Abstract
Introduction
Frozen shoulder is a very common musculoskeletal condition and the evidence related to the additional effects of extracorporeal shockwave therapy (ESWT) with intra-articular (IA) lidocaine injection in individuals with frozen shoulder is rare. Therefore, this study aims to compare and investigate the additional effects of extracorporeal shockwave therapy (ESWT) with intra-articular (IA) lidocaine injection in a frozen shoulder.
Methods
Sixty eligible participants with frozen shoulder were included and the active group (n = 30, age 52.12 ± 5.2 years) received a lidocaine injection (1% lidocaine (Xylocaine) and 2cc (80 mg) methylprednisolone acetate) with active ESWT (3.5 bar air pressure and 2000 pulses with an energy flux density (EFD) ¼ 0.16 mJ/mm2) three sessions a week for 4 weeks. The placebo group (n = 30, age 53.56 ± 5.5 years) received lidocaine injection with placebo treatment (a special head that blocked the shock waves) three sessions a week for 4 weeks. Both groups received progressive resistance exercises (PRE) to the shoulder muscles. The primary outcome was pain intensity, measured with the visual analogue scale. The other outcome measures were the thickness of the coracohumeral ligament (CHL) measured by magnetic resonance imaging (MRI), abduction, and lateral rotation range of motion (ROM), functional disability, kinesiophobia, depression status, and quality of life. Participants were assessed at baseline, after 4 weeks, 8 weeks, and at 6-month follow-up.
Results
The post-intervention at 4 weeks showed an improvement of 2.0 (CI 95% 1.71–2.28) in the active group compared to the placebo group. Similar effects were noted after 8 weeks (2.2) (CI 95% 1.91–2.48) and at the 6-month (1.9) (CI 95% 1.61–2.18) follow-up. Similar improvements were also found in the thickness of the CHL ligament (0.6) (CI 95% 0.46–0.73), abduction and lateral rotation (ROM) (– 23.6) (CI 95% – 27.47 to -19.72), (- 18.10) (CI 95% – 19.72 to – 16.47), functional disability (16.2) (CI 95% 14.85–17.54), kinesiophobia (11.0 (CI 95% 10.21–11.98), depression status (4.4) (CI 95% 4.03–4.76) and quality of life (0.9) (CI 95% 0.79–1.00) (p = 0.001) at the 6-month follow-up period, where mean estimates and their confidence intervals all included worthwhile effects. There were no adverse reactions or side effects noted in either the active or placebo groups during and after the treatment.
Conclusions
The study concluded that the addition of extracorporeal shockwave therapy after intra-articular lidocaine injection improves pain, functional disability, range of motion, kinesiophobia, depression status, and quality of life in people with frozen shoulder.
Trial Registration
https://ctri.nic.in, identifier; CTRI/2020/04/024834 prospectively registered on 24/04/2020.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The outcome of this study found the effect of lidocaine injection combined with active extracorporeal shockwave therapy in frozen shoulder patients. |
The outcome of this study found the effect of lidocaine injection combined with placebo extracorporeal shockwave therapy in frozen shoulder patients. |
The outcome of this study found differences in patient-centred outcomes between lidocaine injection combined with active extracorporeal shockwave therapy versus lidocaine injection combined with placebo extracorporeal shockwave therapy in frozen shoulder patients. |
This study provided better knowledge about this condition and gives clinical evidence for selecting the proper rehabilitation procedure in frozen shoulder patients. |
Introduction
Frozen shoulder is a very common musculoskeletal condition in the upper extremity characterized by gradual and progressive pain in and around the shoulder joint [1]. The pattern of restriction is characterized by a more pronounced limitation in lateral rotation, followed by restrictions in abduction and flexion. Studies show that the incidence of frozen shoulder is 3–5% for the general public and usually resolves within 12 months [2]. The exact etiology and the mechanism of frozen shoulder are idiopathic and not yet defined [2, 3]. Clinically, it is divided into three phases: the painful phase, the stiffening phase, and the thawing phase [4]. Generally, acute patients are treated with physiotherapy, painkillers, muscle relaxants, nonsteroidal anti-inflammatory drugs (NSAIDs), intra-articular steroid injections, and hydrodilatation techniques [5,6,7]. In chronic cases, manipulation under general anesthesia (MUA) and surgical interventions such as capsular release and arthroscopic correction procedures were performed [8, 9]. In physiotherapy, different approaches like thermotherapy, ultrasound, extracorporeal shockwave therapy (ESWT), interferential therapy (IFT), and different therapeutic exercises have been used [10,11,12].
Generally, the medical and surgical treatment will not solve the real pathology of the disease and has a possibility of drug reactions. Also, these are expensive treatment procedures, and not agreed to by most patients [13, 14]. However, the local administration of intra-articular lidocaine injection in the specific painful region is commonly used to treat this problem. It alters the release of endogenous substances and inhibits the formation of collagen fibers, extracellular matrix (ECM), and granulation tissue [15]. Nevertheless, lidocaine injections are discouraged by many clinicians because of the high recurrence rate and short-term effects [16]. From a clinical perspective, orthopedic surgeons encourage the application of intra-articular lidocaine injection followed by physiotherapy, and believe this is a better approach to treating the affected structures [6, 17]. However, the recent literature on these combined interventions shows contradictory results in improving pain and functional activities in treating frozen shoulder [18].
Physiotherapy interventions offered for frozen shoulder include hydrocolloid packs, infrared radiation, shortwave diathermy, ultrasound, laser, and physical exercises [19]. Extracorporeal shockwave therapy (ESWT) is one of the most successful treatment modalities used in physical therapy. It is a non-invasive treatment in which high-amplitude soundwaves are applied to the required part of the body. It is an effective method for stimulating tissue healing by stimulating fibroblast proliferation and differentiation into myofibroblasts. It enhances soft tissue healing, increases local blood circulation, facilitates the inflammatory-mediated healing process, and increases the flexibility of collagen fibers in the affected region [20].
Frozen shoulder can mimic other shoulder conditions such as bicipital tendinitis, supraspinatus tendinitis, rotator cuff tear, and acromioclavicular joint arthritis. Recent studies have shown that magnetic resonance imaging (MRI) can be used to find the thickening of the coracohumeral ligament (CHL) and joint capsule and the obliteration of fat under the coracoid process in frozen shoulder [21]. Therefore, along with regular investigation procedures, there is a need to find the MRI changes after lidocaine injection with shockwave therapy in frozen shoulder. So far, no studies have been conducted to find the additional effects (clinical and radiological changes) of ESWT after lidocaine injection in treating frozen shoulder. Therefore, this study aims to compare and investigate the additional effects (clinical (pain intensity) and radiological changes, MRI) of ESWT after lidocaine injection in treating a frozen shoulder. Radiological analysis through MRI will provide the changes in the soft tissues, which will provide sound evidence for the therapists and clinicians to select an optimum intervention for a frozen shoulder.
Method
Design, Setting, and Participants
This trial was a prospectively registered, randomized, placebo-controlled trial conducted at the Department of Physical Therapy, Prince Sattam bin Abdulaziz University, Al Kharj, Saudi Arabia. Participants were recruited between May 1, 2020 and Jan 31, 2023. The uniqueness of this study is its methodology, which is more comprehensive and understandable. This study was designed and conducted according to the principles of the Declaration of Helsinki 1964 and its later amendments. The study has been approved by the Department Ethics Committee (DEC) with an ethical approval number RHPT/020/013. Written informed consent was obtained from all the study participants as per the ethical guidelines. The study was registered prospectively in a trial registry with reference number CTRI/2020/04/024834 on 24/04/2020.
An orthopedic surgeon with 20 years of clinical experience had diagnosed the frozen shoulder (International Classification of Diseases 10th revision [ICD-10] group M75.1-M75.8, M19.8) patients as per the frozen shoulder diagnosing criteria recommended by American Orthopedic Association (AOA) [1]. Participants that were between 18 and 60 years of age, had pain intensity of 3–8 on the visual analogue scale (VAS), and pain duration of 4–12 months (stiffening stage—pain starts to subside, progressive loss of glenohumeral motion in the capsular pattern) were chosen to participate in the study. Excluded from the study were any participants with prior steroid injection therapy, associated neck or arm pain, glenohumeral osteoarthritis, severe musculoskeletal, neural (Parkinson's disease), cardiovascular (heart disease and stroke) somatic and psychiatric problems, metabolic diseases, waiting for any surgery, having medications, alcohol or drug abuse, or involved in any weight training programs. In addition, participants with other soft tissue injuries, fractures at the upper limb, or those that were immobilized for a prolonged period were also excluded from the study.
All participants received a rehabilitation referral letter from the referring hospital to participate in the trial. They also received a study pamphlet detailing the procedure for participating in the study. After signing the written informed consent form, participants were randomized through a computer-generated randomization method and allocated into two groups: lidocaine injection with ESWT—Active group (n = 30, age 52.12 ± 5.2, male-14, female-16) and lidocaine injection with ESWT—Placebo group (n = 30, age 53.56 ± 5.5, male-13, female-17). Participants were allocated by a physiotherapy assistant through an on-site computer system in which allocation was concealed. The person enrolling the participants should ideally not be the same person generating the sequence to prevent manipulation. Each participant’s group allocation was only informed to the treating therapist immediately before the first intervention. The participants were not aware of which treatment they were receiving (blind participants); however, they were informed that they would receive one of the two interventions. Due to the nature of the interventions, it was not possible to blind the therapist who treated the patients. Both groups received the concerned intervention for a period of three sessions per week for 4 weeks. The primary and secondary outcome measures were collected by a blinded therapist at baseline after 4 weeks, 8 weeks, and at 6 months of follow-up and the methods were followed as per our previous study [22].
Interventions
Lidocaine Injection
An orthopedic surgeon conducted a regular physical and orthopedic examination before the administration of the injection. All the participants were treated with a posterior approach intra-articular injection containing a mixture of 5 cc of 1% lidocaine (Xylocaine) and 2 cc (80 mg) methylprednisolone acetate (Depo-Medrol) injected at a 90° angle via a sterile 18-gauge spinal needle [23]. They were asked to rest and not engage in strenuous activities for 1 week following the injection, even if they experienced pain relief. Any adverse consequences such as allergic reactions, nausea, vomiting, and unusual tiredness and weakness were noted and treated by the treating doctor.
Physical Therapy
One week after receiving the injection therapy, all the participants were allowed to take physical therapy interventions by a licensed physical therapist with 15 years of clinical experience in treating shoulder conditions. Participants in both groups received physical therapy treatment for three sessions per week for 4 weeks and each session lasted for 30–40 min. To avoid intervention bias, a fixed physiotherapy protocol (Fig. 1 Permission from the patient has been received) was prepared.
Initially, the calibration of the ESWT (Zimmer, enPuls Version 2.0, Junkersstrabe, Germany) was done by an expert to obtain better and more consistent output from the device. In the active ESWT group, the participant was asked to sit with the shoulder passively abducted at 80°, the elbow flexed at 90°, and the forearm rested on a flat surface. The treatment started with 250 “warm up” pulses at 1.5 bar of air pressure, which accommodates the participant for the radial ESWT treatment. Once the patient was comfortable with the treatment, the air pressure was increased to 3.5 bar and 2000 pulses with an energy flux density (EFD) ¼ 0.16 mJ/mm2, and the impulses were applied with a 15-mm applicator at a frequency of 8 Hz of dose and were administered in two shoulder regions. The first 1000 impulses were applied in an anterior-to-posterior direction at the anterior shoulder joint, and the upper margin of the treatment zone was about one finger’s breadth lateral to the coracoid process (Fig. 2a). The remaining 1000 impulses of the total 2000 impulses per session were applied in a posterior-to-anterior direction on the posterior side of the shoulder joint located beneath the lateral border of the scapular spine (Fig. 2b) [20]. For the placebo group, the same set of treatments was provided but in providing ESWT, a special head that blocked the shockwaves from occurring was used but it was indistinguishable to the study participants.
Progressive resistance exercises (PRE) (Fig. 1) were prescribed with a Thera band (THERABAND, Akron, OH, USA) for the shoulder muscles based on the assessment of individual muscles and personally tailored. Initially, the painful movements are trained with minimal resistance and then progress to the next level of resistance for the other joint movements. The therapist selected the exercise parameters (intensity, frequency, and duration) in every treatment session purely based on the individual capacities without exaggerating the symptoms. Throughout the treatment session, the participants were instructed to follow the correct form and posture to facilitate healing [24]. The patients were instructed to perform the home exercises daily during the 4 weeks of intervention and after 4 weeks of intervention [pendulum exercise, cane exercises (up, back, and out), wall slides (forward and side) three times 30 repetitions] and isolated stretching of shoulder muscles (shoulder stretch, towel stretch, chest stretch, inferior capsule stretch and sleeper stretch three times daily for 30 s). The treatment adherence at home was monitored by a treating therapist by checking the exercise logbook and frequent contact with the patient through the WhatsApp application.
Outcome Measures
Pain intensity was measured with a visual analogue scale (VAS) and the participant was asked to note the perceived pain intensity on the 10-cm point scale, where scores ranged from 'no pain' (0) to 'worst imaginable pain' (10). VAS is considered a moderate-to-good (r = 0.60–0.77) reliable tool for measuring pain intensity in frozen shoulder patients. [25]
Magnetic resonance imaging (MRI) has been established as a reliable and valid assessment tool for measuring the thickening of coracohumeral ligaments in frozen shoulder patients. It was performed with a 3.0-T MR unit (Siemens Medical Solutions, Germany) with a phased-array surface coil (Philips, Netherlands) centered over the glenohumeral joint and strapped in place. The arm position was standardized, with the thumb pointing upward in a neutral position. In the sagittal oblique plane, parallel to the glenohumeral joint (550/15, 3-mm section thickness, 0.3-mm intersection gap, 1,806,180-mm field of view, 5,126,512 matrix size) T1-weighted sections were taken. [26]
Range of motion Shoulder abduction and lateral rotation were measured with a universal goniometer. The goniometric passive ROM measurements for the shoulder appear to be highly reliable when taken by the same physical therapist, regardless of the size of the goniometer used [27].
Functional disability The Disabilities of the Arm, Shoulder and Hand questionnaire (Quick-DASH) was used to measure the upper limb physical disabilities and symptoms in frozen shoulder. It contains 11 items, which are measured in a five-point Likert scale and summarized into a total score from 0 ‘no disability’ to 100 ‘most severe disability. The Quick-DASH has adequate reliability, validity, and the ability to measure changes in disability among people with shoulder problems [28].
Kinesiophobia The Tampa Scale for Kinesiophobia-adjusted version (TSK-AV) was used to measure the status of fear of injury. The scale consists of 13 items, which are marked on a four-point Likert scale (strongly disagree, disagree, agree, strongly agree). Getting a maximum score indicates more fear of injury and less score such as 13 indicates less fear of injury. The scale shows a high level of test–retest reliability (ICC = 0.887) and moderate validity [29].
Depression The Hospital Anxiety and Depression Scale (HADS) was used to measure the depression status of frozen shoulder patients. It consists of seven items each for depression and anxiety subscales. Scoring for each item ranges from 0 to 3, with 3 denoting the highest anxiety or depression level. A total subscale score of > 8 points out of a possible 21 denotes considerable symptoms of anxiety or depression. It is an acceptable, reliable, and valid practical tool for measuring anxiety and depression [30].
Quality of life The EuroQol EQ-5D was used to measure the health-related quality of life, expressed as utility values ranging from 1 to 3, where 1 represents perfect health. The five domains of mobility, self-care, usual activities, pain or discomfort, and anxiety or depression were measured [31].
Statistical Analysis
With a power of 0.8 and a significance level of 0.05, at least 30 participants were needed to be included in each treatment arm (60 participants in total) to detect a clinically important mean difference between groups of 2 points on the VAS scores at 6 months, when assuming a standard deviation of 0.8 points and considering a 10% drop to follow-up. For other outcomes, we considered a between-group difference of 10% of the outcome measure’s scale to be clinically worthwhile.
The data analysis was performed by a statistician who did not participate in the recruitment, evaluation, and treatment aspects of the study. The study homogeneity was analyzed through the Kolmogorov–Smirnov test. The data analysis was performed on an intention-to-treat analysis, in which the missing data were assumed to be included in the analysis. For the missing data, results obtained in the last available assessment of each participant were repeated. The time and group (4 × 2) linear mixed model (LMM) of all the outcome variables between active and placebo groups at baseline, 4 weeks, 8 weeks, and at 6 months of follow-up was performed and the analysis proceeded stepwise, with initial unadjusted covariates such as age, gender, duration of pain, stage characteristics, medication use, and occupation, etc. Multivariate logistic regression was used to test for heterogeneity between groups, and the maximum likelihood and least squares examined the standard error of each of the parameter estimators. The mean difference (MD) and 95% confidence interval (CI) were also calculated for each between-group comparison. The statistical analyses were processed using commercial statistical software (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY, IBM Corp) and a level of significance of p ≤ 0.05 was adopted for all tests.
Results
Compliance with the Trial Protocol
The study recruitment reached the minimum sample size calculation. Almost 50% of the screened participants met the eligibility criteria and were included in the study trial. All of the outcome measures in the registered protocol were reported and no other outcomes were measured or reported and the treatment compliance rate was 100%.
Flow of Participants
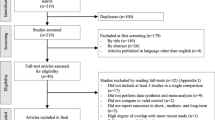
Initially, a total of 114 patients were assessed for eligibility to participate, of whom 15 had the experience of previous physical therapy interventions, 12 had other systemic problems, nine had other shoulder problems, six had undergone some sort of joint surgery, and 12 were not willing to participate in the study. Therefore, 60 participants met the selection criteria and were included in the study and the flow of the participants in the study is shown in Fig. 3. Two participants each from the active and placebo group did not complete the 6-month follow-up period due to personal reasons and time constraints.
Covariates such as the baseline demographic and clinical characteristics of the patients were analyzed through the Kolmogorov–Smirnov test and the scores are shown in Table 1. The mean age ± standard deviation (SD) for the active group (52.12 ± 5.2) and placebo group (53.56 ± 5.5) did not show any difference (p = 0.301). Regarding gender, 27 males (45%) and 33 females (55%) were included, which shows a higher percentage of females in the active and placebo treatment groups. There was no statistically significant difference in the height (p = 0.830) and weight (p = 0.781) measurements between the groups. The data show that the non-dominant side and the left side are involved more in the study groups. Concerning previous episodes of pain, 18.33% (n = 11) of patients complained of previous episodes of pain in both groups. The mean duration of pain for the active group was 8.2 ± 2.6 months and for the placebo group was 7.8 ± 2.8 months, with no significant difference between the groups (p = 0.568). An analysis of the employment history of 60 patients showed that 73.33% (n = 44) were involved in manual work, 18.33% (n = 11) were involved in non-manual work, and 8.33% (n = 5) were not doing any work.
Effects of the Intervention
Primary and Secondary Outcomes
The time and group (4 × 2) linear mixed model (LMM) of the primary variable (pain intensity, VAS) reports a statistically significant difference (p < 0.001) between active and placebo groups at baseline, 4 weeks, 8 weeks, and at 6 months of follow-up. The post-intervention at 4 weeks shows an improvement of 2.0 (CI 95% 1.71–2.28) in the active group than the placebo group. Similar effects have been noted after 8 weeks (2.2) (CI 95% 1.91–2.48) and at 6 months (1.9) (CI 95% 1.61–2.18) of follow-up. The scores show significant changes (p < 0.001) in the active group than the placebo group, which is presented in Tables 2, 3 and 4. The effect size of pain intensity (d = 0.90) shows a larger effect in the active group than in the placebo group.
The time and group (4 × 2) linear mixed model (LMM) of other secondary variables (MRI T2 sagittal section, range of motion (abduction and lateral rotation), functional disability, kinesiophobia, depression and quality of life) report statistically significant differences (p = 0.001) between the active group and placebo group at baseline, 4 weeks, 8 weeks, and at 6 months of follow-up. The post-intervention at 4 weeks shows improvement 0.2 (CI 95% 0.06–0.33), – 15.5 (CI 95% – 19.36 to – 11.63), – 6.6 (CI 95% – 8.20 to – 4.99), 17.10 (CI 95% 15.75–18.44), 8.5 (CI 95% 7.61–9.38), 2.2 (CI 95% 1.83–2.56) and 0.3 (CI 95% 0.19–0.40) in the active group than the placebo group in the MRI T2 sagittal section, range of motion (abduction and lateral rotation), functional disability, kinesiophobia, depression status, and quality of life, respectively. Similar effects have been noted after 8 weeks and at 6 months 0.6 (CI 95% 0.46–0.73), -23.6 (CI 95% – 27.47 to – 19.72), – 18.10 (CI 95% – 19.72 to – 16.47), 16.2 (CI 95% 14.85–17.54), 11.0 (CI 95% 10.21–11.98), 4.4 (CI 95% 4.03–4.76) and 0.9 (CI 95% 0.79–1.00) follow-up period. The corrected Bonferroni post hoc scores show significant changes (p = 0.001) in the active group than the placebo group, which is presented in Tables 2, 3, and 4. The effect size of MRI T2 sagittal section (d = 0.84), abduction (d = 0.65), lateral rotation (d = 0.83), functional disability (d = 0.97), kinesiophobia (d = 0.96), depression status (d = 0.96) and quality of life (d = 0.93) shows a larger effect in the active group than in the placebo group, which is shown in Fig. 4. However, the reports show clinically significant changes in patient healthcare outcomes.
Adverse Events with the Study Intervention
There were no adverse reactions or side effects noted in either the active or placebo groups during and after the treatment.
Discussion
This trial has been executed with the specific objective of finding the additional effects of ESWT after the administration of intra-articular lidocaine injection in patients with frozen shoulder joints and the data are reported subsequently. The report shows that participants in the active group showed significant improvement compared to the placebo group at various intervals in primary (MCID = 2.1) and secondary outcome variables. At the same time, the placebo group also showed a statistically significant improvement over time across all the outcome variables. Hence, the changes noted in our study between these groups found that the problem itself heals spontaneously without any specific treatment.
A study by Rymaruk S et al. investigated that the intra-articular (IA) lidocaine injection improved the pain intensity and functional range of motion of frozen shoulder patients when compared to patients who did not receive IA injection treatment. In addition, intra-articular injection therapy has been observed to be preferable over manipulation under anesthesia (MUA), as it is an easy-to-apply procedure, a safe technique, is cost-effective, and will show early results [32]. The positive effects of these techniques would be due to the capsular stretching effect from the injection of 3 ml of 1% lidocaine into the joint capsule. It is the most widely used local anesthetic drug by orthopedic surgeons and it has the effect of reversibly blocking the conduction of the small nerve fibers, which carry the pain and autonomic information. This immediate effect would be helpful to deteriorate the pain sensation and produce early results [17]. Nevertheless, over the past few years, long-term adverse effect, such as chondrotoxicity, has been reported after the administration of IA lidocaine injection. A recent clinical study investigated that a high-dose administration of IA local anesthetic (377 ml of 0.5% bupivacaine) by a pain pump was related to chondrotoxicity. At the same time, another in vitro study investigated a significant decrease in chondrotoxicity effect after a single-dose 1% lidocaine injection [33]. A study by Hsu WC et al. investigated that the IA injection of lidocaine immediately before a physiotherapy session has a better effect than physiotherapy alone in the treatment of frozen shoulder patients [17]. In the same way, our reports are in agreement with Hsu WC et al., which found an added effect of extracorporeal shockwave therapy (ESWT) after administration of IA lidocaine injection in frozen shoulder conditions. The injection of lidocaine results in considerable pain reduction soon after administration, which facilitates an excessive use of the limb at the earlier stage. This is one of the main causes responsible for the high recurrence rate at the later stage of injection. Therefore, the patients are informed to keep the limb in a resting state for 3 days after the injection.
In addition, Elerian AE et al. investigated that ESWT alone provides a more effective and safer treatment procedure than corticosteroid IA injection for diabetic frozen shoulder patients [34]. The clinically effective ESWT provided in our study involves the administration of the ESWT with 3.5 bar air pressure and 2000 pulses with an energy flux density (EFD) of ¼ 0.16 mJ/mm2 at a frequency of 8 Hz applied in two shoulder regions, which is applied two sessions per week for 4 weeks and it is the ideal parameters for treating frozen shoulder [24]. The present study shows the pain relief effect of ESWT, which could be due to hyper-stimulation analgesia and neovascularization changes in our bodies [35]. Furthermore, Zhang R et al. stated that the application of ESWT induced anti-inflammatory and anti-fibrotic effects and fastened the tissue healing through increased blood flow to the treated site. It also increases the extensibility of the collagen fibers and increases the range of motion in FS patients. Recovering the shoulder movements was thought to demonstrate smoother capsular margins and the reappearance or enlargement of the axillary recess with increased extensibility [20]. Consequently, an overall decrease in pain intensity and an increase in shoulder range of motions increase the functional activities of the joint. Our findings also confirm that ESWT with progressive resistance exercise would be useful for improving the condition even further.
In our study, we measured the thickening of coracohumeral ligaments in frozen shoulder patients through sagittal sections of MRI and found that active ESWT has a significant improvement over placebo ESWT and reached 10% of the minimal clinically important difference (MCID). Mengiardi et al. also found that patients with frozen shoulders had a significantly thickened CHL, which was measured by MRI analysis, which limits the external rotation and induces thickness-related pain [21]. We noticed in both study groups that there were consistent and significant improvements in the Q-DASH scores from the baseline measurements to the follow-up measurements. The changes and the improvement noticed in the muscle strength in our study investigated that progressive resistance training provided faster improvement in muscle quality and enhanced muscle strength. It results in enhanced participants' functional status [24] as well as their psychological (kinesiophobia, depression, and QOL) status. In addition, in our study to find the real effects of ESWT, we included the placebo ESWT group. To obtain the placebo effect, a special cap was used in the transducer head which blocked the extracorporeal shockwaves that transfer to the body. However, the clinical changes observed in the placebo group were due to placebo effects and mentioned as the “Hawthorne effect”.
Strengths and Weaknesses of Study
The strength of this study is its clinical environment and the use of common, well-established physiotherapeutic methods, which assured the external validity of this study. Next, the CONSORT guidelines for randomized controlled trials were rigorously implemented in this study. To eliminate different biases in the study, the randomization, allocation concealment, and the blinding of subjects and therapists were performed. Also, there were a few dropouts and the overall adherence to the study protocol was good. As this is not a serious and self-limiting problem, we picked statistical tests that would reduce type 1 errors. A tight rehabilitation protocol and restricted individual treatment modifications might have altered the results. It would be intriguing to examine the individual effects of ESWT alone in treating acute and subacute conditions using the same inclusion criteria as used in our study. It is possible that those in the placebo group were in more discomfort and thus more frequently engaged in exercises or were more compliant with the exercise regimen (to seek relief from the discomfort) than those in the treatment group. If so, then this would attenuate (or shrink) some of the estimated effects of the active treatment. This renders the reported effects conservative. The confounding factors such as age, gender, duration of pain, stage characteristics, medication use, and occupation may affect the results of the study, which is not controlled during the data analysis. Also, the study did not include a control group, which may present the actual effects of intervention procedures. Hence, future studies can be conducted with the same study design by including a control group and considering the confounding factors in data analysis and long-term follow-up measurements in frozen shoulder patients.
Conclusions
In conclusion, intra-articular lidocaine injection in addition to extracorporeal shockwave therapy and progressive resistance exercise is effective in terms of reducing pain, decreasing coracohumeral ligament thickness, improving range of motion, functional disability, kinesiophobia, depression status, and health-related quality of life in patients with frozen shoulder. This study provided clinicians and therapists with a better understanding of this disease and its intervention.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Georgiannos D, Markopoulos G, Devetzi E, Bisbinas I. Adhesive capsulitis of the shoulder. Is there consensus regarding the treatment? A comprehensive review. Open Orthop J. 2017;11:65–76.
Le HV, Lee SJ, Nazarian A, Rodriguez EK. Adhesive capsulitis of the shoulder: review of pathophysiology and current clinical treatments. Shoulder Elbow. 2017;9(2):75–84.
Ryan V, Brown H, Minns Lowe CJ, Lewis JS. The pathophysiology associated with primary (idiopathic) frozen shoulder: a systematic review. BMC Musculoskelet Disord. 2016;17(1):340.
Tveitå EK, Sandvik L, Ekeberg OM, Juel NG, Bautz-Holter E. Factor structure of the Shoulder Pain and Disability Index in patients with adhesive capsulitis. BMC Musculoskelet Disord. 2008; 9103.
Kumar S, Grimmer K. Nonsteroidal anti-inflammatory drugs (NSAIDs) and physiotherapy management of musculoskeletal conditions: a professional minefield? Ther Clin Risk Manag. 2005;1(1):69–76.
Hsieh L-F, Hsu W-C, Lin Y-J, Chang H-L, Chen C-C, et al. Addition of intra-articular hyaluronate injection to physical therapy program produces no extra benefits in patients with adhesive capsulitis of the shoulder: a randomized controlled trial. Arch Phys Med Rehab. 2012;93:957–64.
Koh KH. Corticosteroid injection for adhesive capsulitis in primary care: a systematic review of randomised clinical trials. Singapore Med J. 2016;57(12):646–57.
Kraal T, Beimers L, The B, Sierevelt I, van den Bekerom M, Eygendaal D. Manipulation under anaesthesia for frozen shoulders: outdated technique or well-established quick fix? EFORT Open Rev. 2019;4(3):98–109.
Patel R, Urits I, Wolf J, Murthy A, Cornett EM, Jones MR, Ngo AL, Manchikanti L, Kaye AD, Viswanath O. A comprehensive update of adhesive capsulitis and minimally invasive treatment options. Psychopharmacol Bull. 2020;50(4 Suppl 1):91–107.
Chan HBY, Pua PY, How CH. Physical therapy in the management of frozen shoulder. Singapore Med J. 2017;58(12):685–9.
Nakandala P, Nanayakkara I, Wadugodapitiya S, Gawarammana I. The efficacy of physiotherapy interventions in the treatment of adhesive capsulitis: a systematic review. J Back Musculoskelet Rehabil. 2021;34(2):195–205.
Gunay Ucurum S, Kaya DO, Kayali Y, Askin A, Tekindal MA. Comparison of different electrotherapy methods and exercise therapy in shoulder impingement syndrome: A prospective randomized controlled trial. Acta Orthop Traumatol Turc. 2018;52(4):249–55.
Tasto JP, Elias DW. Adhesive capsulitis. Sports Med Arthrosc Rev. 2007;15(4):216–21.
Dias R, Cutts S, Massoud S. Frozen shoulder. BMJ. 2005;331(7530):1453–6. https://doi.org/10.1136/bmj.331.7530.1453.
Honda H, Gotoh M, Kanazawa T, et al. Effects of lidocaine on torn rotator cuff tendons. J Orthop Res. 2016;34(9):1620–7.
Donald MJ, Derbyshire S. Lignocaine toxicity; a complication of local anaesthesia administered in the community. Emerg Med J. 2004;21(2):249–50.
Hsu WC, Wang TL, Lin YJ, Hsieh LF, Tsai CM, Huang KH. Addition of lidocaine injection immediately before physiotherapy for frozen shoulder: a randomized controlled trial. PLoS ONE. 2015;10(2): e0118217.
Pandey V, Madi S. Clinical Guidelines in the Management of Frozen Shoulder: An Update! Indian J Orthop. 2021;55(2):299–309.
Tang HY, Wei W, Yu T, Zhao Y. Physical therapy for the treatment of frozen shoulder: a protocol for a systematic review of a randomized controlled trial. Medicine (Baltimore). 2019;98(32): e16784.
Zhang R, Wang Z, Liu R, Zhang N, Guo J, Huang Y. Extracorporeal shockwave therapy as an adjunctive therapy for frozen shoulder: a systematic review and meta-analysis. Orthop J Sports Med. 2022;10(2):23259671211062224.
Mengiardi B, Pfirrmann CW, Gerber C, Hodler J, Zanetti M. Frozen shoulder: MR arthrographic findings. Radiology. 2004;233:486–92.
Nambi G, Alghadier M, Ebrahim EE, et al. Comparative effects of Mulligan’s mobilization, spinal manipulation, and conventional massage therapy in cervicogenic headache-a prospective, randomized, controlled trial. Healthcare (Basel). 2022;11(1):107.
Song C, Song C, Li C. Outcome of manipulation under anaesthesia with or without intra-articular steroid injection for treating frozen shoulder: a retrospective cohort study. Medicine (Baltimore). 2021;100(13): e23893.
Hopewell S, Keene DJ, Marian IR, Dritsaki M, Heine P, Cureton L, Dutton SJ, Dakin H, Carr A, Hamilton W, Hansen Z, Jaggi A, Littlewood C, Barker KL, Gray A, Lamb SE, GRASP Trial Group. Progressive exercise compared with best practice advice, with or without corticosteroid injection, for the treatment of patients with rotator cuff disorders (GRASP): a multicentre, pragmatic, 2 × 2 factorial, randomised controlled trial. Lancet. 2021;398(10298):416–28.
Sindhu BS, Shechtman O, Tuckey L. Validity, reliability, and responsiveness of a digital version of the visual analogue scale. J Hand Ther. 2011;24(4):356–64.
Li JQ, Tang KL, Wang J, et al. MRI findings for frozen shoulder evaluation: is the thickness of the coracohumeral ligament a valuable diagnostic tool? PLoS ONE. 2011;6(12): e28704.
Riddle DL, Rothstein JM, Lamb RL. Goniometric reliability in a clinical setting. Shoulder measurements. Phys Ther. 1987;67(5):668–73.
Alnahdi AH. Validity and reliability of the Arabic quick disabilities of the arm, Shoulder and Hand (QuickDASH-Arabic). Musculoskelet Sci Pract. 2021;53: 102372.
Kortlever JTP, Karyampudi P, Ottenhoff JSE, Ring D, Vagner GA, Reichel LM. Using the Tampa Scale for Kinesiophobia Short Form in patients with upper extremity specific limitations. Hand (N Y). 2021;16(6):847–53.
Cassiani-Miranda CA, Scoppetta O, Cabanzo-Arenas DF. Validity of the Hospital Anxiety and Depression Scale (HADS) in primary care patients in Colombia. Gen Hosp Psychiatry. 2022;74:102–109.
Feng YS, Kohlmann T, Janssen MF, Buchholz I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021;30(3):647–73.
Rymaruk S, Peach C. Indications for hydrodilatation for frozen shoulder. EFORT Open Rev. 2017;2(11):462–8.
Matsen FA, Papadonikolakis A. Published evidence demonstrating the causation of glenohumeral chondrolysis by postoperative infusion of local anaesthetic via a pain pump. J Bone Joint Surg Am. 2013;95:1126–34.
Elerian AE, Rodriguez-Sanz D, Abdelaziz Elsherif A, Dorgham HA, Al-Hamaky DMA, El Fakharany MS, Ewidea M. Effectiveness of shock wave therapy versus intra-articular corticosteroid injection in diabetic frozen shoulder patients’ management: randomized controlled trial. Appl Sci. 2021;11(8):3721.
Engebretsen K, Grotle M, Bautz-Holter E, et al. Supervised exercises compared with radial extracorporeal shockwave therapy for subacromial shoulder pain: 1-year results of a single-blind randomized controlled trial. Phys Ther. 2011;91:37–47.
Linde K, Fässler M, Meissner K. Placebo interventions, placebo effects and clinical practice. Philos Trans R Soc Lond B Biol Sci. 2011;366(1572):1905–12.
Funding
This study was supported via funding for the study from Prince Sattam bin Abdulaziz University project number (PSAU/2023/R/1444).
Author information
Authors and Affiliations
Contributions
Conceptualization: Gopal Nambi, Mshari Alghadier, Mudathir Mohamedahmed Eltayeb, Osama R. Aldhafian, Ayman K. Saleh, Nesreen Alsanousi, Mohamed Nagah Ahmed Ibrahim, Abdehamid A. Attallah, Mohammed Abdelgwad Ismail, Mohamed Elfeshawy, Yaser El Sayed Hasan Wahd, Alaa Jameel A. Albarakati. Data curation: Osama R. Aldhafian, Ayman K. Saleh, Mohamed Nagah Ahmed Ibrahim, Abdehamid A. Attallah, Mohammed Abdelgwad Ismail, Mohamed Elfeshawy, Yaser El Sayed Hasan Wahd, Alaa Jameel A. Albarakati. Formal analysis: Gopal Nambi, Mshari Alghadier, Mudathir Mohamedahmed Eltayeb, Osama R. Aldhafian, Yaser El Sayed Hasan Wahd, Alaa Jameel A. Albarakati. Methodology: Gopal Nambi, Mshari Alghadier, Mudathir Mohamedahmed Eltayeb, Osama R. Aldhafian, Ayman K. Saleh, Nesreen Alsanousi, Mohamed Nagah Ahmed Ibrahim, Abdehamid A. Attallah, Mohammed Abdelgwad Ismail, Mohamed Elfeshawy, Yaser El Sayed Hasan Wahd, Alaa Jameel A. Albarakati. Project administration: Gopal Nambi, Mshari Alghadier, Mudathir Mohamedahmed Eltayeb, Osama R. Aldhafian, Ayman K. Saleh, Alaa Jameel A. Albarakati. Supervision: Gopal Nambi, Mshari Alghadier, Mudathir Mohamedahmed Eltayeb, Osama R. Aldhafian, Ayman K. Saleh, Nesreen Alsanousi, Yaser El Sayed Hasan Wahd, Alaa Jameel A. Albarakati. Writing – review & editing: Gopal Nambi, Mshari Alghadier, Mudathir Mohamedahmed Eltayeb, Osama R. Aldhafian, Ayman K. Saleh, Nesreen Alsanousi, Mohamed Nagah Ahmed Ibrahim, Abdehamid A. Attallah, Mohammed Abdelgwad Ismail, Mohamed Elfeshawy, Yaser El Sayed Hasan Wahd, Alaa Jameel A. Albarakati.
Corresponding author
Ethics declarations
Conflict of Interest
Gopal Nambi, Mshari Alghadier, Mudathir Mohamedahmed Eltayeb, Osama R. Aldhafian, Ayman K. Saleh, Nesreen Alsanousi, Mohamed Nagah Ahmed Ibrahim, Abdehamid A. Attallah, Mohammed Abdelgwad Ismail, Mohamed Elfeshawy, Yaser El Sayed Hasan Wahd, and Alaa Jameel A. Albarakati have nothing to disclose.
Ethical Approval
This study has been approved by the Department Ethics Committee (DEC) with an ethical approval number RHPT/020/013 and registered in the clinical trial registry with reference number: CTRI/2020/04/024834.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nambi, G., Alghadier, M., Eltayeb, M.M. et al. Additional Effect of Extracorporeal Shockwave Therapy with Lidocaine Injection on Clinical and MRI Findings in Frozen Shoulder: A Prospective, Randomized, Double-Blinded, Placebo-Controlled Trial. Pain Ther 13, 251–268 (2024). https://doi.org/10.1007/s40122-024-00575-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-024-00575-9