Abstract
Introduction
Chronic low back pain often comprises mixed pain types and involves multiple factors. Therefore, we hypothesized that the systemic transdermal formulation of diclofenac sodium (DF systemic patch), which is effective for nociceptive pain, and an α2δ Ca2+ channel ligand, which is effective for neuropathic pain, would have additive effects in the treatment of chronic low back pain.
Methods
From among participants in a randomized, double-blind, placebo-controlled study of DF systemic patch (75 or 150 mg) applied once daily for 2 weeks in patients with chronic low back pain, we performed a subpopulation analysis of those who were concomitantly treated with an α2δ Ca2+ channel ligand during the study period. The efficacy endpoint was pain intensity score on a visual analog scale (VAS).
Results
The difference (95% confidence interval) in the least square mean pain VAS score between patients in the 150-mg combination group, who were treated with 150-mg DF systemic patch and an α2δ Ca2+ channel ligand (n = 11), and those in the non-combination group, who were treated with placebo patch and α2δ Ca2+ channel ligand (n = 22), was − 15.09 mm (− 26.45, − 3.73). Because the upper limit of the 95% confidence interval was less than zero, this result indicates that the pain VAS score improved more in the 150-mg combination group than in the non-combination group (placebo group).
Conclusions
The combination of the DF systemic patch and an α2δ Ca2+ channel ligand may be more effective than α2δ Ca2+ channel ligand monotherapy for controlling chronic low back pain.
Trial registration numbers: JPRN-JapicCTI-205134 and jRCT2080225040.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Chronic low back pain is one of the most common chronic pain disorders and often comprises mixed pain types. |
We hypothesized that combining a systemic transdermal patch containing the non-steroidal anti-inflammatory drug (NSAID) diclofenac sodium (DF systemic patch), which is effective for nociceptive pain, with an α2δ Ca2+ channel ligand, which is effective for neuropathic pain, would have additive effects in patients with chronic low back pain. |
What was learned from the study? |
In patients with chronic low back pain, the combination of the DF systemic patch and α2δ Ca2+ channel ligand appears to have additive effects, as shown by improvement in pain visual analog scale scores in the 150-mg combination group than in the α2δ Ca2+ channel ligand monotherapy (placebo group) placebo patch group. |
The population evaluated in this report did not experience gastrointestinal adverse events, which are typical adverse effects of NSAIDs, or lightheadedness or dizziness, which are commonly observed with α2δ Ca2+ channel ligands. |
Introduction
In Japan, low back pain is one of the most frequent conditions in terms of both symptomatic prevalence and consultation rates and is also one of the most common reasons for visits to healthcare facilities in Europe and the US [1]. Chronic low back pain is a highly common chronic pain disorder and is defined as “low back pain that has persisted for more than 3 months” [2]. Chronic pain is classified as nociceptive, neuropathic, and nociplastic pain, but it is rare for chronic pain to be attributed to any one of these three factors, and it often involves mixed pain [3]. Similarly, chronic low back pain, which involves a variety of pathologies, may be due to nociceptive pain, neuropathic pain, or both [4, 5].
In general, the recommended pharmacological therapies for chronic low back pain include non-steroidal anti-inflammatory drugs (NSAIDs) in Europe [6]; NSAIDs, tramadol, and duloxetine in the US [7]; and weak opioids, NSAIDs, and serotonin noradrenaline reuptake inhibitors (SNRIs) [1] in Japan. Although α2δ Ca2+ channel ligands (e.g., pregabalin and mirogabalin) have demonstrated efficacy in controlling neuropathic pain, they are not currently recommended for the treatment of chronic low back pain because of the poor level of evidence, e.g., [8]. However, a systematic review indicated that neuropathic pain was present in 16.7–54.4% of patients with chronic low back pain [9], and studies reported that NSAIDs and α2δ Ca2+ channel ligands have additive effects in the treatment of chronic low back pain [10, 11]. Consequently, the addition of α2δ Ca2+ channel ligands to standard therapy may be expected to have an additive effect in chronic pain patients with neuropathic pain.
Diclofenac sodium is one of the most widely used NSAIDs in clinical practice [12]. The once-daily systemic transdermal patch of diclofenac sodium (DF systemic patch) was newly developed and approved. In Japan, it is indicated for “analgesia and anti-inflammation in low back pain, humeroscapular peri arthritis, cervico-omo-brachial syndrome and tenosynovitis” and “analgesia in various cancers.” To receive the approval for low back pain, we studied a matrix-type formulation of the DF systemic patch containing 75 mg of diclofenac sodium per 70 cm2 (7 × 10 cm) patch in a phase II study (randomized, placebo-controlled, double-blind, parallel-group comparison; unpublished data) and a phase III randomized, placebo-controlled, double-blind comparative study in patients with chronic low back pain [13]. When we performed a post hoc analysis in the subgroup of patients in the phase II study who were concomitantly treated with pregabalin, one of the α2δ Ca2+ channel ligands, the results suggested that efficacy (i.e., change in pain visual analog scale [VAS] score) was better in the combination group (DF systemic patch + pregabalin) than in the non-combination group (placebo patch + pregabalin). This finding suggested that the subpopulation analysis in the phase II study might reveal an additive effect of the combination of DF systemic patches and α2δ Ca2+ channel ligands in chronic low back pain, although the pain classification of chronic low back pain has not been determined.
To test this hypothesis, we performed an exploratory subpopulation analysis of participants from the phase III randomized, placebo-controlled, double-blind comparative study [13] who continued to use an α2δ Ca2+ channel ligand throughout the study period, i.e., we compared the combination groups, which received 75 or 150 mg DF systemic patch plus an α2δ Ca2+ channel ligand, with the non-combination group, which received the placebo patch plus an α2δ Ca2+ channel ligand.
Methods
The analysis was performed in the subpopulation of participants in the phase III study who received concomitant α2δ Ca2+ channel ligands. The phase III study was conducted as a multicenter, randomized, placebo-controlled, double-blind, parallel-group study at 75 Japanese sites [13]. It was performed in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines. The 75 participating institutions acquired approval of the protocol from one of the following institutional review boards: Shinagawa East One Medical Clinic Institutional Review Board; Sugiura Clinic Institutional Review Board; and Jimbo Orthopedics Institutional Review Board. Prior to enrollment, all participating patients provided written informed consent to participate in the study. The study was registered with the Japan Primary Registries Network (JPRN-JapicCTI-205134) and the Japan Registry for Clinical Trials (jRCT2080225040).
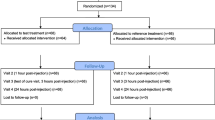
The phase III study was performed in Japanese patients aged 20 years or older who had been diagnosed with chronic low back pain and had been receiving pain control treatment with NSAIDs or acetaminophen for a minimum of 4 weeks. Patients were excluded if they had symptoms that may have affected the clinical assessment, e.g., radiating pain, numbness, or paralysis extending to the knees. After a 1-week wash-out period, patients underwent a 1-week single-blind placebo patch application (1-week placebo run-in period), after which placebo responders or patients with unstable pain status were excluded. Subsequently, eligible patients (e.g., those with a mean pain VAS score ≥ 40 mm in the 3 days immediately preceding enrollment in the double-blind treatment period) were enrolled in the 2-week double-blind treatment period. Patients were randomized in a 1:1:2 ratio to the 75-mg DF systemic patch group (one active drug patch, one placebo patch), 150-mg group (two active drug patches), or placebo group (two placebo patches). The efficacy and safety of the DF systemic patch (75 or 150 mg) were compared with that of the placebo.
With regard to pre-treatment and concomitant medications and therapies, treatment with α2δ Ca2+ channel ligands, SNRIs, and analgesic adjuncts (e.g., anxiolytics, muscle relaxants, tricyclic antidepressants) or with physiotherapy (e.g., heat therapy, traction therapy, lumbar braces) or alternative therapies (e.g., acupuncture, osteopathy) for low back pain was permitted provided that the dose was not modified and treatment was not initiated from at least 4 weeks prior to the wash-out period and throughout the study period (i.e., the wash-out period, placebo run-in period, and double-blind treatment period). However, pharmacologic treatments, such as opioids, vaccinia virus-inoculated rabbit inflammatory skin extract, and corticosteroids, and surgical treatments that may have affected pain assessment, e.g., lumbar trigger point injections and percutaneous electrical nerve stimulation, were not permitted during the study period.
A total of 538 patients with chronic low back pain were enrolled in the study and randomly allocated to the 75-mg group (n = 136), 150-mg group (n = 135), or placebo group (n = 267). At the end of each day throughout the study period (i.e., the wash-out period, placebo run-in period, and double-blind treatment period), patients rated the average degree of pain at the site of interest (lower back) by using a 100-mm VAS and recorded the value in a patient diary [13].
Patients
All 538 patients enrolled in the study were included in the efficacy and safety analysis [13]. In the overall population (full analysis set; FAS), 47 patients were taking concomitant α2δ Ca2+ channel ligands (pregabalin and mirogabalin) and were included in the present subpopulation analysis (75-mg group, n = 14 patients; 150-mg group, n = 11; and placebo group, n = 22).
In the FAS, 114 out of 538 patients took concomitant medications other than α2δ Ca2+ channel ligands for the treatment of chronic low back pain throughout the study period (i.e., the wash-out period, placebo run-in period, and double-blind treatment period). The concomitant medications included muscle relaxants (e.g., eperisone; 48 cases), prostaglandin E1 derivatives (43 cases), vitamin preparations (21 cases), SNRIs (16 cases), Chinese herbal preparations (five cases), and a prostaglandin I2 derivative (one case), whereby some patients took more than one concomitant medication.
Statistical analyses
The subpopulation analysis included patients in the FAS who were concomitantly treated with an α2δ Ca2+ channel ligand. Although this subpopulation analysis was pre-planned, it was positioned as an exploratory analysis. Tabulation of demographic and baseline characteristics and adverse events (AEs) of the subpopulation were performed post hoc. To evaluate efficacy, in each group (i.e., the 75-mg and 150-mg combination groups and placebo group) the results of the pain VAS scores were used to calculate the least square mean, standard error, and 95% confidence interval (CI) of the change from baseline (i.e., the start of the double-blind treatment period) in the mean 3-day pain VAS score after 2 weeks of treatment and the between-group difference in the least square means of the active treatment combination group (75-mg and 150-mg groups) relative to the placebo group and its 95% CI. The mean 3-day pain VAS score was calculated from the pain VAS scores on the 3 days immediately preceding each specified hospital visit (e.g., the mean 3-day pain VAS score at baseline was the mean of the pain VAS scores on the 3 days immediately preceding the start of the double-blind treatment period). In case of premature discontinuation of participants, the mean of the pain VAS scores on the 3 days immediately preceding the end of the wash-out period were used. SAS® version 9.4 was used for statistical analysis. The subpopulation analysis described in this article was performed for exploratory purposes, so no sample size was determined for the subpopulation analysis and no statistical comparisons were performed between groups.
The subpopulation safety analysis included patients in the safety analysis set (SAF; i.e., those who received the study drug) who were concomitantly treated with the α2δ Ca2+ channel ligand. The incidence rates of adverse events (AEs) and adverse drug reactions (ADRs; i.e., AEs for which a causal relationship could not be ruled out) were tabulated for each group in the subpopulation. Event names of AEs were coded with system organ classes and preferred terms according to the Medical Dictionary for Regulatory Activities version 23.1.
Results
Patient Disposition and Baseline Characteristics
Demographic and baseline characteristics of patients in the subpopulation and the overall population (FAS) are shown in Table 1. Although age and disease duration tended to be higher in the subpopulation placebo group than in the two subpopulation combination treatment groups and the overall population, the values were generally similar.
Patient-specific background information and the daily dose and purpose of use of the concomitant α2δ Ca2+ channel ligand used in each patient are shown in Table 2; almost all of the 47 patients received pregabalin (n = 43), and the remaining four patients received mirogabalin. During the study period (i.e., the wash-out period, placebo run-in period, and double-blind treatment period), the dosage of the concomitant α2δ Ca2+ channel ligand was not modified and treatment with these drugs was not initiated in any patients.
Efficacy
The results of the subpopulation analysis are shown in Fig. 1, and the results of the analysis in the subpopulation and FAS in the original phase III study are shown in Table 3. At 2 weeks after treatment, the change from baseline in pain VAS scores was greater in both the 150-mg and 75-mg combination groups than in the placebo group. In particular, the upper limit of the 95% CI for the difference in the change between the 150-mg combination group and the placebo group was less than zero, indicating improvement in pain VAS scores in the 150-mg combination group compared with the placebo group.
Change in mean 3-day pain visual analog score in subpopulations from baseline to week 2. Least square means ± standard error of the least square means are illustrated for the change from baseline in the 3-day mean pain visual analog scale score after 2 weeks of treatment in the 75-mg and 150-mg combination groups (active diclofenac sodium systemic patch + alpha-2-delta calcium channel ligand) and the placebo group (non-combination group; placebo + alpha-2-delta calcium channel ligand). VAS visual analog scale
Safety
The incidence rates of AEs and ADRs in the subpopulation are shown in Table 4. The AE incidence rate was highest in the 75-mg combination group but lower and similar in the 150-mg combination and placebo groups. The same pattern was seen in the incidence rate of ADRs. With the exception of the incidence rate of AEs in the 150-mg combination group, which tended to be lower than in the overall population, the incidence rates were generally similar to those in the overall population (SAF; AE incidence rates in the overall population were 27.2% in the 75-mg group, 29.6% in the 150-mg group, and 28.1% in the placebo group, and ADR incidence rates were 14.0% in the 75-mg group, 12.6% in the 150-mg group, and 19.1% in the placebo group) [13]. The subpopulation showed no gastrointestinal tract disturbances or AEs considered to be associated with such disturbances, which are commonly observed with NSAIDs, no AEs such as lightheadedness or dizziness which are commonly observed with α2δ Ca2+ channel ligands, and no serious AEs. The case of interstitial lung disease reported in the placebo group was present before the start of the study, but the investigator judged it to be an AE because at the time of the tests conducted during the study period it had worsened from the state prior to the start of placebo patch application.
Discussion
This report presents the results of a subpopulation analysis of patients who were continuously treated with α2δ Ca2+ channel ligands throughout a phase III study performed to assess the efficacy of a DF systemic patch (75 and 150 mg) in patients with chronic low back pain [13]. In this subpopulation analysis, the 75-mg and 150-mg combination groups (i.e., the group of patients treated with the active DF systemic patch and an α2δ Ca2+ channel ligand) were defined as combination groups and the placebo group (i.e., the group of patients treated with placebo patch and an α2δ Ca2+ channel ligand) was defined as the non-combination group. The subpopulation analysis evaluated the efficacy and safety of concomitant treatment with DF systemic patch and an α2δ Ca2+ channel ligand by comparing the combination groups with the non-combination group.
In the subpopulation analysis, no restrictions were placed on the reason for using the α2δ Ca2+ channel ligands. As such, although some patients were taking the α2δ Ca2+ channel ligand for reasons other than treating low back pain, the intended use in these patients was primarily for low back pain-related disorders or for neuropathic pain associated with low back pain (Table 2). The 150-mg combination group showed improvement in the 3-day mean pain VAS score after 2 weeks of treatment compared with the non-combination group (placebo group). In addition, a comparison of differences between each of the combination groups and the non-combination group (placebo group) in the subpopulation and between the 75-mg and 150-mg groups and the placebo group in the overall population (FAS) showed greater differences in the subpopulation than in the overall population (Table 3). These results support our hypothesis of an additive effect of the DF systemic patch and α2δ Ca2+ channel ligands in patients with chronic low back pain.
In patients taking α2δ Ca2+ channel ligands to treat chronic low back pain, neuropathic pain is assumed to be a contributing factor to their chronic pain. It is conceivable that in this patient group, the combination with diclofenac sodium, which suppresses nociceptive pain, may have resulted in additional pain improvement compared with the non-combination group (placebo group), resulting in a greater change in pain VAS scores. Furthermore, one study reported that the addition of the α2δ Ca2+ channel ligand mirogabalin in patients treated with NSAIDs for lumbar spinal canal stenosis with peripheral neuropathic pain significantly improved the change in pain VAS scores compared with patients treated with NSAIDs alone [14], and another found that the combination of celecoxib and pregabalin was more effective than monotherapy [10]. Although these reports do not directly support an additive effect of the DF systemic patch itself with α2δ Ca2+ channel ligands, the positive effects of the concomitant use of NSAIDs and α2δ Ca2+ channel ligands indicate that the DF systemic patch may also be highly likely to have an additive effect in controlling chronic pain, such as that associated with the mixed pain of chronic low back pain.
With respect to safety, the incidence rates of AEs and ADRs tended to be higher in the 75-mg combination group than in the placebo group, but this was considered to be due to the limited number of cases in this subpopulation analysis. In support of this, the incidence rates of AEs and ADRs in the 150-mg combination group were similar to those in the placebo group, indicating that there was no dose-related increase in the incidence rates of AEs and ADRs. In addition, AEs such as gastrointestinal disturbance-related AEs, which are commonly observed with NSAIDs, and lightheadedness and dizziness, which are commonly observed with α2δ Ca2+ channel ligands, were not observed in any of the groups. These results suggest that the concomitant use of a DF systemic patch with an α2δ Ca2+ channel ligand is as safe as the administration of an α2δ Ca2+ channel ligand alone. The efficacy and safety of the long-term administration of the DF systemic patch have been confirmed [15]. With the DF systemic patch, the AEs associated with the upper gastrointestinal mucosa (a clinically relevant effect of NSAIDs) tend to be less frequent than with oral agents [16]. Taken together, findings indicate that the DF systemic patch may potentially become a viable option for the long-term administration of NSAIDs in addition to α2δ Ca2+ channel ligands for pain control in chronic low back pain.
This subpopulation analysis has some limitations. First, the results are limited in terms of generalizability: Because the phase III study included patients whose pain was controlled by treatment with NSAIDs or acetaminophen, it may have been biased towards a population with a high responsiveness to these drugs. Second, the subpopulation analysis was exploratory, and the pain classification of chronic low back pain was not determined, so a future prospective study needs to test the hypothesis in a larger population of patients with a definite pain classification of chronic low back pain.
Conclusions
The combination of DF systemic patch and an α2δ Ca2+ channel ligand may have greater efficacy in the treatment of chronic low back pain than monotherapy with α2δ Ca2+ channel ligand.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available because the data are confidential and proprietary to Hisamitsu Pharmaceutical Co., Inc.
References
The Japanese Orthopaedic Association. Japanese Orthopaedic Association (JOA). Clinical practice guidelines on the management of low back pain. 2nd ed. Tokyo: Nankodo Co. Ltd.; 2019.
Snelgrove S, Liossi C. Living with chronic low back pain: a metasynthesis of qualitative research. Chronic Illn. 2013;9(4):283–301.
Health, Labour and Welfare Policy Research Grants (Research on chronic pain) in Japan: Research for the Uniform Accessibility to Chronic Pain Management Systems and Improved Healthcare Utilizing Pain Center Treatment Databases. Clinical practice guideline for the management chronic pain. 1st ed. Tokyo: Shinko Trading Co., Ltd.; 2021.
Morlion B. The relevance of neuropathic components in chronic back pain. Curr Med Res Opin. 2011;27(10):2067–8.
Kaki AM, El-Yaski AZ, Youseif E. Identifying neuropathic pain among patients with chronic low-back pain: use of the Leeds Assessment of Neuropathic Symptoms and Signs pain scale. Reg Anesth Pain Med. 2005;30(5):422–8.
National Guideline Centre (UK). Low back pain and sciatica in over 16s: assessment and management. 2016. https://www.ncbi.nlm.nih.gov/books/NBK401577/. Accessed 14 April July 2023.
Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514. https://doi.org/10.7326/M16-2367.
Koes BW, van Tulder M, Lin CW, Macedo LG, McAuley J, Maher C. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19(12):2075–94.
Fishbain DA, Cole B, Lewis JE, Gao J. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15(1):4–15.
Romanò CL, Romanò D, Bonora C, Mineo G. Pregabalin, celecoxib, and their combination for treatment of chronic low-back pain. J Orthop Traumatol. 2009;10(4):185–91.
Yeole AB, Lakshmi GSR, Selvakumar CJ, et al. Efficacy and safety of pregabalin prolonged release-etoricoxib combination compared to etoricoxib for chronic low back pain: phase 3, randomized study. Pain Ther. 2022;11(4):1451–69.
Gan TJ. Diclofenac: an update on its mechanism of action and safety profile. Curr Med Res Opin. 2010;26(7):1715–31.
Taguchi T, Yamaguchi S, Terahara T, Okawa K, Inakura H. Systemically acting diclofenac sodium patch for control of low back pain: a randomized, double-blind, placebo-controlled study in Japan. Pain Ther. 2023;12(2):529–42.
Nikaido T, Takatsuna H, Tabata S, Shiosakai K, Nakatani T, Konno SI. Efficacy and safety of add-on mirogabalin to NSAIDs in lumbar spinal stenosis with peripheral neuropathic pain: a randomized, open-label study. Pain Ther. 2022;11(4):1195–214.
Taguchi T, Yamaguchi S, Terahara T, Okawa K, Inakura H, Nohara Y. Safety of long-term administration of a systemic-acting diclofenac sodium patch (HP-3150) in Japanese patients with low back pain. Jpn Pharmacol Ther. 2022;50(2):213–27.
Fukase H, Futagami S, Yamamoto T, et al. An endoscopic study of the influence of a systemic patch containing diclofenac sodium on the upper gastrointestinal mucosa in healthy middle-aged and elderly men and women—a randomized controlled trial with patent drug control. Jpn Pharmacol Ther. 2023;51(3):341–50 (translated from Japanese).
Acknowledgements
The authors would like to thank all of the patients, investigators, and site staff involved in the study.
Funding
This study and the journal’s Rapid Service Fee were funded by the Hisamitsu Pharmaceutical Co., Inc., Tokyo, Japan.
Author information
Authors and Affiliations
Contributions
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Shigeki Yamaguchi, Medical Advisor, gave advice on the study design on which this analytical study was based, the conduct of the study, and the interpretation of the data from this analytical study. Takaaki Terahara, Koji Okawa, and Hiroshi Inakura contributed to the study concept and design on which this development was based, the conduct of the study, and the analysis and interpretation of the data from this analytical study. All authors approved the version of the manuscript to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
Shigeki Yamaguchi received medical consultant fees from Hisamitsu Pharmaceutical Co., Inc. Shigeki Yamaguchi received honoraria from The Nakatomi Foundation. Takaaki Terahara, Koji Okawa, and Hiroshi Inakura are employees of Hisamitsu Pharmaceutical Co., Inc., Tokyo, Japan.
Ethical Approval
The phase III study was conducted in compliance with the Declaration of Helsinki of 1964 and its later amendments and Good Clinical Practice guidelines. The 75 participating institutions acquired approval of the protocol from one of the following institutional review boards: Shinagawa East One Medical Clinic Institutional Review Board, Sugiura Clinic Institutional Review Board, and Jimbo Orthopedics Institutional Review Board. Prior to enrollment, all participants provided written informed consent to participate in the study. Throughout the study period, no ethical problems or medical malpractice occurred, and the participants’ identities were kept confidential. The phase III study was registered with the Japan Primary Registries Network (JPRN) as JPRN-JapicCTI-205134 and the Japan Registry for Clinical Trials (jRCT) as jRCT2080225040.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yamaguchi, S., Terahara, T., Okawa, K. et al. Combined Efficacy of Systemically Acting Diclofenac Sodium Patch and Alpha-2-Delta Calcium Channel Ligand in Chronic Low Back Pain: Subanalysis of a Phase III Study. Pain Ther 12, 1439–1454 (2023). https://doi.org/10.1007/s40122-023-00558-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00558-2