated pain patients, especially those with cancer-related pain. IDDS delivers medications locally to the intrathecal space, which increases clinical success, decreases pain ratings, reduces medication doses and eliminates most pain medication-related toxicity. The use of IDDS has also been linked to a longer survival time in cancer patients [18,19,20].
The external pump linked to the IDDS offers a continuous medication infusion that provides better pain control. Additionally, this type of pump allows the patient to administer boluses of medication to themselves in response to breakthrough pain while the pump delivers a baseline infusion. One of the most important benefits of this type of arrangement is that IDDS allows the administration of drugs that cannot be administered via any other route. This may also reduce the overall dose of opioids significantly [21]. However, IDDS also has its own complications, such as urinary retention and nausea and vomiting, which are common side effects and can be medically treated.
Prior studies have shown that common complications of an IDDS and implanted pump include dislodgement, kinking or fracture of the catheter, bleeding, neurological injury, infection and CSFLs after the catheter is removed [19, 22]. Catheter extraction from the intrathecal space often leaves a fistula that goes through the lumbodorsal fascia into the subcutaneous tissue. This is one of the leading causes of CSFLs in patients with IDDS and an implanted pump. However, few patients who have had IDDS implantation with an external pump have CSFLs. There have only been a few cases of a CSFL after IDDS implantation with an external pump in our department, but it is useful to identify any common risk factors. Once CSFLs occur, patients usually have to abandon intrathecal analgesia, which has been shown to be a useful treatment modality for many of our patients.
Our results demonstrated that 7 of the 231 patients studied from January 1, 2021, to December 31, 2022, had postoperative CSFLs. There were no statistically significant differences in the patients’ gender, age, height, weight, BMI, tumour type, albumin levels, haemoglobin levels or history of diabetes. It was also noted that pre- and postoperative chemotherapy, pre- and postoperative radiotherapy, preoperative immunotherapy and postoperative targeted therapy were not independent factors for CSFLs. At the end of the analysis, only preoperative targeted therapy and postoperative immunotherapy were shown to be independent factors associated with an increased postoperative CSFL rate. In our study, target therapy in CSFL patients includes EGFR-TKIs, like osimertinib, anlotinib and cetuximab; the most common anti-VEGF inhibitor is bevacizumab. Immunotherapies applied in CSFL patients are sintilimab (PD-1), navulizumab (PD-1), adebrelimab (PD-L1) and durvalumab (PD-L1). As most of our patients have advanced cancer, chemotherapy was standard treatment, and radiotherapy was usually palliative.
Bevacizumab is an anti-VEGF inhibitor, which has been widely applied in patients with advanced solid tumours. VEGF is expressed in almost all organs and plays a fundamental physiological role. Anti-angiogenesis agents that target the VEGF/VEGF receptor pathway have become an important part of standard therapy in the treatment of multiple cancers [23]. Activation of the HIF-1α/VEGF/VEGFR2 pathway promotes wound healing by increasing angiogenesis, while reduced angiogenesis may result in delayed wound healing [24]. In a study of bevacizumab in the treatment of glioblastoma multiforme, delayed wound healing occurred in first- and (6.7%), second-line treatment (2.9%) and beyond [25]. Preoperative VEGF targeted therapy may have caused delayed healing of the dura mater and any incision, which could have led to the development of CSFLs. In contrast to preoperative targeted therapy, postoperative targeted therapy did not appear to be associated with an increased risk of CSFLs. This may because postoperative targeted therapy would not have a significant impact on tissue that had already healed.
This study also showed that postoperative immunotherapy is an associated risk factor for increased postoperative CSFLs. Wang et al. have showed that PD-L1 is dynamically expressed in wound granulation tissue, and PD-L1 deficiency leads to increased local inflammation and delayed wound healing. Therefore, applying PD-L1 inhibitor in cancer patients could affect wound healing [26]. PD-1/PD-L1 pathway is very important to tumour immune surveillance. PD-1/PD-L1 signalling also plays a critical role in the occurrence and development of inflammatory and immune diseases [27]. Furthermore, it probably enhanced local inflammation effects and attacked implants in our CSFL cases. However, the mechanism of immunotherapy related to increases in CSFLs still requires further research.
Prior studies have noted that in endoscopic endonasal skull base surgery, the incidence of postoperative CSFLs ranges from 8.1 to 15.9% and has an increased incidence with elevated BMI [17, 28]. Increased BMI is also a risk factor for developing spontaneous CSFLs [29]. However, in our study, increased BMI was not associated with an increased incidence of CSFLs. One possible explanation is that few high BMI patients with advanced cancer require intrathecal analgesia, and the sample size may have been too small.
In a study including 200 children who underwent intrathecal baclofen pump implantation, the incidence of CSFLs was 11% [30]. In another study, the percentage of CSFLs was lower in the Ascender catheter group compared to the silicone catheter group (1.1% vs. 5.5%) [31]. Although the proportion of CSFLs in our study was low, there may ultimately be a benefit to using the Ascenda catheter rather than a silicone one.
This study demonstrated that there were two locations where CSFLs occurred, one located in the back at the point of the puncture site for the catheter and the other located in the hypochondriac region where the infusion port was implanted. According to the data, the time of CSFLs at the catheter puncture site was earlier compared to the CSFLs that occurred in the hypochondriac region. We deduced the potential reason for these CSFLs differing by location and timing was related to the fact that, in the early stage of healing, cerebrospinal fluid may have flowed from the back incision, which could have worsened the healing of the incision. Even more significantly, the catheter was exposed. If the CSFL was small, the back incision could still heal. In the presence of an existing leak, cerebrospinal fluid would flow along the catheter and pass through the lumbodorsal fascia into the subcutaneous tissue. CSF can flow out from the puncture site (Fig. 4). Hence, the amount of leakage might be the reason for the time difference between the two types of CSFLs.
Persistent CSFLs would not only disturb the dynamics of CSF circulation but could also lead to the occurrence of systemic positional headaches and dizziness and could further increase poor meningoencephalitis wound healing, even causing complications such as intracranial haemorrhages [32, 33]. Conservative treatment of CSFLs includes bed rest, increased fluid intake, simple analgesia and cephalexin [34]. Severe cases may require repositioning of the catheter, an epidural blood patch, purse-string sutures over the dura around the catheter [20] or even removal of the IDDS. Currently, based on our previous clinical experience, there was only one case of successful treatment of CSFLs without withdrawing IDDS. Preventing the occurrence of CSFLs is particularly important. From our study, we find preoperative target therapy and postoperative immunotherapy are risk factors associated with CSFLs after IDDS and external pump implantation. These data could aid physicians in developing treatment plans for those patients who have had preoperative target therapy and postoperative immunotherapy and are being considered for IDDS with an external pump. More research will ultimately be required to confirm whether our findings are supported and to aid in a search for possible mechanisms contributing to CSFLs.
This study was a single-centre retrospective study. To prevent the evolution of cancer therapy from effecting our outcomes, the study period was limited to 2 years. Therefore, the number of cases is relatively small. Large-sample multicentre studies may be required.
Conclusion
The results of this study suggest that preoperative targeted therapy and postoperative immunotherapy were associated with an increased postoperative CSFL rate in patients receiving an IDDS and external pump implantation. Moreover, CSFLs occurred earlier at the catheter's puncture site compared to the infusion port's location. Consequently, application of preoperative targeted therapy and postoperative immunotherapy in cancer pain patients who have an IDDS and external pump should be considered.
Data Availability
The datasets analyzed during the current study are not publicly available due to confidentiality principle of the Hunan Cancer Hospital.
References
Snijders RAH, Brom L, Theunissen M, van den Beuken-van Everdingen MHJ. Update on prevalence of pain in patients with cancer 2022: a systematic literature review and meta-analysis. Cancers (Basel). 2023. https://doi.org/10.3390/cancers15030591.
Gallagher R, Hawley P, Yeomans W. A survey of cancer pain management knowledge and attitudes of British Columbian physicians. Pain Res Manag. 2004;9(4):188–94. https://doi.org/10.1155/2004/748685.
Singh V, Manchikanti L, Benyamin RM, Helm S, Hirsch JA. Percutaneous lumbar laser disc decompression: a systematic review of current evidence. Pain Physician. 2009;12(3):573–88.
Bhaskar A. Interventional pain management in patients with cancer-related pain. Postgrad Med. 2020;132(sup3):13–6. https://doi.org/10.1080/00325481.2020.1807796.
The Polyanalgesic Consensus Conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation. 2017;20(4):405–6. https://doi.org/10.1111/ner.12618.
Hayek SM, Deer TR, Pope JE, Panchal SJ, Patel VB. Intrathecal therapy for cancer and non-cancer pain. Pain Physician. 2011;14(3):219–48.
Albright AL, Gilmartin R, Swift D, Krach LE, Ivanhoe CB, McLaughlin JF. Long-term intrathecal baclofen therapy for severe spasticity of cerebral origin. J Neurosurg. 2003;98(2):291–5. https://doi.org/10.3171/jns.2003.98.2.0291.
Christo PJ, Mazloomdoost D. Interventional pain treatments for cancer pain. Ann N Y Acad Sci. 2008;1138:299–328. https://doi.org/10.1196/annals.1414.034.
Brogan SE, Winter NB, Okifuji A. Prospective observational study of patient-controlled intrathecal analgesia: impact on cancer-associated symptoms, breakthrough pain control, and patient satisfaction. Reg Anesth Pain Med. 2015;40(4):369–75. https://doi.org/10.1097/aap.0000000000000251.
Stearns L, Boortz-Marx R, Du Pen S, Friehs G, Gordon M, Halyard M, et al. Intrathecal drug delivery for the management of cancer pain: a multidisciplinary consensus of best clinical practices. J Support Oncol. 2005;3(6):399–408.
Manchikanti L, Boswell MV, Datta S, Fellows B, Abdi S, Singh V, et al. Comprehensive review of therapeutic interventions in managing chronic spinal pain. Pain Physician. 2009;12(4):E123–98.
Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50(2):149–51. https://doi.org/10.1097/00000542-197902000-00013.
Follett KA, Naumann CP. A prospective study of catheter-related complications of intrathecal drug delivery systems. J Pain Symptom Manag. 2000;19(3):209–15. https://doi.org/10.1016/s0885-3924(99)00153-0.
Nagel SJ, Reddy CG, Frizon LA, Holland MT, Machado AG, Gillies GT, et al. Intrathecal therapeutics: device design, access methods, and complication mitigation. Neuromodulation. 2018;21(7):625–40. https://doi.org/10.1111/ner.12693.
Zhang X, Yuan L, Tan Z, Wu H, Chen F, Huang J, et al. CD64 plays a key role in diabetic wound healing. Front Immunol. 2024;15:1322256. https://doi.org/10.3389/fimmu.2024.1322256.
Cho SK, Mattke S, Gordon H, Sheridan M, Ennis W. Development of a model to predict healing of chronic wounds within 12 weeks. Adv Wound Care (New Rochelle). 2020;9(9):516–24. https://doi.org/10.1089/wound.2019.1091.
Dlouhy BJ, Madhavan K, Clinger JD, Reddy A, Dawson JD, O’Brien EK, et al. Elevated body mass index and risk of postoperative CSF leak following transsphenoidal surgery. J Neurosurg. 2012;116(6):1311–7. https://doi.org/10.3171/2012.2.Jns111837.
Smith TJ, Coyne PJ, Staats PS, Deer T, Stearns LJ, Rauck RL, et al. An implantable drug delivery system (IDDS) for refractory cancer pain provides sustained pain control, less drug-related toxicity, and possibly better survival compared with comprehensive medical management (CMM). Ann Oncol. 2005;16(5):825–33. https://doi.org/10.1093/annonc/mdi156.
Duarte R, Copley S, Nevitt S, Maden M, Al-Ali AM, Dupoiron D, et al. Effectiveness and safety of intrathecal drug delivery systems for the management of cancer pain: a systematic review and meta-analysis. Neuromodulation. 2022. https://doi.org/10.1016/j.neurom.2022.03.003.
Singh PK, Jain R, Mishra S, Kumar S, Bhatnagar S, Deo S. Management of pericatheter cerebrospinal fluid leak after intrathecal implantation of a drug delivery system. Am J Hosp Palliat Care. 2008;25(3):237–9. https://doi.org/10.1177/1049909108315520.
Dupoiron D. Intrathecal analgesia in cancer pain. Cancer Treat Res. 2021;182:225–37. https://doi.org/10.1007/978-3-030-81526-4_14.
Padalia D, Jassal N, Patel S. Near death experience from placement of an intrathecal catheter. Pain Physician. 2016;19(4):E621–3.
Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6(8):465–77. https://doi.org/10.1038/nrclinonc.2009.94.
Khandia R, Munjal A, Dhama K, Malik YS. Bacterial toxins: a hope towards angiogenic ailments. Curr Drug Metab. 2017;18(10):926–41. https://doi.org/10.2174/1389200218666170911150948.
Mamo A, Baig A, Azam M, Rho YS, Sahebjam S, Muanza T, et al. Progression pattern and adverse events with bevacizumab in glioblastoma. Curr Oncol. 2016;23(5):e468–71. https://doi.org/10.3747/co.23.3108.
Wang XH, Guo W, Qiu W, Ao LQ, Yao MW, Xing W, et al. Fibroblast-like cells promote wound healing via PD-L1-mediated inflammation resolution. Int J Biol Sci. 2022;18(11):4388–99. https://doi.org/10.7150/ijbs.69890.
Scandiuzzi L, Ghosh K, Hofmeyer KA, Abadi YM, Lázár-Molnár E, Lin EY, et al. Tissue-expressed B7–H1 critically controls intestinal inflammation. Cell Rep. 2014;6(4):625–32. https://doi.org/10.1016/j.celrep.2014.01.020.
Sun I, Lim JX, Goh CP, Low SW, Kirollos RW, Tan CS, et al. Body mass index and the risk of postoperative cerebrospinal fluid leak following transsphenoidal surgery in an Asian population. Singap Med J. 2018;59(5):257–63. https://doi.org/10.11622/smedj.2016159.
Gendeh BS, Salina H. Observation of spontaneous cerebrospinal fluid rhinorrhoea to body mass index: a preliminary report. Med J Malaysia. 2007;62(5):368–9.
Motta F, Buonaguro V, Stignani C. The use of intrathecal baclofen pump implants in children and adolescents: safety and complications in 200 consecutive cases. J Neurosurg. 2007;107(1 Suppl):32–5. https://doi.org/10.3171/ped-07/07/032.
Motta F, Antonello CE. Comparison between an Ascenda and a silicone catheter in intrathecal baclofen therapy in pediatric patients: analysis of complications. J Neurosurg Pediatr. 2016;18(4):493–8. https://doi.org/10.3171/2016.4.Peds15646.
Eser MT, Hanalioglu S, Cetiner MZ, Dinc S, Peker HO, Sorar M, et al. Identification of risk factors for postoperative cerebrospinal fluid leakage and comparison of two alternative dural augmentation techniques in posterior fossa and spinal surgeries. Turk Neurosurg. 2019;29(3):377–85. https://doi.org/10.5137/1019-5149.Jtn.24432-18.0.
Kinaci A, Moayeri N, van der Zwan A, van Doormaal TPC. Effectiveness of sealants in prevention of cerebrospinal fluid leakage after spine surgery: a systematic review. World Neurosurg. 2019;127:567-75.e1. https://doi.org/10.1016/j.wneu.2019.02.236.
Turnbull DK, Shepherd DB. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91(5):718–29. https://doi.org/10.1093/bja/aeg231.
Acknowledgements
We appreciate the data analysis by Dr. Songlin Zhu.
Funding
This work supported by the Funding of Hunan Provincial Health Commission, NO. 202204114480 and The Natural Science Foundation of Hunan Province, NO. 2022JJ80079. The Rapid Service fee will be funded by corresponding author.
Author information
Authors and Affiliations
Contributions
Yuan Li and Shuwu Zhao: data collection and data analysis. Chen Su: design this project, organization and manuscript preparation. Keith Candiotti: manuscript polishing and project consult.
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts of interest are reported from any of the author.
Ethical approval
The study was approved by the Academic Committee of Hunan Cancer Hospital (2023 Scientific Research Quick Review No. 59). As this is a retrospective study and most patients have passed away or lost contact, informed consent has been exempted.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, Y., Zhao, S., Candiotti, K. et al. Risk Factors Associated with Postoperative Cerebrospinal Fluid Leaks After Intrathecal Drug Delivery System and an External Pump Implantation in Cancer Patients: A Retrospective Study. Pain Ther 13, 637–650 (2024). https://doi.org/10.1007/s40122-024-00608-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-024-00608-3