Abstract
Introduction
In the treatment of patients with chronic pain, whole-body electrostatic therapy using the Elosan Cabin C1 has been proposed as an adjunctive therapy. So far, data on the use of this cabin are limited. Promising results with a significant reduction in pain scores have been obtained in a small group of patients. However, treatment with Elosan Cabin C1 has not been the subject of evaluation in a larger patient population. The aim of this study was to investigate the efficacy and adverse effects of electrostatic treatment in such a population.
Methods
Prospective, multi-center, observational clinical trial conducted in daily practice in a large adult ambulatory population with chronic pain. Each patient received eight weekly Elosan C1 treatment sessions for up to 9 weeks. Treatment was added to an established conservative pain management. Pain scores (visual analog scale (VAS) 0–100, primary outcome) and sleep quality (seven-point Likert scale, secondary outcome) were assessed before, during, and at the end of the treatment period; quality of life (SF-12: Physical Component Summary = PCS, Mental Component Summary = MCS; secondary outcome) was assessed before and at the end of the treatment period. Subgroup analyses were performed for sex, age, duration of pain, initial pain location, pain entity, and pain medication at the start of treatment.
Results
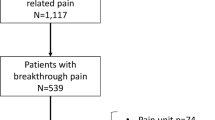
A total of 192 patients were enrolled, 143 patients (74.5%) had a complete set of 8 treatment sessions. A reduction in pain scores from 68 ± 14 points to 47 ± 22 points was observed (p < 0.001), 65% of patients (responders) had a reduction of > 15 points. Female patients had a significantly better response than male patients with a higher number of responders (76% vs. 38%; p < 0.001). Patients with a pain history < 1 year had a significantly better response than patients with a pain history > 1 year. The Physical Component Summary (PCS) increased from 36 ± 11 to 41 ± 11 (+ 18%, p < 0.001) and the Mental Component Summary (MCS) from 41 ± 7 to 43 ± 7 (+ 6%, p = 0.3). Overall sleep quality improved significantly from 4.6 ± 1.7 to 3.73 ± 1.7 points (p < 0.001), with a higher proportion of responders in the female group (37 vs. 18%; p < 0.034). No serious adverse events were observed during treatment.
Conclusions
Electrostatic therapy with Elosan Cabin C1 may be a useful and effective adjunct therapy for patients with chronic pain. The results suggest that female patients and those with a recent history of pain experience the greatest benefit.
Trial Registration
NCT04818294 (clinicaltrials.gov).
Plain Language Summary
Electrostatic treatment with the Elosan Cabin C1 is a form of pain therapy that works by applying an electrical charge to the outside of the whole body without a corresponding current flowing inside. The treatment is painless and lasts 8 min per session. The study investigated the effect of the Elosan treatment in 143 patients over a period of 8 weeks with sessions once per week. Investigated outcomes were changes in pain, quality of life, sleep quality, and side effects of the treatment. 65% of the patients had a relevant improvement in pain levels, the average pain reduction in all patients was 30.9%. Quality of life and sleep quality also improved significantly. There were no relevant side effects of the treatment. The best effect was seen in female patients and if the duration of pain was less than 1 year. It was found that by applying the electrostatic field once a week, various types of pain can be reduced with a long-lasting effect. The treatment is ideally combined with physiotherapy and other complementary pain therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Electrostatic therapy has been proposed as an effective treatment for chronic pain, a growing burden in society with many challenges in treatment approaches. |
To date, there has not been much scientific evidence investigating the effects of electrostatic therapy. |
This study investigates the efficacy and adverse events of electrostatic therapy for chronic pain. |
Electrostatic therapy can significantly reduce chronic pain and improve quality of life and sleep. |
It may be a useful adjunct to a multimodal approach to pain management. |
Introduction
Chronic pain is a common and increasingly prevalent health problem. At the individual level, treatment options are often limited and inadequate [1]. Its prevalence ranges from 11 to 40% [2, 3] and has been shown to be associated with sociodemographic and socioeconomic factors such as increasing age, female gender, or lower educational level [2, 4, 5], as reported in several studies in the US and Europe in recent years. The spine and large joints, often following trauma or surgery, are the most commonly reported sites of chronic pain [6]. Patients with chronic pain have been shown to have a poorer general health status and are more likely to report depression, anxiety, or feelings of helplessness [7]. Chronic pain is therefore highly detrimental to the quality of life, daily activities, and employment of these patients [1, 8]. The etiology of chronic pain is multifactorial, usually described as a combination of biochemical, psychosocial, and sociodemographic determinants [9]. As such, the adequate management of chronic pain is a challenge, and a multimodal approach has been widely accepted as an adequate measure to deal with the complexity of the therapeutic approach [10]. However, the most commonly used type of treatment is still medication alone [11], even though patients have reported their dissatisfaction with the often-inadequate treatment they receive. Unsurprisingly, patients tried other therapies and reported a willingness to try anything to improve their condition [1]. Common treatments included physiotherapy and massage, followed by acupuncture and relaxation therapy, or psychological interventions such as biofeedback or mindfulness therapy. All of these treatments have shown varying degrees of success [12], but they can be effective as complementary therapies in a multidisciplinary approach to pain management. Electrostatic treatment is an old technology that has been in use for a long time in complementary and physical medicine [13] and has recently been the subject of renewed interest in clinical practice [14]. A novel Elosan Cabin C1 is aimed at treating patients with chronic pain by applying an electrostatic field [15]. To date, very limited data on its use and efficacy are available. Promising results were obtained in a small pilot study. It showed that treatment with Elosan Cabin C1 significantly reduced pain by about 25% in 34 chronic pain patients. However, the aim of achieving a clinically relevant reduction in pain in a significant number of patients was missed [16].
Aim and Objectives
The objective of this study was to evaluate the efficacy and safety of whole-body electrostatic therapy using the Elosan Cabin C1 in daily practice in a large adult ambulatory population with chronic pain. We hypothesized that, when comparing the beginning and end of the study period, there would be a significant reduction in the VAS pain score. The outcomes studied were the relief of pain, the effect on quality of life and the improvement of sleep disturbance due to the intervention. In addition, the adverse effects associated with the Elosan therapy were also evaluated.
Methods
Study Design
The study was a prospective, multi-center, observational clinical trial (phase IV trial). The trial was registered at clinicaltrials.gov (NCT04818294) and at the SNCTP (000004304). This study was a category A study according to the Swiss Federal Law on Human Research (HRA-A). Results are reported according to the STROBE guidelines [17]. Nine centers participated in this study. Patients were recruited and the study was conducted on an outpatient basis in office-based pain management clinics. The list of participating centers, i.e., the ELES study group, can be found in the Supplementary Material (Table A1).
Patients
Patients aged > 18 years with a history of chronic pain, defined as pain lasting more than 3 months [18] with visual analog scale (VAS) > 50 (scale 0–100 points) were included in the study after written informed consent. Patients with typical chronic pain entities (i.e., degenerative, neuropathic, and rheumatic pain as well as patients suffering from fibromyalgia) participated in this study. This also included patients with diffuse whole-body pain on one or both body sides and patients with strong stabbing constant pain, especially in muscles and tendon insertions.
Exclusion criteria were significant comorbidity (such as cardiac arrhythmias), implanted electronic devices (such as pacemakers, intracardiac defibrillators, or pain stimulators), diagnosis of psychosis, history of epilepsy, participation in another study and unwillingness to comply with an 8-week study protocol.
Ethics and Ethical Approval
Ethical approval was obtained from the ethic committee Zurich (Kantonale Ethikkommission Zürich—KEK), BASEC-ID 2021–00074, the ethic committee Eastern Switzerland (Ethikkommission Ostschweiz—EKO) and the ethic committee Northwest—Central Switzerland (Ethikkommission Nordwest-Zentralschweiz—EKNZ). Each patient participating provided physical written informed consent before inclusion. A study code was allocated to each patient and any personal patient information is being stored separately from anonymized data. All data were handled according to the Swiss Data Protection Act. The study was performed in accordance with the Helsinki Declaration. Good clinical practice regarding the use of medical products was followed according to the European Guideline for medical products (93/42/EGW; MDR 2017/745) and ISO 14971. Safety procedures were applied as required (ClinO Art 37–43; ICH/E6 6.8; ISO14155 8.2.5., A.14).
The Electrostatic Cabin and its Mode of Operation
Elosan Cabin C1 (Elosan AG, 9472 Grabs, Switzerland) is a registered CE-marked medical device (Medical Device IIa, TÜV Rheinland 2020; CE 0197). A high-voltage electrostatic field of up to 50,000 V is generated inside the cabin by a direct current (DC) voltage generator [19]. A metal handle serves as the electrode. The counter-electrode is formed by the entire inner surface of the cabin, which is electrically insulated and acts as a Faraday cage [15]. After the patient stands in the booth (Fig. 1), the electrical voltage is applied to the hands via the metal handle and a polarizing electrostatic field is generated over the entire surface of the body. A treatment cycle consists of three phases of charging and discharging. This can be felt on the skin with the patient's hair bristling.
The physiological mechanisms involved in the reduction of pain by means of an electrostatic field are not yet fully understood and are the subject of ongoing research. Two main mechanisms may be involved in inducing biological effects at the cellular and tissue level using DC electrostatic fields [20]: Electric fields can modulate transmembrane glycoproteins and induce glycocalyx fluid shearing, which is transmitted via the cellular cytoskeleton to activate signaling pathways. At the tissue level, this may induce activation of cutaneous receptors and neurons. This may result in centrally mediated effects on muscle relaxation, the autonomic nervous system and pain. There is increasing evidence that exposure to electrostatic fields does not cause significant adverse effects in humans [21].
Elosan Cabin C1 Treatment Protocol
Each patient received a total of eight treatment sessions in the Elosan Cabin C1 (Fig. 2). These were carried out on a weekly basis over a maximum period of nine weeks. During a treatment session, a patient spent a total of 8 min in the cabin while six cycles of electrostatic charging and discharging were carried out. The treatment was always given as an adjunct to an already established conservative pain management regimen consisting of drug therapy and physical therapy. Patients were interviewed at enrolment and before and after each Elosan treatment session. A standardized questionnaire was used to assess pain, quality of life, and sleep quality.
Flow chart of study design. After inclusion, the following parameters were recorded: At Treatment session (TS) 1: constant pain medication, pain score as visual analog scale (VAS), sleep quality, Mental Component Summary (MCS) and Physical Component Summary (MCS) with SF-12, adverse events. At TS 2–7: constant pain medication, pain score as visual analog scale (VAS), sleep quality, adverse events. At TS 8: Same parameters as TS 1
Primary Outcome Measure
Change in pain intensity during the study was assessed using a 0–100 visual analogue scale [12]. The values of the baseline pain scores were compared with the values of the scores obtained at the end of the treatment period. Pain scores were also assessed before and after each treatment session.
Secondary Outcome Measures
To assess quality of life, the SF-12 questionnaire was used with its composite scores Physical Component Summary (PCS) and Mental Component Summary (MCS) [22, 23]. The PCS score is a reflection of physical limitations in daily life, while the MCS score is a measure of psychological distress and depressive symptoms. The quality of life was assessed before and at the end of the entire treatment period. The baseline values were compared with the values obtained at the end of the treatment period. Sleep quality was also assessed at these time points using a seven-point Likert scale (1 = always restful sleep, 7 = never restful sleep).
Adverse Events/Side Effects
All potentially treatment-related adverse events such as dizziness, marked changes in pain perception, nausea and other vegetative symptoms were recorded.
Power Analysis
Sample size was calculated based on previous clinical experience with the Elosan Cabin C1 where a pain reduction of 24.3% (12) with a standard deviation of 32.6% could be achieved. Assuming that a minimal reduction of pain score values of 15 points will occur, we computed an effect size of − 0.46. Aiming for significance α at 0.05 and a study power at 90%, a minimum of 43 patients had to be included for any targeted subgroup analysis. Considering a drop-off during the treatment period of 30% of the included patients, at least 56 patients for each subgroup had to be available for statistical analysis. R package pwr was used for this power calculation [24].
Statistics
Data are presented as mean ± standard deviation for continuous variables and frequency (n) and percent (%) for categorical variables. One-way repeated measures analysis of variance (ANOVA) with time (= 8 treatment sessions) as repeated measure was done for the primary outcome (pain scores). The patients were categorized as responders when having at least a 15-point reduction of VAS pain scale, non-responders otherwise. For secondary outcome measures (SF-12 variables and sleep quality), one-way ANOVA with repeated measures (= 2 timepoints: baseline and final) was used to assess change. The patients were categorized as responders when having at least a 15-point improvement of SF-12 scores, respectively, 2 points improvement in sleep quality, non-responders otherwise. Welch two-sample t test, Pearson’s chi-squared test and Fisher’s exact test were applied for continuous and categorical variables for analyzing differences in subgroups (gender, age, pain duration, initial pain localization, pain entity and pain medication between the responders and non-responders sub-groups). Finally, logistic regression (generalized linear with stepwise elimination of subgroups at baseline) was applied in order to assess significant associations. For all analyses, significance was set at α = 0.05 (two-tailed). All analyses were performed using R version 4.3.1 [25].
Results
Patient population
A total of 192 patients with chronic pain were enrolled between May 2021 and June 2022; 143 patients (74.5%) completed eight treatment sessions in Elosan Cabin C1 and were available for statistical analysis (Table 1). Of these, 105 (73.4%) were female and 38 (26.6%) were male. The mean age was 55.4 ± 13.6 years. The female patients were significantly younger than the male patients, but they did not differ in terms of their initial assessment of pain. In the patients' general health status assessed by the consulting physician, there was no significant difference between beginning and end of the study period (Fig. 4A). Pain medication use was similar between the sexes with the exception of the use of antidepressants and gabapentin/pregabalin.
Pain Assessment
In the patient cohort, a significant reduction in initial pain scores was observed from 68 ± 14 VAS points (range, 50–100 points) to 47 ± 22 points (range, 0–100 points) at the end of treatment with Elosan Cabin C1 (Fig. 3A). Overall, the pain score was reduced by 30.9% (p < 0.001). A pain score reduction of ≥ 15 VAS points was achieved by 61% of patients (responders). A significant difference in pain scores occurred between female and male patients during the treatment period (Fig. 3B). Compared to baseline, female patients had significantly lower pain scores at the end of the treatment (− 36 ± 31%, p < 0.001), whereas males had moderate decreases (− 15 ± 28%, p = 0.056) (Table 2). In addition, the rate of responders was significantly higher in the female group (67%) compared to the male group (45%, p = 0.018), i.e., patients with a pain reduction of ≥ 15 points on the VAS. To assess responder and non-responder characteristics, a stepwise logistic regression model was used for the entire cohort and the male/female subgroups. The model showed that sex was the only significant variable for the overall cohort (male sex − 1.63 OR, p < 0.001). In the female subgroup, the duration of pain was the only significant variable: the estimated effect of a pain duration of ≤ 1 year was + 1.49 OR (p < 0.05) compared to a longer duration of symptoms. Finally, in the male subgroup, a lower quality of sleep at the start of the study had an estimated effect on the response of + 0.86 OR (p < 0.05). In practical terms, this means that female patients, and in particular those with a pain duration of ≤ 1 year, are more likely to respond to treatment with Elosan; and only males with a poor quality of sleep had a better chance of responding to the treatment. Subgroup analysis of patients with different chronic pain durations showed no difference in pain scores at baseline, but the most pronounced reduction in pain scores was seen in patients with a history of pain of less than one year (Table 3). The most significant improvement in pain scores could be observed within the first five treatment sessions (Fig. 3A).
Assessment of Quality of Life (SF-12)
Quality of life showed an improvement during the treatment period as assessed by the SF-12 (Fig. 4): Physical Component Summary (PCS) increased from 36 ± 11 to 41 ± 11 (+ 18%, p < 0.001) and Mental Component Summary (MCS) from 41 ± 7 to 43 ± 7 (+ 6%, p = 0.3). Female and male patients had a distinctly different pattern of quality of life at the beginning and the end of the therapy period (Table 2). The impact of the Elosan therapy had a comparable effect in female and male patient groups with an increase of PCS of + 18% (p = 0.0078) and + 19% (p = 0.2), and both groups showed no significant changes in MCS. Patients with a history of chronic pain > 5 years had the lowest quality of life at the beginning of the treatment and the lowest impact of treatment as compared to those with a shorter history of chronic pain (Table 3).
Quality of Sleep
The overall quality of sleep improved significantly during the treatment period from 4.6 ± 1.7 points (seven-point Likert scale) to 3.73 ± 1.7 points (p < 0.001, Fig. 4). Female patients started with a lower sleep quality than male patients. They responded better to the treatment (Table 2). Patients with a history of chronic pain > 5 years had the lowest sleep quality and least improvement as a result of Elosan Cabin C1 treatment (Table 3). No significant differences in pain scores, quality of life or sleep quality were found in all other subgroup analyses for age, primary pain location, initial pain entity or pain medication.
Adverse Events/Side Effects
There were no reports of serious adverse events at any of the study centers during the course of the study. Adverse events such as transient increase in pain, vertigo and mild vegetative symptoms occurred in n = 6 patients (4.2%), n = 8 patients (5.6%) and n = 4 patients (2.8%) respectively and were limited to a few hours after each treatment with Elosan Cabin C1. Table 4 gives an overview of the recorded adverse events in relation to all 1144 treatment sessions.
Discussion
This study evaluated the efficacy and safety of Elosan Cabin C1 whole-body electrostatic therapy in adults with chronic pain over a study period of eight weeks (Fig. 2). The outcomes assessed were pain relief, impact on quality of life and sleep quality in 192 patients. Elosan therapy, in combination with continued conservative treatment, was effective in relieving chronic pain: A total of 61% of the patients experienced an improvement in their pain condition, defined as a reduction of at least 15 points on the VAS pain scale. The overall VAS pain score reduction was 30.9%. Along with pain reduction, there was significant improvement in mobility and physical activity as measured by the SF-12 Physical Component Summary (PCS, + 18%). Regarding sleep quality, 32% of patients reported improvement, with an overall increase of 13% (seven-point Likert scale). These results are in line with the results of the preliminary ELO02 study, where similar improvements in pain, quality of life and sleep quality have been reported [16]. The subgroup analysis showed that patients with long-standing pain conditions and those on prolonged pharmacotherapy also benefited from the treatment. However, female patients were significantly more likely to respond to Elosan treatment if their pain had lasted less than a year. Considering the pathophysiology of pain chronification and the potential development of a chronic pain syndrome over time, this effect is plausible, as the longer the duration of pain, the more challenging the treatment becomes.
Pain on the one hand and inactivity, psychological distress, and sleep problems on the other have a bidirectional relationship and influence each other [12]. The aim of multimodal pain management must therefore be not only to reduce pain, but also to increase mobility despite pain, restore psychological resilience and improve sleep quality. Physical therapy, using either an exercise or a manipulative approach, attempts to restore flexibility, normal muscle function and muscle strength. Exercise can influence the body's pain modulation system and slightly reverse central sensitization [26]. In the long term, overall pain and functional status can be improved. However, the success of physical therapy is often limited by the underlying pain and the short duration of the treatment sessions in a limited window of time [27]. In addition to exercise, multimodal approaches may also include relaxation techniques such as progressive muscle relaxation and mindfulness meditation. These have been shown to be effective in several types of chronic pain, by reducing central sensitization and pain levels [28]. However, not all patients are open to and suitable for such techniques. The evidence for relaxation techniques is weak [29, 30]. Another possible adjunct in the treatment of chronic pain is the use of massage therapy. There is a large body of literature on different types of massage for different types of pain. Overall, a review of the current literature by Lin et al. found limited positive evidence that massage therapy is effective in pain management, at least in the short term [30]. A complementary method with the largest body of evidence is acupuncture. There is a considerable number of RCTs investigating acupuncture for the treatment of chronic pain and associated comorbidities such as depression and sleep disorders. Most of the reviewed trials show some efficacy in improving pain, reducing opioid use, and improving sleep quality and depression [30,31,32,33]. However, there is a lack of standardization in the way acupuncture is studied. This makes it difficult to compare different studies and the level of evidence varies [33, 34]. As well as the methods mentioned above, there is a wide range of other modalities that can be used to treat chronic pain, such as Yoga, Pilates, Tai Chi, osteopathy, spine manipulation, low level laser, or operant and cognitive behavioral therapies. Overall, complementary methods are in high demand by patients and are recommended in the literature as an adjunct to pharmacological and invasive pain treatments, but lack high quality scientific evidence [12, 29,30,31, 35].
In addition to the conservative methods described, there has been a development of electrical and magnetic therapies for acute and chronic pain: magnetic or electrical transcranial stimulation, transcutaneous electrical nerve stimulation (TENS) and the electrostatic treatment under investigation. Direct electrical or magnetic transcranial stimulation of the brain has been validated as an effective treatment for various types of chronic pain in Grade B trials, but there is still a great deal of heterogeneity in the literature, which makes it difficult to give precise recommendations. The treatment is also not yet widely available to patients [36,37,38,39]. TENS has been shown to be effective for musculoskeletal and neuropathic pain, but the effect is short-lived and has little effect on central pain modulation [40,41,42]. The underlying mechanisms of pain relief by TENS and transcranial stimulation are not fully understood but appear to be different. Whereas TENS is thought to have a peripheral target that may affect central pain processing over time, transcranial stimulation is thought to have a direct effect on the brain's neuroplasticity [36, 37].
The neurological mechanisms behind the effects of the electrostatic treatment are also still the subject of research. Clinically, our results show a significant improvement of various pain entities and sleep quality with a lasting effect, at least for the period observed. Because of this non-pain specific and sustained effect, we also suggest a central effect on neuroplasticity in the brain. As the electrostatic field has only superficial penetration into the skin [43], this central effect is most likely achieved by influencing afferent pathways via receptors in the skin. The significant improvement in mobility seen in the PCS could be explained by centrally mediated muscle relaxation. In comparison to the above methods of complementary pain treatment, electrostatic therapy is a promising treatment option that has both immediate and long-term effects on chronic pain and quality of life. However, to achieve the best possible effect in pain management, several methods should be combined to address the different comorbidities of chronic pain. Electrostatic treatment with the Elosan Cabin C1 can be a useful adjunct to rapidly reduce pain and increase mobility and resilience to exercise, especially in cases where musculoskeletal and soft tissue pain are dominant. As a result, physiotherapy can be used more effectively as part of the treatment regimen. Better pain control and improvements in sleep and quality of life could then improve psychological resilience and support psychosomatic therapy. Elosan treatment should therefore not be seen as a stand-alone treatment, but as an adjunct to physical therapy, psychosomatic therapy and other complementary treatment options aimed at restoring resilience in the patient's daily life. All these options can work in conjunction with pharmacology but may allow the patient to reduce the use of analgesics and especially opioids.
The Elosan treatment itself takes a minimal amount of time (8 min) and causes no pain. There were no safety events or serious side effects throughout the study. The treatment is always carried out according to the same schedule and does not need to be adapted to the individual patient. Therefore, after an initial consultation with the doctor, the treatment can be administered by the staff of a medical practice. In our study, this was the case in most of the trial sites. Due to the simplicity of the treatment and the possibility to delegate the process, Elosan therapy can be easily implemented in the daily routine of an ambulatory pain clinic.
As with all subjective outcomes, we found a very high variance in the measured outcome parameters. Therefore, our study must be interpreted with caution. A further limitation is the lack of a control group (continuation of the standard therapy without Elosan treatment or a sham treatment). The idea of a randomized control trial has been rejected on the basis of experiences made during the previous Elosan study ELO02 [16]: The nature of electrostatic treatment, with its clear perceptible effect on the patients' skin, precluded sham treatment during the planned study period, and no reliable control group could have been established. In addition, in the outpatient setting, it would not have been feasible to have a control group with standard treatment only. Without a control group, the measured effect of improved pain is based on the combined treatment of Elosan and continued standard therapy. However, the temporal relationship between pain improvement and the start of Elosan therapy strongly suggests efficacy, as most of the patients had a long history of pain and were in a steady state with their conservative pain therapy. Moreover, it was not possible to control for non-specific effects of the treatment under the given circumstances. A certain degree of placebo effect is to be expected with the treatment set-up in the cabin. In this trial, patients were followed for 8 weeks. This is a relatively short time interval compared with the long period of chronic pain before treatment. Long-term pain development and recurrence after treatment were not followed beyond the treatment period. In contrast to the precursory study ELO02 (16), the current study had sufficient power for the outcomes studied, with 143 complete data sets. However, the male subject group was smaller than the expected sample size of 56 (underpowered) and was underrepresented compared to the female subjects (105 women vs. 38 men). This may explain the significantly higher response rate in the female population (67%) compared to the male subjects (45%).
There is still insufficient data on the potential benefits of different configurations of an electrostatic field. Electrostatic exposure of the human body is a natural phenomenon with no evidence of harmful effects (21). To date, the treatment regimen (8 min with a total of six charges of a fixed voltage dose) has been established empirically without scientific analysis of the ideal voltage or duration of the pulses. Further research should investigate the most effective forms of electrostatic field application for the desired indications. The underlying mechanisms and optimal treatment combinations in a multimodal treatment approach should also be further investigated.
The study included only patients with chronic pain. From the experience gained in this study, one mechanism of pain reduction in musculoskeletal pain could be muscle relaxation. Therefore, Elosan treatment could be helpful in the treatment of various acute musculoskeletal conditions, such as low back pain, shoulder pain and acute postoperative pain. Extrapolating from our results, there may also be a positive effect on recovery after intense exercise in athletes. Further research is needed to investigate both the effects on acute pain and musculoskeletal recovery after exercise and surgery.
Conclusions
The Elosan Cabin C1 can be an effective, well-tolerated, and non-invasive addition to the range of treatment options available for chronic pain conditions. It fits well into a multimodal treatment approach and can be a useful adjunct to pharmacological and physical pain management. By rapidly reducing musculoskeletal pain and improving exercise tolerance, there is also potential for use in acute musculoskeletal pain (e.g., lumbago) and in the postoperative period following extremity and spinal surgery. This acute and perioperative use should be the subject of further investigation in future studies.
Data Availability
The datasets generated and analyzed during the current study are available from the author on reasonable request.
References
Breivik H, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333.
Dahlhamer J, et al. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–6.
Todd A, et al. The European epidemic: Pain prevalence and socioeconomic inequalities in pain across 19 European countries. Eur J Pain. 2019;23(8):1425–36.
Dorner TE, et al. How are socio-demographic and psycho-social factors associated with the prevalence and chronicity of severe pain in 14 different body sites? A cross-sectional population-based survey. Wien Klin Wochenschr. 2018;130(1–2):14–22.
Fayaz A, et al. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6): e010364.
Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022;163(2):e328–32.
Jaremo P, et al. Illness beliefs among patients with chronic widespread pain - associations with self-reported health status, anxiety and depressive symptoms and impact of pain. BMC Psychol. 2017;5(1):24.
Sylwander C, et al. The impact of chronic widespread pain on health status and long-term health predictors: a general population cohort study. BMC Musculoskelet Disord. 2020;21(1):36.
Meints, S.M., et al., Behavioral, Psychological, Neurophysiological, and Neuroanatomic Determinants of Pain. J Bone Joint Surg Am, 2020. 102 Suppl 1(Suppl 1): p. 21–27.
Dale R, Stacey B. Multimodal Treatment of Chronic Pain. Med Clin North Am. 2016;100(1):55–64.
Giordano J, Schatman ME. An ethical analysis of crisis in chronic pain care: facts, issues and problems in pain medicine. Part I Pain Physician. 2008;11(4):483–90.
Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082–97.
King, P., The Uses of Static Electricity as an Aid to Recovery. Bristol Med Chir J (1883), 1906. 24(93): p. 233–237.
Dai Y, et al. Effects of electrostatic therapy on nighttime sleep and daytime symptoms in patients with chronic insomnia: Evidences from an open label study. Front Neurosci. 2023;16:1047240.
Bischof, A., Static electricity pain treatment cabin. 2018: Switzerland.
Hoederath P, Kuhnke O, Bohn W. Elosan therapy: a new treatment option for chronic pain. Schmerz. 2020;34(1):4–12.
Ghaferi AA, Schwartz TA, Pawlik TM. STROBE Reporting Guidelines for Observational Studies. JAMA Surg. 2021;156(6):577–8.
Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl, 1986. 3: p. S1–226.
Reinhardt, B., Device for treating the human body by means of static electricity. 1981: Germany.
Hart, F.X.P.J.R., The Application of Electric Fields in Biology and Medicine, in Electric Field. 2018, InTech.
Petri AK, et al. Biological effects of exposure to static electric fields in humans and vertebrates: a systematic review. Environ Health. 2017;16(1):41.
Drixler, K., et al., [Validation of the Short-Form-Health-Survey-12 (SF-12 Version 2.0) assessing health-related quality of life in a normative German sample]. Z Psychosom Med Psychother, 2020. 66(3): p. 272–286.
Luo X, et al. Reliability, validity, and responsiveness of the short form 12-item survey (SF-12) in patients with back pain. Spine. 2003;28(15):1739–45.
Champely, S., Ekstrom, C, Dalgaard, P, Gill, J, Weibelzahl, S, Anandkumar, A, Ford, C, Volcic, R & De Rosario, H. pwr: Basic functions for power analysis. R package version 1.3–0. 2017 [cited 2023 16.08.2023]; Available from: https://cran.r-project.org/web/packages/pwr/.
R Core Team, R., R: A language and environment for statistical computing. 2013.
Arribas-Romano A, et al. Efficacy of physical therapy on nociceptive pain processing alterations in patients with chronic musculoskeletal pain: a systematic review and meta-analysis. Pain Med. 2020;21(10):2502–17.
Fattal C, et al. What is the efficacy of physical therapeutics for treating neuropathic pain in spinal cord injury patients? Ann Phys Rehabil Med. 2009;52(2):149–66.
Jinich-Diamant A, et al. Neurophysiological Mechanisms Supporting Mindfulness Meditation-Based Pain Relief: an Updated Review. Curr Pain Headache Rep. 2020;24(10):56.
Qaseem A, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514–30.
Lin YC, Wan L, Jamison RN. Using integrative medicine in pain management: an evaluation of current evidence. Anesth Analg. 2017;125(6):2081–93.
Chou R, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166(7):493–505.
Liu F, et al. Acupuncture for chronic pain-related insomnia: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019:5381028.
Zhang N, et al. Overall reporting descriptions of acupuncture for chronic pain in randomized controlled trials in English journals. J Pain Res. 2021;14:2369–79.
Paley, C.A. and M.I. Johnson, Acupuncture for the Relief of Chronic Pain: A Synthesis of Systematic Reviews. Medicina (Kaunas), 2019. 56(1).
Urits I, et al. A comprehensive review of alternative therapies for the management of chronic pain patients: acupuncture, tai chi, osteopathic manipulative medicine, and chiropractic care. Adv Ther. 2021;38(1):76–89.
Hamid P, Malik BH, Hussain ML. Noninvasive transcranial magnetic stimulation (TMS) in chronic refractory pain: a systematic review. Cureus. 2019;11(10): e6019.
Lefaucheur JP, et al. The use of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) to relieve pain. Brain Stimul. 2008;1(4):337–44.
Attal N, et al. Repetitive transcranial magnetic stimulation for neuropathic pain: a randomized multicentre sham-controlled trial. Brain. 2021;144(11):3328–39.
Fernandes AM, Graven-Nielsen T, de Andrade DC. New updates on transcranial magnetic stimulation in chronic pain. Curr Opin Support Palliat Care. 2022;16(2):65–70.
Johnson MI, et al. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: a systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open. 2022;12(2): e051073.
Rodriguez Lagos L, et al. Effects of percutaneous and transcutaneous electrical nerve stimulation on endogenous pain mechanisms in patients with musculoskeletal pain: a systematic review and meta-analysis. Pain Med. 2023;24(4):397–414.
Beltran-Alacreu, H., et al., Percutaneous versus transcutaneous electrical nerve stimulation for the treatment of musculoskeletal pain. a systematic review and meta-analysis. Pain Med, 2022. 23(8): p. 1387–1400.
Maruyama Y, et al. Electric-field penetration depth and dielectric spectroscopy observations of human skin. Skin Res Technol. 2020;26(2):255–62.
Acknowledgements
We thank all patients within this study for their participation and their contribution to creating scientific evidence for this new method in pain therapy.
Medical writing, Editorial Assistance
Initial study design, ethical approval, and recruitment of the study centers was conducted by Elosan AG in Grabs, Switzerland. Principal investigators of the study centers are listed in Table A1.
Funding
The study was sponsored by Elosan AG, 9472 Grabs, Switzerland, the manufacturer of the cabin. The sponsor provided the Elosan Cabin at each center. After the accomplishment of the study, the involved centers were able to purchase the cabin at reduced costs. None of the clinical investigators received any financial gratuity nor had they any financial interest related to the company.
Author information
Authors and Affiliations
Consortia
Contributions
Initial study design, conception, and data collection by the sponsor. Data analysis was performed by Vincent Stadelmann and Christoph Karl Hofer. The first draft of the manuscript was written by Stephan Steinhauser and all authors commented on previous versions of the manuscript. All authors read and approved the submitted manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no competing interests.
Ethics and Ethical Approval
Ethical approval was obtained from the ethic committee Zurich (Kantonale Ethikkommission Zürich – KEK), BASEC-ID 2021–00074, the ethic committee Eastern Switzerland (Ethikkommission Ostschweiz – EKO) and the ethic committee Northwest – Central Switzerland (Ethikkommission Nordwest-Zentralschweiz – EKNZ). Each patient participating provided physical written informed consent before inclusion. A study code was allocated to each patient and any personal patient information is being stored separately from anonymized data. All data were handled according to the Swiss Data Protection Act. The study was performed in accordance with the Helsinki Declaration. Good clinical practice regarding the use of medical products was followed according to the European Guideline for medical products (93/42/EGW; MDR 2017/745) and ISO 14971. Safety procedures were applied as required (ClinO Art 37–43; ICH/E6 6.8; ISO14155 8.2.5., A.14.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Steinhauser, S., Ganter, M.T., Stadelmann, V. et al. Whole-Body Electrostatic Pain Treatment in Adults with Chronic Pain: A Prospective Multicentric Observational Clinical Trial. Pain Ther 13, 69–85 (2024). https://doi.org/10.1007/s40122-023-00560-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00560-8