Abstract
Introduction
Renal colic is one of the most common urological emergencies, and is usually caused by ureteral colic spasms. Pain management in renal colic remains the central focus of emergency treatment. The purpose of this meta-analysis is to identify the efficacy and safety of ketamine versus opioids in the treatment of patients with renal colic.
Methods
We searched PubMed, EMBASE, Cochrane Library, and Web of Science databases for published randomized controlled trials (RCTs) that referred to the use of ketamine and opioids for patients with renal colic. The methodology was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The mean difference (MD) or odds ratio (OR) with a 95% confidence interval (CI) was used to analyze the data. The results were pooled using a fixed-effects model or random-effects model. The primary outcome measure was patient-reported pain scores 5, 15, 30, and 60 min after drug administration. The secondary outcome measure was side effects.
Results
The data analysis revealed that ketamine was similar to opioids in pain intensity at the time of 5 min post-dose (MD = − 0.40, 95% CI − 1.82 to 1.01, P = 0.57), 15 min post-dose (MD = − 0.15, 95% CI − 0.82 to 0.52, P = 0.67), 30 min post-dose (MD = 0.38, 95% CI − 0.25 to 1.01, P = 0.24). Also, the pain score of ketamine was better than that of opioids at 60 min after administration (MD = − 0.12, 95% CI − 0.22 to − 0.02, P = 0.02). As for safety, the ketamine group was linked to a significant decrease in the incidence of hypotensive (OR = 0.08, 95% CI 0.01–0.65, P = 0.02). The two groups did not statistically differ in the incidence of nausea, vomiting, and dizziness.
Conclusions
Compared with opioids, ketamine showed a longer duration of analgesia in renal colic, with satisfactory safety.
Trial Registration
The PROSPERO registration number is CRD42022355246.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Renal colic caused by nephrolithiasis is common in urological and emergency clinical practice. Timely pain management is an essential component of renal colic treatment at the time of the patient visit. Given the adverse side effects of nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, the development of new mechanism-based analgesics with fewer side effects and better pain relief properties appeared crucial. Thus, we conduct a meta-analysis of randomized controlled trials (RCTs) to systematically assess the efficacy and safety of ketamine versus opioids in the treatment of patients with renal colic for the first time. To the best of our knowledge, no previous studies have been conducted on this subject matter. |
Compared to opioids, the use of ketamine produces more persistent relief in patients with renal colic and has a much better safety profile. Our meta-analysis concluded that ketamine holds promise as an alternative to opioids for renal colic patients in pain management in the emergency department. |
Introduction
Renal colic, resulting from urinary tract obstruction mainly due to stone impaction [1], was the most common reason for urological emergencies [2]. Approximately 5–15% of the population worldwide suffered from renal colic [3], and typical symptoms of the disease included colic in the lower back, which can radiate to the groin, perineum, and other areas, accompanied by nausea and vomiting [4]. Timely pain management is an essential component of renal colic treatment at the time of the patient visit [5].
Currently, nonsteroidal anti-inflammatory drugs (NSAIDs) or opioids serve as the conventional treatment options for renal colic [6, 7]. These drugs have analgesic action primarily through two very different pharmacological mechanisms. The NSAIDs were mainly the inhibition of cyclooxygenase (COXs) and thus the synthesis of inflammatory substances such as prostaglandins [8], and opioids suppressed nociception by binding to opioid receptors [9]. Although many studies have reported that NSAIDs and opioids provided pain relief in renal colic [10, 11], the side effects of these drugs must not be overlooked either. Pathan et al. [12] concluded that patients with coronary artery disease, asthma, or chronic obstructive pulmonary disease have limitations in the availability of NSAIDs, and opioids can also cause considerable side effects, including decreased blood pressure, vomiting, dizziness, and lightheadedness [13]. Given their adverse side effect profile, the development of new mechanism-based analgesics with fewer side effects and better pain relief properties appeared crucial.
Ketamine is a derivative of phencyclidine (PCP) and represented one of the most widely used anesthetics throughout the world [14, 15]. As a result of its providing adequate analgesia without significant respiratory depression, this medicine presented low and predictable side effects, which set it apart from other analgesics. In addition, ketamine can be administered through multiple routes (injection, intranasal, oral, skin, topical, epidural, and subcutaneous) [16], and quickly distributed into organs and tissues after administration with a short half-life of about 2–3 h. Ketamine can also promote relaxation of smooth muscle that facilitated the expulsion of stones. Their potential in the treatment of renal colic has gradually begun to receive attention in more recent years. However, there were few evidence-based assessments available of ketamine for renal colic.
Thus, the present study was designed to conduct a meta-analysis of randomized controlled trials (RCTs) to systematically assess the efficacy and safety of ketamine versus opioids in the treatment of patients with renal colic. This meta-analysis was performed in agreement with the latest Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) statements.
Methods
Search Strategy
Three authors independently searched and screened all the relevant published literature in PubMed (inception to November 2022), EMBASE (inception to November 2022), Cochrane Library (inception to November 2022), and Web of Science databases (inception to November 2022). This search strategy combined the following specific subject (MeSH) headings: “ketamine”, “kidney calculi”, “opioid peptides”, “pain management”, “randomized controlled trial”, and “renal colic”. The PICOS (populations, interventions, comparators, outcomes, and study designs) strategies were utilized to guide the search. Reference lists of included articles were also traced by the researchers to avoid missing literature. The specific retrieval strategy is listed in Table 1. Inter-investigator reliability was assessed using kappa scores. Our study was registered at PROSPERO, registration number CRD42022355246.
Inclusion Criteria
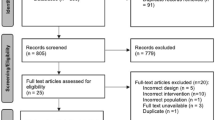
Included articles were identified based on the following inclusion criteria: (I) all RCTs analyzing the efficacy and safety of ketamine and opioids in the treatment of renal colic; (II) the full text can be obtained; (III) the study contained complete and accurate data available for analysis. The benefits of RCTs are multiple and the risk of bias is lower for high-quality RCTs compared to non-RCTs. The PRISMA flowchart was presented in Fig. 1. A PRISMA checklist is shown in Supplementary Material “PRISMA Checklist”.
Quality Assessment
Assessing the quality of the included RCTs using the Cochrane Handbook [17]. By random sequence generation, allocation concealment, blinding method, incomplete outcome data, and selective reporting, articles were graded into three categories: ( +) low risk of bias; (?) unclear risk of bias; (–) high risk of bias.
Data Extraction
Data extraction was carried out by three authors independently. The extracted data included: (I) the name of the first author and year of publication; (II) the type of article; (III) treatment modalities for each group; (IV) the sample size of each group; (V) the mode of administration; (VI) the dose of administration; (VII) the outcome of the article. The main outcome was changes in pain scores and the secondary outcome was the incidence of hypotensive, nausea, vomiting, and dizziness after administration.
Statistical and Meta-analysis
Outcomes analysis was performed with Review Manager software (RevMan, version 5.3.0, Cochrane Collaboration) [18]. Mean difference (MD) and 95% confidence interval (CI) was adopted to portray the continuous results and odds ratio (OR) and 95% CI were adopted to analyze the dichotomous results. The I-square (I2) test was applied to evaluate the effect of heterogeneity on the results of a meta-analysis. If we found statistical heterogeneity, the random-effect model was utilized (P < 0.05). Instead, we considered the study homogeneous and selected a fixed effects model for the analysis. The results were visualized by forest plot, and a P value of less than 0.05 was considered significant.
Compliance with Ethics Guidelines
This article is based on previously conducted research and does not contain any new studies on human participants or animals by any of the authors.
Results
Characteristics of Included Studies
Based on the search strategy developed above, a total of 59 articles were obtained (PubMed: 21; EMBASE: 13; Cochrane Controlled Trials Register: 18; Web of Science: seven). Of these, 46 irrelevant or duplicated articles were excluded. Following a screening of titles and abstracts, five articles were excluded. After examining the tables and figures in each article, three articles were excluded due to the inability to obtain full text or the absence of effective data. Finally, five RCTs were selected for final analysis [19,20,21,22,23]. The characters of included studies are given in Table 2.
The Quality of Included Studies
All five included studies were RCTs, among which four studies were double-blind RCTs [19,20,21,22]. One study [23] was conducted without blindness, and therefore graded the quality of evidence as high risk of bias “-”. In terms of selective reporting and other bias, two studies were judged as having unclear risk of bias “?”. The results of the risk of bias assessment are reported in Fig. 2. The agreement between reviewers reached a kappa score of 0.82.
Efficacy
The severity of pain was quantified by the pain score in the questionnaire. We considered the changes in the mean value of pain scores as the main outcome to determine the efficacy of treatment with ketamine.
Changes in Pain Score at 5 min
Three RCTs reported the data that the changes in the pain score at 5 min after treatment of renal colic with ketamine and opioids (Fig. 3A). Considering P < 0.05, we regarded the study as heterogeneous and therefore chose a random-effects model for the analysis. The results identified that therapy with ketamine exhibited similar effects on pain scores as opioids after 5 min of treatment (MD = − 0.40, 95% CI: − 1.82 to 1.01, P = 0.57).
Changes in Pain Score at 15 min
Four RCTs, incorporating a total of 554 patients, demonstrated the changes in the pain score 15 min after treatment (Fig. 3B). The test of heterogeneity proved the existence of statistical heterogeneity across groups, and therefore a random-effects model was applied for evaluating the results, which reflected MD was − 0.15, with a 95% CI of − 0.82 to − 0.52 (P = 0.67). We concluded that the effect of ketamine on pain score was similar to that of opioids after 15 min of treatment.
Changes in Pain Score at 30 min
Five RCTs with a sample of 690 patients revealed in the pain score at 30 min after treatment (Fig. 3C). As notable statistical heterogeneity was observed among the groups, a random-effects model was applied for the meta-analysis. The model revealed that the MD was 0.38, the 95% CI was − 0.25 to 1.01, the I2 was 83%, and the Chi-squared value was 23.81 (P = 0.24). We concluded that the ketamine and opioids groups were similar in terms of the pain score 30 min after treatment.
Changes in pain score at 60 min
Three RCTs analyzed the changes in the pain score at 30 min after treatment of renal colic with ketamine and opioids (Fig. 3D). Pooled results from a fixed-effects model visualized by the forest plot (MD − 0.12, 95% CI – 0.22 to − 0.02, I2 = 68%, Chi2 = 6.19, P = 0.02). From these results, we suggested superior effects of ketamine for pain reduction compared to opioids at 60 min after treatment.
Safety
Hypotensive
Two of the RCTs included in our study examined the risk of hypotensive after treatment of renal colic with ketamine and opioids (Fig. 4A). A fixed-effects model was selected to perform the analysis based on the heterogeneity test (P > 0.05), and it was observed that the OR was 0.08 and the 95% CI was 0.01 to 0.65 (P = 0.02). Our analysis revealed that the risk of hypotensive after treatment in the ketamine group was significantly better than that in the opioids group.
Nausea
Five RCTs with a sample of 690 patients explored the risk of nausea after treatment (Fig. 4B). With a random-effects model, the OR was 0.42 (95% CI 0.04–4.87, P = 0.49). Based on the above results, we found no significant differences in the risk of nausea after treatment between the two groups.
Vomiting
Two RCTs investigated the data that the incidence of vomiting after treatment of renal colic with ketamine and opioids (Fig. 4C). Due to P > 0.05, we performed a fixed-effects model for the analysis. The results showed that there was no significant difference between the two groups in the incidence of vomiting (OR = 1.25, 95% CI 0.50–3.14, P = 0.64).
Dizziness
Four RCTs with a sample of 554 patients detected the occurrence of dizziness after treatment between the two groups (Fig. 4D). A random-effects model showed that OR was 2.47 (95% CI 0.50–12.33, P = 0. 27). By combining with the above results, we found no significant differences in the risk of dizziness after treatment between the two groups.
Blood Pressure
Two RCTs compared the changes in blood pressure (systolic pressure) after treatment of renal colic with ketamine and opioids (Fig. 4E). Due to P > 0.05, a fixed-effects model was applied for the analysis. The results showed that the difference between the groups was not statistically significant in blood pressure after treatment (OR = 1.11, 95% CI − 2.00 to 4.23, P = 0.48).
Grading of Evidence
By the GRADE methodology, we collected evidence from systematic reviews to summarize the outcomes (Table 3).
Discussion
Abdominal pain due to kidney stone accounts for 1% of emergency department visits [24]. Epidemiological studies have revealed that the prevalence of kidney stones is around 1–5% in Asia, 5–9% in Europe, 13% in North America, and 20% in Saudi Arabia [25]. Also, the incidence of kidney stones in Western countries was also increasing year by year [26]. Most kidney stones are discovered when patients seek treatment in the emergency department due to an attack of renal colic. Sufficient, safe, and timely analgesia was an essential component of the management of patients with renal colic in emergency medicine.
Ketamine, a non-competitive N-methyl-d-aspartate receptor (NMDAR) antagonist, is a widespread analgesic and dissociative anesthetic agent. Despite being best characterized by its dissociative anesthetic properties, various new indications for ketamine have been identified in a variety of clinical settings including anesthesia, pain medicine, and psychiatry in recent years [27]. Zarate et al. demonstrated that intravenous NMDAR produced robust and rapid antidepressant effects [28]. In addition, ketamine is also increasingly used to treat acute and chronic pain [29]. The primary receptor target of ketamine is the NMDAR. As a non-competitive open-channel blocker, ketamine exerts analgesic effects by binding to the allosteric PCP site located within the pore of the NMDAR channel [30, 31]. The NMDAR has been proven to play an essential role in learning, memory, synaptic plasticity, and pain perception [32]. However, the molecular mechanisms of ketamine are not restricted to NMDAR, and some studies also pointed out that ketamine could bind to opioid receptors, monoamines, cholinergic, and adrenoceptor systems [33]. In the management of acute pain, sub-dissociative doses of ketamine (0.1–0.6 mg/kg) have been shown to provide favorable analgesia effects in patients with opioid-tolerant pain and opioid-induced nociceptive hypersensitivity states. Because ketamine has sympathomimetic activity, it may cause tachycardia, hypertension, increased intracranial pressure, and vomiting [34]. Despite these side effects, ketamine is an ideal drug because of its short half-life and the absence of clinically significant respiratory depression [35].
Many published studies now compare the analgesic effects of ketamine with those of opioids in patients with acute pain. Motov et al. [36] found that ketamine is as efficacious as morphine in pain relief and showed a favorable safety profile. In another study, Abdolkarimi et al. [37] proved that ketamine showed a better clinical effect in pain relief than meperidine. An RCT carried out by Shimonovich et al. [38] analyzed the efficacy and safety of intranasal ketamine for acute traumatic pain in the emergency department. They concluded that intranasal ketamine and intravenous, intranasal morphine provided similar safety and efficacy. Frouzan et al. [39] also explored the analgesic effects of ketamine and morphine in patients with fractures, revealing that morphine had a better analgesic effect than ketamine. Furthermore, Lester et al. [40] reported that the treatment of sub-dissociative ketamine in the emergency department may be a safe and effective analgesic adjunct.
In our research, we scrutinized five RCTs enrolled 690 participants with renal colic to systematically analyze the efficacy and safety of ketamine and opioids in the treatment of renal colic. We found that both groups showed a similar degree of improvement in their pain symptoms at 5, 15, and 30 min after administration. However, patients in the ketamine group exhibited significantly improved pain levels at 60 min than the opioids group, suggesting that it could provide a more persistent analgesic effect for renal colic. Regarding safety, the ketamine group was superior to the opioids group in terms of the risk of hypotensive after treatment. Regarding the other safety outcome measures, the incidence of nausea, vomiting, and dizziness was similar between the two groups.
Based on the above results, we considered the satisfactory safety of ketamine in treating renal colic. Moreover, in the case of emergency room visits, respiratory mechanics and hemodynamics monitoring is a difficult challenge. The study by Shimonovich et al. [38] found that ketamine could be efficient not only in alleviating pain but also in reducing the risks of hemodynamic instability and respiratory side effects. Thus, ketamine has some advantages in emergency pain management and may serve as an alternative to conventional drugs for pain relief in patients with renal colic. These findings offered an alternative option to clinicians and provided a new therapeutic strategy for renal colic in urological emergencies.
As far as we are aware, no previous meta-analyses are reporting on the efficacy and safety of ketamine for renal colic. Our study includes studies that are all findings from RCTs, which we considered to have a low risk of bias and may be thought to be the main strength of this study. The results of meta-analysis carry great importance from a scientific standpoint but also in clinical practice. However, our study still has some shortcomings and there is still much work to be done in the future. First, the mode of administration of ketamine in the study by Arash et al. [20] was intravenous, but other RCTs included in our meta-analysis reported that those of ketamine were intranasal. Such differences could potentially lead to bias in the results. Future studies concentrating on the most recent RCTs are needed to solve this problem. Second, the pain assessment tools used in the study by Mahboub et al. [19] are different from those used in other included RCTs, which might also result in bias. Third, the included RCTs are all from Iran, which limits the applicability of the findings of our study. Therefore, further high-quality RCTs are recommended to determine the efficacy and safety of ketamine in the treatment of renal colic.
Conclusions
Compared to opioids, the use of ketamine produces more persistent relief in patients with renal colic and has a much better safety profile. Our meta-analysis concluded that ketamine holds promise as an alternative to opioids for renal colic patients in pain management in the emergency department.
References
Yasui T, Okada A, Hamamoto S, et al. Pathophysiology-based treatment of urolithiasis. Int J Urol. 2017;24(1):32–8. https://doi.org/10.1111/iju.13187.
Jokar A, Cyrus A, Babaei M, et al. The effect of magnesium sulfate on renal colic pain relief: a randomized clinical trial. Emerg (Tehran). 2017;5(1):e25.
Walls R, Hockberger R, Gausche-Hill M. Rosen’s emergency medicine-concepts and clinical practice e-book. Elsevier Health Sciences; 2017.
Loftus C, Nyame Y, Hinck B, et al. Medical expulsive therapy is underused for the management of renal colic in the emergency setting. J Urol. 2016;195(4 Pt 1):987–91. https://doi.org/10.1016/j.juro.2015.11.026.
O’Connor A, Schug SA, Cardwell H. A comparison of the efficacy and safety of morphine and pethidine as analgesia for suspected renal colic in the emergency setting. J Accid Emerg Med. 2000;17(4):261–4. https://doi.org/10.1136/emj.17.4.261.
Schmidt S, Kroeger N. Pain therapy for acute renal colics: Nonsteroidal anti-inflammatory drugs (NSAIDs) and non-opioids. Urologe A. 2016;55(3):386–90. https://doi.org/10.1007/s00120-016-0027-3.
Zamanian F, Jalili M, Moradi-Lakeh M, Kia M, Aghili R, Aghili SM. Morphine suppository versus indomethacin suppository in the management of renal colic: randomized clinical trial. Pain Res Treat. 2016;2016:4981585. https://doi.org/10.1155/2016/4981585.
Larkin GL, Peacock WF, Pearl SM, Blair GA, D’Amico F. Efficacy of ketorolac tromethamine versus meperidine in the ED treatment of acute renal colic. Am J Emerg Med. 1999;17(1):6–10. https://doi.org/10.1016/s0735-6757(99)90003-7.
Wilkerson RG, Kim HK, Windsor TA, Mareiniss DP. The opioid epidemic in the United States. Emerg Med Clin N Am. 2016;34(2):e1–23. https://doi.org/10.1016/j.emc.2015.11.002.
Faridaalaee G, Mohammadi N, Merghati SZ, et al. Intravenous morphine vs intravenous ketofol for treating renal colic: a randomized controlled trial. Emerg (Tehran). 2016;4(4):202–6.
Shirazi M, Salehipour M, Afrasiabi MA, et al. Analgesic effects and safety of desmopressin, tramadol and indomethacin in patients with acute renal colic: a randomized clinical trial. Bull Emerg Trauma. 2015;3(2):41–5.
Pathan SA, Mitra B, Cameron PA. A systematic review and meta-analysis comparing the efficacy of nonsteroidal anti-inflammatory drugs, opioids, and paracetamol in the treatment of acute renal colic. Eur Urol. 2018;73(4):583–95. https://doi.org/10.1016/j.eururo.2017.11.001.
Pathan SA, Mitra B, Straney LD, et al. Delivering safe and effective analgesia for management of renal colic in the emergency department: a double-blind, multigroup, randomised controlled trial. Lancet. 2016;387(10032):1999–2007. https://doi.org/10.1016/S0140-6736(16)00652-8.
Domino EF. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113(3):678–84. https://doi.org/10.1097/ALN.0b013e3181ed09a2.
WHO. Fact file on ketamine. World Health Organization; 2016.
Pasero C, McCaffery M. Pain control: ketamine: low doses may provide relief for some painful conditions. Am J Nurs. 2005;105(4):60–4. https://doi.org/10.1097/00000446-200504000-00028.
Vader JP. Randomised controlled trials: a user’s guide. BMJ. 1998;317:1258. https://doi.org/10.1136/bmj.317.7167.1258.
Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142. https://doi.org/10.1002/14651858.ED000142.
Pouraghaei M, Moharamzadeh P, Paknezhad SP, et al. Intranasal ketamine versus intravenous morphine for pain management in patients with renal colic: a double-blind, randomized, controlled trial. World J Urol. 2021;39(4):1263–7. https://doi.org/10.1007/s00345-020-03319-4.
Forouzan A, Masoumi K, Motamed H, et al. Comparison of the analgesic effect of intravenous ketamine versus intravenous morphine in reducing pain of renal colic patients: double-blind clinical trial study. Rev Recent Clin Trials. 2019;14(4):280–5. https://doi.org/10.2174/1574887114666190705122727.
Farnia MR, Jalali A, Vahidi E, Momeni M, Seyedhosseini J, Saeedi M. Comparison of intranasal ketamine versus IV morphine in reducing pain in patients with renal colic. Am J Emerg Med. 2017;35(3):434–7. https://doi.org/10.1016/j.ajem.2016.11.043.
Mozafari J, Maleki Verki M, Motamed H, Sabouhi A, Tirandaz F. Comparing intranasal ketamine with intravenous fentanyl in reducing pain in patients with renal colic: a double-blind randomized clinical trial. Am J Emerg Med. 2020;38(3):549–53. https://doi.org/10.1016/j.ajem.2019.05.049.
Maryam Z, Ali A. The analgesic effect of intranasal ketamine and intravenous morphine in patients with flank pain (renal colic) in the emergency department: a clinical trial study. Immunopathol Persa. 2022. https://doi.org/10.34172/ipp.2022.xx.
Marx J, Walls R, Hockberger R. Rosen’s emergency medicine-concepts and clinical practice E-book. Elsevier Health Sciences; 2013.
Ramello A, Vitale C, Marangella M. Epidemiology of nephrolithiasis. J Nephrol. 2000;13(Suppl 3):S45-50.
Amato M, Lusini ML, Nelli F. Epidemiology of nephrolithiasis today. Urol Int. 2004;72(Suppl 1):1–5. https://doi.org/10.1159/000076582.
Linda L, Phillip EV. Ketamine: 50 years of modulating the mind. Front Hum Neurosci. 2016;29(10):612. https://doi.org/10.3389/fnhum.2016.00612.
Carlos A, Jaskaran B, Paul J, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64. https://doi.org/10.1001/archpsyc.63.8.856.
Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol. 2014;77(2):357–67. https://doi.org/10.1111/bcp.12094.
Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621–60. https://doi.org/10.1124/pr.117.015198.
Zorumski CF, Izumi Y, Mennerick S. Ketamine: NMDA receptors and beyond. J Neurosci. 2016;36(44):11158–64. https://doi.org/10.1523/JNEUROSCI.1547-16.2016.
Zoghbi HY, Gage FH, Choi DW. Neurobiology of disease. Curr Opin Neurobiol. 2000;10(5):655–60. https://doi.org/10.1016/s0959-4388(00)00135-5.
Shrestha R, Pant S, Shrestha A, Batajoo KH, Thapa R, Vaidya S. Intranasal ketamine for the treatment of patients with acute pain in the emergency department. World J Emerg Med. 2016;7(1):19–24. https://doi.org/10.5847/wjem.j.1920-8642.2016.01.003.
Bg K. Anesthetic agents basic and clinical pharmacology. 1st ed. McGraw Hill; 2010. p. 345–7.
Andrew W, David M, Harish R. Intravenous sub-anesthetic ketamine for perioperative analgesia. J Anaesthesiol Clin Pharmacol. 2016;32(2):160–7. https://doi.org/10.4103/0970-9185.182085.
Motov S, et al. Intravenous sub dissociative-dose ketamine versus morphine for analgesia in the emergency department: a randomized controlled trial. Ann Emerg Med. 2015;66(3):222–9.
Abdolkarimi B, Zareifar S, Golestani Eraghi M, Saleh F. Comparison effect of intravenous ketamine with pethidine for analgesia and sedation during bone marrow procedures in oncologic children: a randomized, double-blinded, crossover trial. Int J Hematol Oncol Stem Cell Res. 2016;10(4):206–11.
Shimonovich S, Gigi R, Shapira A, et al. Intranasal ketamine for acute traumatic pain in the emergency department: a prospective, randomized clinical trial of efficacy and safety. BMC Emerg Med. 2016;16(1):43. https://doi.org/10.1186/s12873-016-0107-0.
Forouzan A, Masoumi K, Motamed H, Mozafari J, Gharibi S. Comparison of intranasal ketamine versus intravenous morphine in pain relief of patient with bone fracture. Int J Adv Biotechnol Res. 2017;1(8):1636–43.
Lester LM, Braude DM, Niles CM. Low-dose ketamine for analgesia in the ED: a retrospective case series. Am J Emerg Med. 2010;28:820–7. https://doi.org/10.1016/j.ajem.2009.07.023.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
Xiaopeng Hu and Dongxu Zhang designed the research, interpreted the data and revised the paper. Dongxu Zhang, Pu Liang, Bowen Xia, and Xin Zhang performed the data extraction and carried out the meta-analysis. Dongxu Zhang and Pu Liang drafted the paper. All of the authors approved the submitted and final versions.
Disclosures
The authors declare that they have no competing interests.
Compliance with Ethics Guidelines
This article is based on previously conducted research and does not contain any new studies on human participants or animals by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhang, D., Liang, P., Xia, B. et al. Efficacy and Safety of Ketamine Versus Opiates in the Treatment of Patients with Renal Colic: A Systematic Review and Meta-analysis. Pain Ther 12, 1079–1093 (2023). https://doi.org/10.1007/s40122-023-00530-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00530-0