Abstract
Introduction
Chronic refractory low back and lower extremity pain recalcitrant to conservative management and epidural injections secondary to postsurgery syndrome, spinal stenosis, and disc herniation are sometimes managed with percutaneous adhesiolysis. Consequently, this systematic review and meta-analysis was undertaken to assess the efficacy of percutaneous adhesiolysis in managing low back and lower extremity pain.
Methods
A systematic review and meta-analysis of randomized controlled trials (RCTs) utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was performed. A comprehensive literature search of multiple databases from 1966 to July 2022, including manual searches of the bibliography of known review articles was performed. Quality assessment of the included trials, meta-analysis, and best evidence synthesis was performed.
The primary outcome measure was a significant reduction in pain (short term up to 6 months and long term more than 6 months).
Results
The search identified 26 publications, with 9 trials meeting the inclusion criteria. The results of dual-arm and single-arm analyses showed significant improvement in pain and function at 12 months. Opioid consumption was also significantly reduced at 6 months with dual-arm analysis, whereas single-arm analysis showed a significant decrease from baseline to treatment at the 3-, 6-, and 12-month analyses. At 1 year follow-up, seven of seven trials were positive for improvements in pain relief, function, and diminution of opioid use.
Discussion
Based on the present systematic review of nine RCTs, the evidence level is I to II, with moderate to strong recommendation for percutaneous adhesiolysis in managing low back and lower extremity pain. The limitations of the evidence include paucity of literature, lack of placebo-controlled trials, and the majority of the trials studying post lumbar surgery syndrome.
Conclusion
The evidence is level I to II or strong to moderate based on five high-quality and two moderate-quality RCTs, with 1 year follow-up that percutaneous adhesiolysis is efficacious in the treatment of chronic refractory low back and lower extremity pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Chronic refractory low back and lower extremity pain secondary to post lumbar surgery syndrome, spinal stenosis, and disc herniation is common. |
2. Disc herniation, spinal stenosis, and postsurgery syndrome is managed with multiple interventional techniques, implantable therapies, or repeat surgical interventions. |
3. The present systematic review identified seven high-quality and two moderate-quality randomized controlled trials (RCTs) evaluating the role of percutaneous adhesiolysis in managing chronic recalcitrant low back and lower extremity pain. |
4. The evidence is level I to II with moderate to strong recommendation in managing low back and lower extremity pain after failure of conservative management and fluoroscopically directed epidural injections. |
5. Significant paucity of the literature and heterogeneity among available trials continues to be an issue, resulting in an ongoing debate regarding efficacy, effectiveness, indications, and medical necessity. |
Introduction
Chronic refractory low back pain with or without lower extremity pain that does not resolve after conservative therapy or even surgical treatment can present a therapeutic dilemma with limited options for proper management [1,2,3,4,5,6,7,8]. Low back and lower extremity pain recalcitrant to conservative management and epidural injections may be secondary to postsurgery syndrome, spinal stenosis, and disc herniation [1,2,3,4,5,6,7,8]. Disc herniation and spinal stenosis are often managed with surgical interventions and postsurgery syndrome may also be managed with repeat surgical interventions or implantable therapies. However, for those patients who are not responsive to or candidates for surgical interventions and/or have not adequately responded to epidural injections, percutaneous adhesiolysis may be an option [1,2,3,4,5,6,7,8,9,10,11,12]. Percutaneous adhesiolysis is also considered an option in patients not amenable to or having an inadequate response to neuromodulation therapies [1, 7,8,9,10,11,12,13,14,15,16]. Changing coverage policies have impacted utilization patterns of interventional techniques in general and percutaneous adhesiolysis in particular [1, 9,10,11,12,13,14,15,16].
National health care expenditures are an important issue, specifically since the COVID-19 pandemic, which has drastically altered health care delivery and modes of treatment [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. The COVID-19 pandemic and the opioid epidemic have negatively impacted access to treatment and costs in chronic pain sufferers [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. The analysis of national health care spending in the USA showed an increase of 9.7% to reach $4.1 trillion in 2020, compared with a 4.3% increase seen in 2019 [17, 18]. The acceleration in 2020 was related to a 36% increase in federal expenditures for health care that occurred largely in response to the COVID-19 pandemic. Multiple other factors, including consolidation of providers into an employment model by health systems, which has increased substantially, has been contributing to increasing health care expenses [17, 18, 23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Multiple effects due to COVID-19, with increased psychological stress and suffering, may also have a significant effect on outcomes [27, 30,31,32]. An analysis of the utilization patterns in the fee-for-service (FFS) Medicare population, including the impact of COVID-19, showed declining interventional techniques with an overall decrease of interventional techniques at an annual rate of 0.4% per 100,000 Medicare population from 2010 to 2019, and a decrease of 24.5% for epidural injections and adhesiolysis procedures [19]. However, the decrease from 2019 to 2020 due to the COVID-19 pandemic was 18.7% for all interventions compared with 19.0% for epidural and adhesiolysis procedures [19]. Additionally, epidural-specific utilization patterns [22] showed an overall decrease of utilization of epidural injections of 24.1% annually from 2010 to 2019, with a significant effect of the COVID-19 pandemic showing a 19.0% decrease from 2019 to 2020 [22]. Further, compared with multiple other interventions, including epidural injections, facet joint interventions, and sacroiliac joint interventions, augmentation procedures [10, 11, 14,15,16] and percutaneous adhesiolysis [12] have faced a substantial decline at a rapid rate. There is discordance of opinions on the efficacy and effectiveness of medical necessity and indications among various authorities [1,2,3,4,5,6,7,8].
Helm et al. [6] published a systematic review of percutaneous adhesiolysis in 2016 utilizing seven randomized controlled trials (RCTs) and three observational studies, concluding with level I or strong evidence of the efficacy of percutaneous adhesiolysis in the treatment of chronic refractory low back and lower extremity pain. In subsequent reports, Cho et al. [7] and Manchikanti et al. [2,3,4] have shown level II–I evidence for post lumbar surgery syndrome, spinal stenosis, and disc herniation. In fact, Cho et al. [7] have shown significant evidence for both percutaneous adhesiolysis and spinal cord stimulation (SCS) with a recommendation of level A for epidural adhesiolysis for 6–12 months of pain relief and functional improvement and level B for SCS.
In contrast, Brito-García et al. [8] in a systematic review without meta-analysis provided a rather poor methodological quality assessment of the rating of the trials, with downgrading to low quality, which have been rated as high quality in multiple other evaluations. Overall, they concluded that there was no evidence for percutaneous adhesiolysis. Thus, of the five systematic reviews, three of them, including a meta-analysis and one systematic review without meta-analysis, showed positive results compared with only one systematic review that, although it looked at similar studies, concluded very differently. Manchikanti et al. [5], in assessing systematic reviews with a systematic analysis, identified multiple issues in one of the systematic reviews. Further, Manchikanti et al. [1, 9, 37,38,39,40] and others [41, 42] have also described extensively the issues related to performance of evidence synthesis in systematic reviews and meta-analysis.
Consequently, to assess the efficacy of percutaneous adhesiolysis in managing low back and lower extremity pain this systematic review and meta-analysis of RCTs was undertaken..
Methods
A systematic review and meta-analysis were performed based on the methodological and reporting quality of systematic reviews, as described by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [43]. Methodology from other reviews was also utilized [2,3,4,5, 37,38,39, 44].
Eligibility Criteria
Randomized trials of interest included patients suffering from chronic low back and lower extremity pain due to postsurgery syndrome, spinal stenosis, and disc herniation and treated with percutaneous epidural neurolysis or adhesiolysis. Trials of patients with fractures, malignancies, acute trauma, and inflammatory diseases were excluded. All RCTs were included.
This review focused on lumbar percutaneous adhesiolysis/neurolysis for postsurgery syndrome, central spinal stenosis, and disc herniation with multiple approaches. All trials that provided appropriate outcome data and analysis for 6 months were reviewed. Book chapters, case reports, and reports without an appropriate diagnosis were not considered.
Information Sources
All available studies in the English language, or with available translation, with appropriate reporting of outcomes data for 6 months were included. Searches were performed using multiple databases, including PubMed, www.ncbi.nlm.nih.gov/pubmed; Cochrane Library, www.thecochranelibrary.com; US National Guideline Clearinghouse (NGC), www.guideline.gov/; clinical trials, www.clinicaltrials.gov/; and Google Scholar, https://scholar.google.com; from 1966 to July 2022 [4].
Search Strategy
The search terminology was as follows:
(chronic low back pain OR nerve root compression OR lumbosciatic pain OR radicular pain OR radiculitis OR sciatica OR disc herniation, postsurgery syndrome, failed surgery syndrome, spinal stenosis) AND (epidural injection OR epidural adhesiolysis OR neurolysis OR epidural neuroplasty OR epidural lysis of adhesions OR percutaneous adhesiolysis OR neurolysis OR transforaminal injection OR corticosteroid OR methylprednisolone OR bupivacaine OR lidocaine) AND (meta-analysis [pt] OR randomized controlled trial [pt] OR controlled clinical trial [pt] OR systematic review OR randomized controlled trials [mh] OR nonrandomized studies OR observational studies OR random allocation [mh] OR double-blind method [mh] OR single-blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR (“clinical trial” [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp]).
Data Selection
In the identification of the relevant literature, the article selection and extraction of the data from the included studies was conducted independently, by two review authors (N.N.K. and M.R.S.). Any disagreement among the reviewer authors were resolved by the third author (A.D.K.). All conflicts of interest of the reviewers with authorship of the article was resolved by assigning them to other reviewers.
Study Risk of Bias Assessment
Two authors completed the quality assessment of each individual article. Three authors completed evidence synthesis. All conflicts were resolved as stated above by a fourth author.
The quality of each RCT was assessed using the Cochrane Review rating system (see Table S1 in the electronic supplementary material for details) [45] and the Interventional Pain Management Techniques–Quality Appraisal of Reliability and Risk of Bias Assessment Tool (IPM–QRB) for RCTs (aee Table S2 in the electronic supplementary material for details) [46].
Randomized trials meeting at least 9 of the 13 inclusion criteria of the Cochrane Review were considered high quality. The trials meeting 5–8 criteria were considered moderate quality, and those meeting fewer than 5 criteria were considered low quality and were excluded.
Based on the IPM–QRB criteria, randomized trials with scores of 32–48 were considered high quality, studies scoring 16–31 were considered moderate quality, and studies scoring less than 16 were considered low quality and were excluded.
Analysis of the Evidence
Analysis of the evidence was performed by two authors, N.N.K. and E.K., with consultation from A.D.K., M.R.S., and J.A.H. Any disagreements among the authors was resolved by consensus or by A.D.K. and J.A.H.
Outcome of the Studies
Clinically important outcome measures were 50% significant improvement from the baseline pain score or a change of at least 3 points on an 11-point pain scale of 0 to 10 and a change of 30% or more on disability scores [4].
Based on the relevance and effectiveness of the adhesiolysis, either compared with a control group or from baseline to follow-up, a trial was categorized as positive or negative neutral. Reference point measurements were considered at 3 months, 6 months, and 1 year [4].
The best-evidence synthesis developed by American Society of Interventional Pain Physicians (ASIPP), modified, and collated using multiple criteria, was used for qualitative analysis (Table 1) [47]. The evidence synthesis varied from strong to opinion- or consensus-based using five levels of evidence.
Table 2 presents guidance for the strength of recommendations from weak to strong [48].
The results of best evidence as per grading were utilized and the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) system of appraisal was used for determining the body of evidence [49]. Clinical relevance and pragmatism of all studies were assessed [50].
Meta-analysis
Dual-Arm Meta-analysis
For the dual-arm meta-analysis, Review Manager version 5.4 (The Cochrane Collaboration) 2020, software was used. For pain and functionality improvement data, the studies were reported as the standardized mean differences (SMD) with 95% confidence intervals (CI). Data were plotted using forest plots to evaluate treatment effects using a random effects model. Heterogeneity was interpreted through I2 statistics [40].
Single-Arm Meta-analysis
For the single-arm meta-analysis, Comprehensive Meta-Analysis version 3.0 (Biostat Inc., Englewood, NJ) software was used. For pain and functionality improvement data, the studies were reported as the mean differences with 95% confidence intervals. Data were plotted using forest plots to evaluate treatment effects. Heterogeneity was interpreted through I2 statistics [40].
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Study Selection
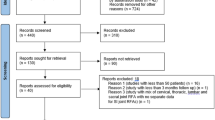
Figure 1 shows a flow diagram of the study selection using the PRISMA study selection process.
Based on the search criteria, 26 publications were identified and considered for inclusion [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76]. A total of 12 trials [51,52,53,54,55,56,57,58,59, 61, 62, 76] met the inclusion criteria and 9 trials were included after exclusion of duplicates, follow-up evaluations, and qualifications [51,52,53,54, 56, 58, 61, 62, 76]. Three trials reported follow-up results, consequently, these were not considered as separate studies [54,55,56,57,58,59]. Of the nine trials included, six of them studied postsurgery syndrome [51,52,53,54, 62, 76], two trials studied spinal stenosis [56, 61], and one trial studied disc herniation [58]. Of the included trials, only one trial was placebo controlled [58], and eight were active controlled trials [51,52,53,54, 56, 61, 62, 76].
Methodological Quality and Risk of Bias Assessment
Tables 3 and 4 present the methodological quality assessment and risk of bias of the nine RCTs utilizing the Cochrane review criteria and the IPM–QRB criteria, respectively [51,52,53,54, 56, 58, 61, 62, 76]. Assessment by the Cochrane review criteria showed all of them as high-quality trials, scoring at least 9 of 13. However, based on the IPM–QRB instrument, seven of the nine trials [52, 54, 56, 58, 61, 62, 76] scored as high, with scores of over 32 of 48. The remaining two studies [51, 53] showed moderate quality, with scores above 16.
Study Characteristics
Table 5 presents the characteristics and outcomes of the studies meeting the inclusion criteria for receiving percutaneous adhesiolysis/neurolysis for lumbar disc herniation.
Results of Individual Studies
Qualitative Analysis
Qualitative analysis at 6-months follow-up, showed that one of the nine trials had negative results [61]; however, at 1-year follow-up, seven trials showed positive results [51,52,53,54, 56, 58, 62].
Qualitative analysis was also performed, utilizing a modified approach of grading of evidence with moderate (level II) evidence from seven relevant high-quality RCTs [52, 54, 56, 58, 61, 62, 76] and two relevant moderate-quality RCTs [51, 53].
Utilizing the GRADE criteria [49], there was no change in the evidence level. All the trials were considered to meet clinical relevance and pragmatism [50]. All the included trials met pragmatic criteria for clinical relevance and pragmatism [50]. In addition, the evidence was assessed by qualitative and quantitative evidence synthesis utilizing conventional dual-arm and single-arm meta-analyses. Further, the results of grading utilizing the GRADE system of appraisal for determining the body of evidence showed no change in the evidence levels.
Quantitative Analysis
Pain Level at 3 Months
Figure 2A–C shows the change in pain level using the Numeric Rating Scale (NRS) or Visual Analog Scale (VAS) at 3 months.
Assessment of pain levels at 3 months utilizing dual-arm and single-arm meta-analyses. A Pain at 3 months, percutaneous adhesiolysis versus control, dual-arm meta-analysis. B Pain at 3 months in percutaneous adhesiolysis groups with single-arm meta-analysis. C Pain at 3 months in control groups with single-arm meta-analysis
Dual-Arm Meta-analysis
There were seven trials [52,53,54,55,56, 58, 61] with 548 patients that compared percutaneous adhesiolysis with the control group in a dual-arm meta-analysis. The results showed a statistically significant difference in pain levels between these two groups [SMD −1.21 (−1.67, −0.75), p < 0.0001] (Fig. 2A).
Single-Arm Meta-analysis
Figure 2B shows the results of a single-arm meta-analysis utilizing the percutaneous adhesiolysis group. There were eight trials [52,53,54,55,56,57,58, 61] that assessed pain scores at 3 months using NRS or VAS in patients who underwent percutaneous adhesiolysis. As shown in Fig. 2B, the pooled mean difference of pain scores from the baseline to 3 month follow-up was a 4.499 point decrease (95% CI −4.608 to −4.390, p < 0.0001).
Figure 2C shows the results of a single-arm meta-analysis utilizing a control group. There were seven trials [52,53,54,55,56, 58, 61] that assessed pain scores at 3 months using NRS or VAS in patients from the control group. As shown in Fig. 2C, the pooled mean difference of pain scores from the baseline to 3 month follow-up was a 2.585 point decrease (95% CI −2.750 to −2.419, p < 0.001).
Functionality at 3 Months
Figure 3A–C shows the change in functionality level using the Oswestry Disability Index (ODI) at 3 months.
Assessment of functional status at 3 months utilizing dual-arm and single-arm meta-analyses. A Functionality at 3 months, percutaneous adhesiolysis versus control, dual-arm meta-analysis. B Functionality at 3 months in percutaneous adhesiolysis groups with single-arm meta-analysis. C Functionality at 3 months in the control single-arm meta-analysis
Dual-Arm Meta-analysis
There were seven trials [52,53,54,55,56, 58, 61] with 548 patients that compared percutaneous adhesiolysis with a control group in a dual-arm meta-analysis. The results showed a statistically significant difference in functionality levels between these two groups [SMD −1.10 (−1.53, −0.67), p < 0.0001] (Fig. 3A).
Single-Arm Meta-analysis
Figure 3B shows the results of a single-arm meta-analysis utilizing the percutaneous adhesiolysis group. There were eight trials [52,53,54,55,56,57,58, 61] that assessed the functionality scores at 3 months using ODI in patients who underwent percutaneous adhesiolysis. As shown in Fig. 3B, the pooled mean difference of functionality scores from the baseline to 3 month follow-up was a 15.914 point decrease (95% CI −16.458 to −15.371, p < 0.0001).
Figure 3C shows the results of a single-arm meta-analysis utilizing a control group. There were seven trials [52,53,54,55,56, 58, 61] used to assess functionality scores at 3 months using ODI in patients from the control group. As shown in Fig. 3C, the pooled mean difference of functionality scores from the baseline to 3 month follow-up was a 7.819 point decrease (95% CI: 8.616 to −7.021, p < 0.0001).
Opioid Consumption at 3 Months
Figure 4A–C shows the change in opioid intake using the morphine milligram equivalent scale (MMEq) at 3 months.
Assessment of opioid consumption at 3 months utilizing dual-arm and single-arm meta-analyses. A Opioid consumption at 3 months, percutaneous adhesiolysis versus control, dual-arm meta-analysis. B Opioid consumption at 3 months in percutaneous adhesiolysis groups with single-arm meta-analysis. C Opioid consumption at 3 months in control groups with a single-arm meta-analysis
Dual-Arm Meta-analysis
There were three trials [54,55,56] with 287 patients that compared percutaneous adhesiolysis with the control group in a dual-arm meta-analysis. The results showed no statistically significant difference in opioid intake between these two groups [SMD −0.32 (−0.78, 0.13) p = 0.16] (Fig. 4A).
Single-Arm Meta-analysis
Figure 4B shows the change in opioid intake using the MMEq at 3 months for patients undergoing percutaneous adhesiolysis. There were four trials [54,55,56,57] with a pooled mean reduction in opioid intake from baseline to 3 months of follow-up of 13.493 MMEq (95% CI −18.266 to −8.720, p < 0.0001).
Figure 4C shows the change in opioid intake using the MMEq at 3 months for patients in the control treatment. There were three trials [54,55,56] with a pooled mean reduction in opioid intake from baseline to 3 months of follow-up of 2.736 MMEq (95% CI −7.406 to 1.935, p = 0.251).
Overall, at 3 months, there was a significant improvement with percutaneous adhesiolysis utilizing dual- and single-arm meta-analyses with pain and function. In reference to opioid consumption, while there was no significant difference with the dual-arm analysis, with the single-arm analysis, opioid consumption was decreased by 13.5 MMEq in percutaneous adhesiolysis groups, whereas in the control groups, it was reduced by 2.7 MMEq. Further, there was significant decrease in pain relief of 4.5 points in adhesiolysis groups compared with 2.6 points in control groups.
Pain at 6 Months: Percutaneous Adhesiolysis versus Control
Figures 5A–C showed the change in pain level using the NRS or VAS at 6 months.
Assessment of pain levels at 6 months utilizing dual-arm and single-arm meta-analyses. A Pain at 6 months, percutaneous adhesiolysis versus control, dual-arm meta-analysis, single-arm meta-analysis. B Pain at 6 months in percutaneous adhesiolysis groups with single-arm meta-analysis. C. Pain at 6 months in control groups with single-arm meta-analysis
Dual-Arm Meta-analysis
There were seven trials [52,53,54,55,56, 58, 61] with 504 patients that compared percutaneous adhesiolysis with a control group in a dual-arm meta-analysis. The results showed a statistically significant difference in pain levels between these two groups [SMD −1.49 (−2.20, −0.78), p < 0.0001] (Fig. 5A).
Single-Arm Meta-analysis
Figure 5B shows the results of a single-arm meta-analysis utilizing a percutaneous adhesiolysis group. There were eight trials [52,53,54,55,56,57,58, 61] that assessed pain scores at 6 months using NRS or VAS in patients who underwent percutaneous adhesiolysis. As shown in Fig. 5B, the pooled mean difference of pain scores from the baseline to 6 month follow-up was a 4.420 point decrease (95% CI −4.536 to −4.304, p < 0.0001).
Figure 5C shows the results of a single-arm meta-analysis with a control group. There were seven trials [52,53,54,55,56, 58, 61] used to assess pain scores at 6 months using NRS or VAS in patients from the control group. As shown in Fig. 5C, the pooled mean difference of pain scores from the baseline to 6 month follow-up was a 2.141 point decrease (95% CI −2.313 to −1.970, p < 0.0001).
Functionality at 6 Months
Figure 6A–C shows the change in functionality level using the ODI at 6 months.
Assessment of functional status at 6 months utilizing dual-arm and single-arm meta-analyses. A Functionality at 6 months in percutaneous adhesiolysis versus control, in a dual-arm meta-analysis. B Functionality at 6 months in the percutaneous adhesiolysis group, single-arm meta-analysis. C Functionality at 6 months in the control group, single-arm meta-analysis
Dual-Arm Meta-analysis
There were seven trials [52,53,54,55,56, 58, 61] with 505 patients that compared percutaneous adhesiolysis with a control group in a dual-arm meta-analysis. The results showed a statistically significant difference in functionality levels between these two groups [SMD −1.43 (−2.13, −0.73), p < 0.0001] (Fig. 6A).
Single-Arm Meta-analysis
Figure 6B shows the results of a single-arm meta-analysis of percutaneous adhesiolysis. There were eight trials [52,53,54,55,56,57,58, 61] that assessed functionality scores at 6 months using ODI in patients who underwent percutaneous adhesiolysis. As shown in Fig. 6B, the pooled mean difference of functionality scores from the baseline to 6 month follow-up was a 16.307 point decrease (95% CI –16.875 to –15.739, p < 0.0001).
Figure 6C shows the results of a single-arm meta-analysis from the control group. There were seven trials [52,53,54,55,56, 58, 61] that assessed functionality scores at 6 months using ODI in patients from the control group. As shown in Fig. 6C, the pooled mean difference of functionality scores from the baseline to 6 month follow-up was a 6.286 point decrease (95% CI −7.097 to −5.475, p < 0.0001).
Opioid Consumption at 6 Months
Figure 7A–C shows the change in opioid intake using the MMEq at 6 months.
Assessment of opioid consumption at 6 months utilizing dual-arm and single-arm meta-analyses. A Opioid consumption at 6 months, percutaneous adhesiolysis versus control, dual-arm meta-analysis. B Opioid consumption at 6 months in percutaneous adhesiolysis groups with a single-arm meta-analysis. C. Opioid consumption at 6 months in control groups with a single-arm meta-analysis
Dual-Arm Meta-analysis
There were three trials [54,55,56] with 276 patients that compared percutaneous adhesiolysis with a control group in a dual-arm meta-analysis. The results showed a statistically significant difference in opioid intake between these two groups [SMD −0.27 (−0.51, −0.03) p = 0.03] (Fig. 7A).
Single-Arm Meta-analysis
Figure 7B shows the change in opioid intake using the MMEq at 6 months for patients undergoing percutaneous adhesiolysis. There were four trials [54,55,56,57], with a pooled mean decrease in opioid intake from baseline to 6 months of follow-up of 15.311 MMEq (95% CI −16.317 to −14.306, p < 0.0001).
Figure 7C shows the change in opioid intake using the MMEq at 6 months for patients from the control group. There were three trials [54,55,56] with a pooled mean decrease in opioid intake from baseline to 6 months of follow-up of 0.544 MMEq (95% CI −6.660 to 5.571, p = 0.861).
Overall, at 6 months follow-up, pain and function improved significantly on dual- and single-arm analyses. Marked changes were observed with the single-arm analysis from baseline to the treatment. In addition, opioid consumption at 6 months also showed a significant difference with dual-arm analysis. However, these differences were significant with single-arm analysis, with a decrease of 15.3 MMEq in percutaneous adhesiolysis groups compared with 0.5 MMEq in control groups.
Pain at 12 Months
Figure 8A–C shows the change in pain level using the NRS or VAS at 12 months.
Assessment of pain levels at 12 months utilizing dual-arm and single-arm meta-analyses. A Pain at 12 months, percutaneous adhesiolysis versus control, dual-arm meta-analysis. B Pain at 12 months in percutaneous adhesiolysis groups with single-arm meta-analysis. C Pain at 12 months in control groups with single-arm meta-analysis
Dual-Arm Meta-analysis
There were six trials [52,53,54,55,56, 58] with 362 patients that compared percutaneous adhesiolysis with a control group in a dual-arm meta-analysis. The results showed a statistically significant difference in pain levels between these two groups [SMD −1.71 (−2.19, −1.22), p < 0.0001] (Fig. 8A).
Single-Arm Meta-analysis
Figure 8B shows the results of a single-arm meta-analysis utilizing a percutaneous adhesiolysis group. There were seven trials [52,53,54,55,56,57,58] used to assess pain scores at 12 months using NRS or VAS in patients who underwent percutaneous adhesiolysis. As shown in Fig. 8B, the pooled mean difference of pain scores from the baseline to 12 month follow-up was a 4.226 point decrease (95% CI: –4.352 to –4.099, p < 0.0001).
Figure 8C shows the results of a single-arm meta-analysis utilizing the control group. There were six trials [52,53,54,55,56, 58] used to assess pain scores at 12 months using NRS or VAS in patients who from the control group. As shown in Fig. 8C, the pooled mean difference of pain scores from the baseline to 12 months follow-up was a 2.156 point decrease (95% CI −2.409 to −1.904, p < 0.0001).
Functionality at 12 Months
Figure 9A–C shows the change in functionality level using the ODI at 12 months.
Assessment of functional status at 12 months utilizing dual-arm and single-arm meta-analyses. A Functionality at 12 months, percutaneous adhesiolysis versus control, dual-arm meta-analysis. B Functionality at 12 months in percutaneous adhesiolysis groups with single-arm meta-analysis. C Functionality at 12 months in control groups with single-arm meta-analysis
Dual-Arm Meta-analysis
There were six trials [52,53,54,55,56, 58] with 362 patients that compared percutaneous adhesiolysis with a control group in a dual-arm meta-analysis. The results showed a statistically significant difference in functionality levels between these two groups [SMD −1.65 (−2.09, −1.21), p < 0.0001] (Fig. 9A).
Single-Arm Meta-analysis
Figure 9B shows the results of a single-arm meta-analysis in patients undergoing percutaneous adhesiolysis. There were seven trials [52,53,54,55,56,57,58] used to assess functionality scores at 12 months using ODI in patients who underwent percutaneous adhesiolysis. As shown in Fig. 9B, the pooled mean difference of functionality scores from the baseline to 12 months follow-up was a 15.881 point decrease (95% CI −16.485 to −15.277, p < 0.0001).
Figure 9C shows the results of a single-arm meta-analysis utilizing a control group. There were six trials [52,53,54,55,56, 58] used to assess functionality scores at 12 months using ODI in patients from the control group. As shown in Fig. 9C, the pooled mean difference of functionality scores from the baseline to 12 months follow-up was a 5.387 point decrease (95% CI −6.646 to −4.129, p < 0.0001).
Opioid Consumption at 12 Months
Figure 10A–C shows the change in opioid intake using the MMEq at 12 months.
Assessment of opioid consumption at 12 months utilizing dual-arm and single-arm meta-analyses. A Opioid consumption at 12 months, percutaneous adhesiolysis versus control, dual-arm meta-analysis. B Opioid consumption at 12 months in percutaneous adhesiolysis groups with single-arm meta-analysis. C Opioid consumption at 12 months in control groups with single-arm meta-analysis
Dual-Arm Meta-analysis
There were three trials [54,55,56] with 182 patients that compared percutaneous adhesiolysis with the control group in a dual-arm meta-analysis. The results showed no statistically significant difference in opioid intake between these two groups [SMD −0.31 (−0.69, 0.06) p = 0.10] (Fig. 10A).
Single-Arm Meta-analysis
Figure 10B shows the change in opioid intake using the MMEq at 12 months for patients undergoing percutaneous adhesiolysis. There were four trials [54,55,56,57] with a pooled mean decrease in opioid intake from baseline to 12 months of follow-up of 15.094 MMEq (95% CI −16.141 to −14.048, p < 0.0001).
Figure 10C shows the change in opioid intake using the MMEq at 12 months for patients from the control group. There were three trials [54,55,56] with a pooled mean decrease in opioid intake from baseline to 12 months of follow-up of 2.664 MMEq (95% CI −11.399 to 6.070, p = 0.550).
Overall, at 1 year of follow-up, pain and function improved significantly in percutaneous adhesiolysis groups compared with the control groups with dual- and single-arm analyses. Opioid intake was not significantly different with dual-arm analysis between both groups, even though single-arm analysis showed a significant difference, with a decrease of 15 MMEq in percutaneous adhesiolysis groups compared with a decrease of 2.7 MMEq in control groups.
Discussion
The present systematic review and meta-analysis of percutaneous adhesiolysis for low back and lower extremity pain secondary to postsurgery syndrome, central spinal stenosis, and chronic disc herniation showed level I–II or strong to moderate evidence with nine relevant RCTs with moderate to strong strength of recommendation. The RCTs were from six trials studying postsurgery syndrome, two trials studying spinal stenosis, and one randomized placebo-controlled trial studying disc herniation. All other trials were active controlled. Past analysis of evidence synthesis based on individual conditions showed level I evidence for postsurgery syndrome [1, 3, 5, 6] and level II evidence for central spinal stenosis [1, 2] and chronic disc herniation [1, 3]. In contrast to previous reviews, the present meta-analysis combines all updates with utilization of all nine RCTs for three conditions [1,2,3,4].
Failed back surgery syndrome (FBSS) was defined by the International Association for the Study of Pain (IASP) as a phenomenon of persistent or recurrent pain, mainly in the lower back or legs, even after previously anatomically successful surgeries [77]. FBSS has been described extensively [72, 78,79,80,81,82]. The most common causes of FBSS have been identified as epidural fibrosis, arachnoiditis, recurrent disc herniation, and lateral and central spinal stenosis. Spinal stenosis is the result of abnormal narrowing of the spinal canal, lateral recess, or the intervertebral foramina, resulting in pressure on the spinal cord and/or nerve roots [83,84,85,86].
Among the studies that met the inclusion criteria, seven trials provided 1 year results and all of them reported positive results. However, at 6 months follow-up of the nine trials, one trial reported negative results [61]. The one negative trial [61] essentially compared percutaneous adhesiolysis with an inflatable balloon catheter. The inflatable balloon catheter had better results.
The results of this systematic review are in agreement with the majority of previous studies [1,2,3,4,5,6,7], except for one notable exception [5, 8]. The systematic review performed by Brito-García et al. [8] did not include a meta-analysis. Since the publication of the Brito-García review [8], other RCTs have been published. While multiple systematic reviews showed positive evidence ranging from level I to II [1,2,3,4,5,6], Cho et al. [7] showed a higher level of evidence for adhesiolysis than SCS.
Systematic reviews and meta-analysis are performed to meet the goals of evidence-based medicine using the best available evidence in determining clinical care for an individual patient or population. While systematic reviews and meta-analyses are expected to provide information from high-quality research, they may vary and do not guarantee high methodological and reporting rigor [39]. In the scientific world, multiple biases may be present, with publication bias, outcome reporting bias, multiple publication bias, place of publication bias, citation bias, and interpretation bias, which appear to be crucial and relevant to systematic reviews in interventional pain management [39]. Of importance is the interpretation bias referring to the researchers’ or reviewers’ abilities to synthesize and objectively judge and weigh the results found in a study. Consequently, two researchers of different backgrounds might look at the same result in a different way, thus drawing different conclusions based on their own background [87,88,89]. This is common when the data are debatable or qualitative, leading to some conclusions being overstated while others are understated [89]. The major issue is the erroneous classification of trials as “pragmatic” and “real world”. Dal-Ré et al. [50] described that a genuinely pragmatic RCT should fulfill at least two fundamental features, including conduct of the study, which should resemble usual clinical practice, and the applicability of the results to multiple other settings, i.e., real world, not only the one where the trial was conducted. They also showed that some RCTs overtly deviate from usual clinical care and pragmatism, yet many RCTs are classified as pragmatic for purposes of convenience since pragmatic trials are set to represent real-world evidence. A recent publication of epidural steroids in disc herniation and sciatica in response to Cochrane review [90] illustrated multiples of these issues [38, 39]. Further, the role of placebo also has been a seeming source of continuous debate and has been the cause of discordance [40]. In fact, Manchikanti et al. [5] performed a systematic analysis of findings of systematic reviews in post lumbar surgery syndrome showing high compliance in only one systematic review [6] and moderate compliance with two systematic reviews [3, 7], whereas, one systematic review showed negative results with low compliance rate [8] with the PRISMA checklist. A Measurement Tool to Assess Systematic Reviews (AMSTAR) scoring also showed similar results, with high compliance for three systematic reviews [3, 6, 7] and poor compliance for one systematic review [8]. They also evaluated with Scottish Intercollegiate Guidelines Network (SIGN) scoring system showing similar results, thus one systematic review [8] consistently showed lower methodological quality. The present systematic review showed that all the trials included resembled clinical practice, with applicability of the results in a real-world setting.
Epidural steroid injections have been used extensively in managing low back and lower extremity pain [1, 10,11,12,13,14,15,16, 19, 22, 37,38,39, 91, 92]. Causes of chronic radicular pain include mechanical compression of nerve roots, as well as different proinflammatory substances [1] that trigger ectopic neuron firing [1]. Chronic radicular pain secondary to postsurgery syndrome, central spinal stenosis, and disc herniation are managed with mechanical decompression around the compressed nerve root, and inhibition of the inflammatory mediators by injecting targeted steroids into the epidural space or around the affected nerve. While results of studies of epidural injections continue to be debated and differ, the proportion of patients who failed to respond to epidural steroid injections are candidates for percutaneous epidural adhesiolysis [1, 42, 91, 92]. Thus far, the evidence has been in favor of percutaneous adhesiolysis in managing post lumbar surgery syndrome, spinal stenosis, and chronic disc herniation [1,2,3,4,5,6]. The mechanism described in percutaneous adhesiolysis is the combined effect of local lavage of proinflammatory cytokines, reduction of swelling, lysis of adhesions, desensitization and modification of neuromodulation, and local anesthesia. The presence of epidural adhesions may be diagnosed with magnetic resonance imaging (MRI), followed by epidurography based on filling defects. These filling defects by epidurography are minimized in size after successfully performing epidural lysis of adhesions. The epidural space is opened by injection of solutions if the catheter is placed directly into the zone of adhesions. However, the mechanical effect of adhesiolysis with a catheter has been debated [83]. It has been shown that the catheter itself was not able to have a significant mechanical effect in an experimental study setup. However, the authors themselves discussed the obvious limitations that the experimental setup did not represent the real clinical and anatomical environment. However, based on extensive clinical experience, we believe that the mechanical effects are real.
The limitations of this systematic review include the continued paucity of literature despite nine eligible trials that looked at various conditions separately (i.e., postsurgery syndrome, central spinal stenosis, and disc herniation). The other limitation is the lack of placebo-controlled trials despite significant differences noted among the active-controlled trials utilizing epidural injection as control.
Conclusions
This systematic review with meta-analysis utilizing appropriate methodology with qualitative and quantitative evidence synthesis with conventional dual- and single-arm analyses shows level II–I, or moderate to strong evidence of effectiveness based on five high-quality and two moderate-quality RCTs with 1 year follow-up, showing improvement in pain and function as well as a decrease in opioid consumption.
References
Manchikanti L, Knezevic NN, Navani A, et al. Epidural interventions in the management of chronic spinal pain: American Society of Interventional Pain Physicians (ASIPP) comprehensive evidence-based guidelines. Pain Physician. 2021;24:S27-208 (PMID: 33492918).
Manchikanti L, Knezevic NN, Sanapati MR, Boswell MV, Kaye AD, Hirsch JA. Effectiveness of percutaneous adhesiolysis in managing chronic central lumbar spinal stenosis: a systematic review and meta-analysis. Pain Phys. 2019;22:E523–50 (PMID: 31775400).
Manchikanti L, Knezevic NN, Sanapati SP, Sanapati MR, Kaye AD, Hirsch JA. Is percutaneous adhesiolysis effective in managing chronic low back and lower extremity pain in post-surgery syndrome: A systematic review and meta-analysis. Curr Pain Headache Rep. 2020;24:30. https://doi.org/10.1007/s11916-020-00862-y. (PMID: 32468418).
Manchikanti L, Knezevic E, Knezevic NN, et al. The role of percutaneous neurolysis in lumbar disc herniation: Systematic review and meta-analysis. Korean J Pain. 2021;34:346–68. https://doi.org/10.3344/kjp.2021.34.3.346. (PMID:34193641;PMCID:PMC8255147).
Manchikanti L, Soin A, Boswell MV, Kaye AD, Sanapati M, Hirsch JA. Effectiveness of percutaneous adhesiolysis in post lumbar surgery syndrome: a systematic analysis of findings of systematic reviews. Pain Phys. 2019;22:307–22 (PMID: 31337160).
Helm S 2nd, Racz GB, Gerdesmeyer L, et al. Percutaneous and endoscopic adhesiolysis in managing low back and lower extremity pain: a systematic review and meta-analysis. Pain Phys. 2016;19:E245–82 (PMID: 26815254).
Cho JH, Lee JH, Song KS, et al. Treatment outcomes for patients with failed back surgery. Pain Phys. 2017;20:E29-43 (PMID: 28072795).
Brito-García N, García-Pérez L, Kovacs FM, et al. Efficacy, effectiveness, safety, and cost-effectiveness of epidural adhesiolysis for treating failed back surgery syndrome. A systematic review. Pain Med. 2019;20:692–706. https://doi.org/10.1093/pm/pny233. (PMID: 30590850).
Manchikanti L, Kaye AD, Soin A, et al. Comprehensive evidence-based guidelines for facet joint interventions in the management of chronic spinal pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Phys. 2020;23:S1-127 (PMID: 32503359).
Manchikanti L, Sanapati MR, Soin A, et al. An updated analysis of utilization of epidural procedures in managing chronic pain in the Medicare population from 2000 to 2018. Pain Phys. 2020;23:111–26 (PMID: 32214288).
Manchikanti L, Pampati V, Soin A, Sanapati MR, Kaye AD, Hirsch JA. Declining utilization and inflation-adjusted expenditures for epidural procedures in chronic spinal pain in the Medicare population. Pain Phys. 2021;24:1–15 (PMID: 33400424).
Manchikanti L, Kosanovic R, Pampati V, Kaye AD. Declining utilization patterns of percutaneous adhesiolysis procedures in the fee-for-service (FFS) Medicare population. Pain Phys. 2021;24:17–29 (PMID: 33400425).
Manchikanti L, Senapathi SHV, Milburn JM, et al. Utilization and expenditures of vertebral augmentation continue to decline: An analysis in fee-for-service (FFS) Recipients from 2009 to 2018. Pain Phys. 2021;24:401–15 (PMID: 34554681).
Manchikanti L, Pampati V, Vangala BP, et al. Spinal cord stimulation trends of utilization and expenditures in fee-for-service (FFS) Medicare population from 2009 to 2018. Pain Physician. 2021;24:293–308 (PMID: 34323431).
Manchikanti L, Simopoulos TT, Pampati V, et al. Impact of COVID-19 pandemic and updated utilization patterns of sacroiliac joint injections from 2000 to 2020 in the fee-for-service (FFS) Medicare population. Pain Phys. 2022;25:239–50 (PMID: 35652764).
Manchikanti L, Kaye AD, Latchaw RE, et al. Impact of COVID-19 pandemic on utilization patterns of facet joint interventions in managing spinal pain in Medicare population. Pain Ther. 2023;12:505–27.
Martin AB, Hartman M, Lassman D, Catlin A, National Health Expenditure Accounts Team. National Health Care Spending In 2019: steady growth for the fourth consecutive year. Health Aff (Millwood). 2021;40:14–24. https://doi.org/10.1377/hlthaff.2020.02022. (Epub 2020 Dec 16 PMID: 33326300).
Cox C, Amin K, Kamal R. How have health spending and utilization changed during the coronavirus pandemic? Health System Tracker. March 22, 2021. https://www.healthsystemtracker.org/chart-collection/how-have-healthcare-utilization-and-spending-changed-so-far-during-the-coronavirus-pandemic/. Accessed 6 Jan 2022.
Manchikanti L, Pampati V, Sanapati MR, et al. COVID-19 pandemic reduced utilization of interventional techniques 18.7% in Managing chronic pain in the Medicare population in 2020: Analysis of utilization data from 2000 to 2020. Pain Phys. 2020;25:223–38 (PMID: 35652763).
San AU, Kesikburun S, Tezel K. The effect of social isolation during the COVID-19 pandemic on patients with chronic low back pain who underwent a spine intervention. Pain Physician. 2021;24:319–25 (PMID: 34323433).
Manchikanti L, Pampati V, Jha SS, et al. The impact of COVID-19 on interventional pain management practices is significant and long-lasting: an interventional pain management physician survey. Pain Physician. 2022;25:131–44 (PMID: 35322967).
Manchikanti L, Pampati V, Knezevic NN, et al. The influence of COVID-19 on utilization of epidural procedures in managing chronic spinal pain in the Medicare population. Spine (Phila Pa 1976). 2023;10:269–86.
Manchikanti L, Singh VM, Staats PS, et al. Fourth wave of opioid (illicit drug) overdose deaths and diminishing access to prescription opioids and interventional techniques: cause and effect. Pain Physician. 2022;25:97–124 (PMID: 35322965).
Manchikanti L, Vanaparthy R, Atluri S, Sachdeva H, Kaye AD, Hirsch JA. COVID-19 and the opioid epidemic: two public health emergencies that intersect with chronic pain. Pain Ther. 2021;10:269–86. https://doi.org/10.1007/s40122-021-00243-2. (Epub 2021 Mar 14. PMID: 33718982; PMCID: PMC7955940).
Dale R, Kinch L, Kohan L, et al. Pain medicine fellowship video interviews: a COVID-19 trend or here to stay? Pain Physician. 2022;25:125–30 (PMID: 35322966).
Gonder ME, Orr WN, Khan TW. The impact of isolation during COVID-19 on chronic musculoskeletal pain in the geriatric population: a narrative review. Pain Phys. 2022;25:E185–91 (PMID: 35322970).
Eccleston C, Blyth FM, Dear BF, et al. Managing patients with chronic pain during the COVID-19 outbreak: Considerations for the rapid introduction of remotely supported (eHealth) pain management services. Pain. 2020;161:889–93. https://doi.org/10.1097/j.pain.0000000000001885. (PMID:32251203;PMCID:PMC7172975).
Yih C, Chokshi K, Kyriakides C, et al. Point to area of pain: a clinically useful telehealth physical exam technique for focal nociceptive and neuropathic pain. Pain Phys. 2022;25:209–19 (PMID: 35322979).
Stone S, Malanga GA, Capella T. Corticosteroids: review of the history, the effectiveness, and adverse effects in the treatment of joint pain. Pain Phys. 2021;24:S233–46 (PMID: 33492920).
Puntillo F, Giglio M, Brienza N, et al. Impact of COVID-19 pandemic on chronic pain management: looking for the best way to deliver care. Best Pract Res Clin Anaesthesiol. 2020;34:529–37. https://doi.org/10.1016/j.bpa.2020.07.001. (Epub 2020 Jul 17. PMID: 33004164; PMCID: PMC7366114).
Zhang M, Zheng H, Wang J. Considerations when COVID-19 pandemic collides opioid crisis: what we should know? J Anes Perio Manag. 2020;4:009.
Dubey MJ, Ghosh R, Chatterjee S, Biswas P, Chatterjee S, Dubey S. COVID-19 and addiction. Diabetes Metab Syndr. 2020;14:817–23. https://doi.org/10.1016/j.dsx.2020.06.008. (Epub 2020 Jun 9. PMID: 32540735; PMCID: PMC7282772).
Hartman M, Martin AB, Washington B, Catlin A, The National Health Expenditure Accounts Team. National health care spending in 2020: Growth driven by federal spending in response to the COVID-19 pandemic. Health Aff (Millwood). 2022;41:13–25. https://doi.org/10.1377/hlthaff.2021.01763. (Epub 2021 Dec 15 PMID: 34910596).
Bankhead C. Private practices’ share of U.S. medicine continues to shrink – AMA survey: Majority of physicians work in nonphysician-owned practice settings. MedPage Today. May 6, 2021. https://www.medpagetoday.com/practicemanagement/practicemanagement/92451. Accessed 12 Sep 2022.
Trends in health care expenditures, American Medical Association. https://www.ama-assn.org/about/research/trends-health-care-spending. Accessed 6 Jan 2022.
Pipes SC. Will the independent medical practice become extinct in America? https://www.tennessean.com/story/opinion/2022/02/07/decrease-independent-medical-practice-impacts-americans/6656073001/. Accessed 12 Sep 2022
Manchikanti L, Knezevic E, Knezevic NN, et al. Epidural injections for lumbar radiculopathy or sciatica: A comparative systematic review and meta-analysis of Cochrane review. Pain Phys. 2021;24:E539–54 (PMID: 34323441).
Manchikanti L, Knezevic E, Knezevic NN, et al. A Comparative systematic review and meta-analysis of 3 routes of administration of epidural injections in lumbar disc herniation. Pain Phys. 2021;24:425–40 (PMID: 34554683).
Manchikanti L, Knezevic E, Latchaw RE, et al. Comparative systematic review and meta-analysis of Cochrane Review of epidural injections for lumbar radiculopathy or sciatica. Pain Phys. 2022;25:E889–916 (PMID: 36288577.f).
Manchikanti L, Knezevic NN, Sanapati J, Kaye AD, Sanapati MR, Hirsch JA. Is epidural injection of sodium chloride solution a true placebo or an active control agent? A systematic review and meta-analysis. Pain Phys. 2021;24:41–59 (PMID: 33400427).
Helm S II, Harmon PC, Noe C, et al. Transforaminal epidural steroid injections: a systematic review and meta-analysis of efficacy and safety. Pain Phys. 2021;24:S209–32 (PMID: 33492919).
Fang Z, Yuan C, Cheng L, et al. Comparison of clinical efficacy of epidural injection with or without steroids in the treatment of degenerative disc disease: meta-analysis. Pain Physician. 2022;25:145–60 (PMID: 35322968).
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71 (PMID: 33782057; PMCID: PMC8005924).
Manchikanti L, Atluri S, Boswell MV, et al. Methodology for evidence synthesis and development of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Pain Physician. 2021;24:S1-26 (PMID: 33492917).
Furlan AD, Malmivaara A, Chou R, Editorial Board of the Cochrane Back, Neck Group, et al. 2015 updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine (Phila Pa 1976). 2015;40:1660–73. https://doi.org/10.1097/BRS.0000000000001061. (PMID: 26208232).
Manchikanti L, Hirsch JA, Cohen SP, et al. Assessment of methodologic quality of randomized trials of interventional techniques: Development of an interventional pain management specific instrument. Pain Physician. 2014;17:E263–90 (PMID: 24850111).
Manchikanti L, Falco FJE, Benyamin RM, Kaye AD, Boswell MV, Hirsch JA. A modified approach to grading of evidence. Pain Physician. 2014;17:E319–25 (PMID: 24850113).
Agency for Healthcare Research and Quality (AHRQ). National Guideline Clearinghouse. www.guideline.gov. Access 28 Jul 2021
Ryan R, Hill S. How to GRADE the quality of the evidence. Version 3.0. December 2016. http://cccrg.cochrane.org/author-resources. Accessed 28 Jul 2021.
Dal-Ré R, Janiaud P, Ioannidis JPA. Real-world evidence: How pragmatic are randomized controlled trials labeled as pragmatic? BMC Med. 2018;16:49. https://doi.org/10.1186/s12916-018-1038-2. (PMID:29615035; PMCID:PMC5883397).
Heavner JE, Racz GB, Raj P. Percutaneous epidural neuroplasty: prospective evaluation of 0.9% NaCl versus 10% NaCl with or without hyaluronidase. Reg Anesth Pain Med. 1999;24:202–7. https://doi.org/10.1016/s1098-7339(99)90128-1. (PMID: 10338168).
Manchikanti L, Rivera JJ, Pampati V, et al. One day lumbar epidural adhesiolysis and hypertonic saline neurolysis in treatment of chronic low back pain: a randomized, double-blind trial. Pain Physician. 2004;7:177–86 (PMID: 16868590).
Veihelmann A, Devens C, Trouillier H, Birkenmaier C, Gerdesmeyer L, Refior HJ. Epidural neuroplasty versus physiotherapy to relieve pain in patients with sciatica: a prospective randomized blinded clinical trial. J Orthop Sci. 2006;11:365–9. https://doi.org/10.1007/s00776-006-1032-y. (PMID: 16897200).
Manchikanti L, Singh V, Cash KA, Pampati V, Datta S. A comparative effectiveness evaluation of percutaneous adhesiolysis and epidural steroid injections in managing lumbar post surgery syndrome: a randomized, equivalence controlled trial. Pain Physician. 2009;12:E355–68 (PMID: 19935992).
Manchikanti L, Singh V, Cash KA, Pampati V, Datta S. Assessment of effectiveness of percutaneous adhesiolysis and caudal epidural injections in managing lumbar post surgery syndrome: a 2-year follow-up of randomized, controlled trial. J Pain Res. 2012;5:597–608. https://doi.org/10.2147/JPR.S38999. (Epub 2012 Dec 20. PMID: 23293536; PMCID: PMC3533727).
Manchikanti L, Cash KA, McManus CD, Pampati V, Singh V, Benyamin R. The preliminary results of a comparative effectiveness evaluation of adhesiolysis and caudal epidural injections in managing chronic low back pain secondary to spinal stenosis: a randomized, equivalence controlled trial. Pain Physician. 2009;12:E341–54 (PMID: 19935991).
Manchikanti L, Cash KA, McManus CD, Pampati V. Assessment of effectiveness of percutaneous adhesiolysis in managing chronic low back pain secondary to lumbar central spinal canal stenosis. Int J Med Sci. 2013;10:50–9. https://doi.org/10.7150/ijms. (Epub 2012 Dec 10. PMID: 23289005; PMCID: PMC3534877).
Gerdesmeyer L, Wagenpfeil S, Birkenmaier C, et al. Percutaneous epidural lysis of adhesions in chronic lumbar radicular pain: a randomized, double-blind, placebo-controlled trial. Pain Physician. 2013;16:185–96 (PMID: 23703406).
Gerdesmeyer L, Noe C, Prehn-Kristensen A, et al. Long-term efficacy of percutaneous epidural neurolysis of adhesions in chronic lumbar radicular pain: 10 year follow-up of a randomized controlled trial. Pain Phys. 2021;24:359–67 (PMID: 34323437).
Fabris LK, Suput A, Gusic N, Mamontov P. Epidural adhesiolysis in the management of chronic low back pain in failed back surgery syndrome and in lumbar radicular pain: First year of experience at Pula General Hospital, Pula, Croatia—a randomized trial. Acta Med Croatica. 2019;73:57–65.
Karm MH, Choi SS, Kim DH, et al. Percutaneous epidural adhesiolysis using inflatable balloon catheter and balloon-less catheter in central lumbar spinal stenosis with neurogenic claudication: a randomized controlled trial. Pain Phys. 2018;21:593–606 (PMID: 30508987).
Akbas M, Elawamy AR, Salem HH, Fouad AZ, Abbas NA, Dagistan G. Comparison of 3 approaches to percutaneous epidural adhesiolysis and neuroplasty in post lumbar surgery syndrome. Pain Phys. 2018;21:E501–8 (PMID: 30282398).
Hossieni B, Dadkhah P, Moradi S, Hashemi SM, Safdari F. The results of treatment failed back surgery syndrome by adhesiolysis: Comparing the one- and three-day protocols. Anesth Pain Med. 2017;7:e60271. https://doi.org/10.5812/aapm.60271. (PMID: 29696119 PMCID: PMC5903221).
Park SH, Ji GY, Cho PG, et al. Clinical significance of epidurography contrast patterns after adhesiolysis during lumbar percutaneous epidural neuroplasty. Pain Res Manag. 2018. https://doi.org/10.1155/2018/6268045. (PMID:29808106;PMCID:PMC5901487).
Choi EJ, Yoo YJ, Lee PB, Kim YC, Lee SC, Moon JY. A retrospective study to evaluate the effect of concentration of hypertonic saline on efficacy and safety of epidural adhesiolysis. Anesth Analg. 2017;124:2021–9. https://doi.org/10.1213/ANE.0000000000001925. (PMID: 28448392).
Manchikanti L, Pakanati RR, Bakhit CE, Pampati V. Role of adhesiolysis and hypertonic saline neurolysis in management of low back pain: Evaluation of modification of the Racz protocol. Pain Digest. 1999;9:91–6.
Ji GY, Oh CH, Moon B, et al. Efficacy of percutaneous epidural neuroplasty does not correlate with dural sac cross-sectional area in single level disc disease. Yonsei Med J. 2015;56:691–7. https://doi.org/10.3349/ymj.2015.56.3.691. (PMID:25837174;PMCID:PMC4397438).
Hazer DB, Acarbaş A, Rosberg HE. The outcome of epiduroscopy treatment in patients with chronic low back pain and radicular pain, operated or non-operated for lumbar disc herniation: a retrospective study in 88 patients. Korean J Pain. 2018;31:109–15. https://doi.org/10.3344/kjp.2018.31.2.109. (Epub 2018 Apr 2. PMID: 29686809; PMCID: PMC5904345).
Cho PG, Ji GY, Yoon YS, Shin DA. Clinical effectiveness of percutaneous epidural neuroplasty according to the type of surgical level lumbar disc herniation: a 12 month follow up study. J Korean Neurosurg Soc. 2019;62:681–90. https://doi.org/10.3340/jkns.2019.0070. (Epub 2019 Oct 8. PMID: 31591998; PMCID: PMC6835144).
Moon SH, Park JY, Cho SS, et al. Comparative effectiveness of percutaneous epidural adhesiolysis for different sacrum types in patients with chronic pain due to lumbar disc herniation: a propensity score matching analysis. Medicine (Baltimore). 2016;95:e4647. https://doi.org/10.1097/MD.0000000000004647. (PMID: 27631213; PMCID: PMC5402556).
Taheri A, Khajenasiri AR, Nazemian Yazdi NA, Safari S, Sadeghi J, Hatami M. Clinical evaluation of percutaneous caudal epidural adhesiolysis with the Racz technique for low back pain due to contained disc herniation. Anesth Pain Med. 2016;6:e26749. https://doi.org/10.5812/aapm.26749. (PMID: 27826538; PMCID: PMC5097854).
You KH, Park HJ, Son IS, Chung HJ, Kang MS. Contralateral retrodiscal transforaminal approach for percutaneous epidural adhesiolysis: a technical description and retrospective comparative study. Pain Pract. 2022;22:424–31. https://doi.org/10.1111/papr.13096. (Epub 2021 Dec 9 PMID: 34837304).
Rapčan R, Kočan L, Mláka J, et al. A randomized, multicenter, double-blind, parallel pilot study assessing the effect of mechanical adhesiolysis vs adhesiolysis with corticosteroid and hyaluronidase administration into the epidural space during epiduroscopy. Pain Med. 2018;19:1436–44. https://doi.org/10.1093/pm/pnx328. (PMID: 29584916).
Guner D, Asik I, Ozgencil GE, Peker E, Erden MI. The correlation of epidural fibrosis with epiduroscopic and radiologic imaging for chronic pain after back surgery. Pain Phys. 2021;24:E1219–26 (PMID: 34793648).
Manchikanti L, Helm S 2nd, Pampati V, Racz GB. Cost utility analysis of percutaneous adhesiolysis in managing pain of post-lumbar surgery syndrome and lumbar central spinal stenosis. Pain Pract. 2015;15:414–22. https://doi.org/10.1111/papr.12195. (Epub 2014 Mar 26 PMID: 24666747).
Chun-jing H, Hao-xiong N, jia-xiang N. The application of percutaneous lysis of epidural adhesions in patients with failed back surgery syndrome. Acta Cir Bras. 2012;27:357–62. https://doi.org/10.1590/s0102-86502012000900004. (PMID: 22936085).
Merskey H, Bogduk N (editors). Classification of chronic pain: Descriptions of chronic pain syndromes and definition of pain terms. 2nd ed. Seattle: IASP Press; 1994.
Ross JS, Obuchowski N, Modic MT. MR evaluation of epidural fibrosis: proposed grading system with intra- and inter-observer variability. Neurol Res. 1999;21:S23–6. https://doi.org/10.1080/01616412.1999.11758604. (PMID: 10214567).
Fritsch EW, Heisel J, Rupp S. The failed back surgery syndrome: reasons, intraoperative findings, and long term results: a report of 182 operative treatments. Spine (Phila Pa 1976). 1996;21:626–33. https://doi.org/10.1097/00007632-199603010-00017. (PMID: 8852320).
Bosscher HA, Heavner JE. Incidence and severity of epidural fibrosis after back surgery: an endoscopic study. Pain Pract. 2010;10:18–24. https://doi.org/10.1111/j.1533-2500.2009.00311.x. (Epub 2009 Sep 3 PMID: 19735365).
McCarron RF, Wimpee MW, Hudkins PG, Laros GS. The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine (Phila Pa 1976). 1987;12:760–4. https://doi.org/10.1097/00007632-198710000-00009. (PMID: 2961088).
Lee GW, Mun JU, Ahn MW. The impact of posterior epidural adipose tissue on postoperative outcomes after posterior decompression surgery for lumbar spinal stenosis: A prospectively randomized non-inferiority trial. J Orthop Surg (Hong Kong). 2020;28:2309499019896871. https://doi.org/10.1177/2309499019896871. (PMID: 31908178).
Moon SH, Lee JI, Cho HS, Shin JW, Koh WU. Factors for predicting favorable outcome of percutaneous epidural adhesiolysis for lumbar disc herniation. Pain Res Manag. 2017;2017:1494538. (PMID: 28246488; PMCID: PMC5299181).
Birkenmaier C, Baumert S, Schroeder C, Jansson V, Wegener B. A biomechanical evaluation of the epidural neurolysis procedure. Pain Physician. 2012;15:E89-97 (PMID: 22270752).
Haig AJ, Tomkins CC. Diagnosis and management of lumbar spinal stenosis. JAMA. 2010;303:71–2. https://doi.org/10.1001/jama.2009.1946. (PMID: 20051574).
Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009;9:545–50. https://doi.org/10.1016/j.spinee.2009.03.005. (Epub 2009 Apr 23. PMID: 19398386; PMCID: PMC3775665).
Booth A, Papaioannou D, Sutton A. Systematic approaches to a successful literature review. London: Sage Publications Inc; 2012.
Song F, Parekh S, Hooper L, et al. Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess. 2010;14:iii. https://doi.org/10.3310/hta14080. (PMID: 20181324 ix-xi, 1-193).
Jansen S. Bias within systematic and non-systematic literature reviews: The case of the balanced scorecard. University of Twente, Student Theses. 2017. http://essay.utwente.nl/73771/. Accessed 11 June 2021.
Oliveira CB, Maher CG, Ferreira ML, et al. Epidural corticosteroid injections for lumbosacral radicular pain. Cochrane Database Syst Rev. 2020;4:CD013577. https://doi.org/10.1002/14651858.CD013577. (PMID: 32271952; PMCID: PMC7145384).
Celenlioglu AE, Sencan S, Bilim S, Sancar M, Gunduz OH. Comparison of caudal versus transforaminal epidural steroid injection in post lumbar surgery syndrome after single-level discectomy: a prospective, randomized trial. Pain Phys. 2022;25:161–9 (PMID: 35322972).
Manchikanti L, Singh V, Cash KA, Pampati V, Datta S. Fluoroscopic caudal epidural injections in managing post lumbar surgery syndrome: two-year results of a randomized, double-blind, active-control trial. Int J Med Sci. 2012;9:582–91. https://doi.org/10.7150/ijms.4672. (Epub 2012 Sep 8. PMID: 23028241; PMCID: PMC3461763).
Acknowledgements
The authors wish to thank Bert Fellows, MA, Director Emeritus of Psychological Services at Pain Management Centers of America, for manuscript review, and Tonie M. Hatton and Diane E. Neihoff, transcriptionists, for their assistance in preparation of this manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Laxmaiah Manchikanti, MD, Nebojsa Nick Knezevic, MD, PhD, and Emilija Knezevic. The first draft of the manuscript was written by Laxmaiah Manchikanti, MD, and Nebojsa Nick Knezevic, MD, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.”
Disclosures
Dr. Hirsch is a consultant for Medtronic and Relievant. He is a grant supported Senior Affiliate Research Fellow at the Neiman Policy Institute. Laxmaiah Manchikanti has nothing to disclose. Nebojsa Nick Knezevic has nothing to disclose. Emilija Knezevic has nothing to disclose. Rachana Pasupuleti has nothing to disclose. Alan D. Kaye has nothing to disclose. Mahendra R. Sanapati has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The data used in this review are available from multiple sources, including public databases PubMed, Google Scholar Cochrane library, US national Guidelines clearing house and are included in the references section of this article.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Manchikanti, L., Knezevic, N.N., Knezevic, E. et al. Efficacy of Percutaneous Adhesiolysis in Managing Low Back and Lower Extremity Pain: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Pain Ther 12, 903–937 (2023). https://doi.org/10.1007/s40122-023-00508-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00508-y