Abstract
Introduction
This study aimed to investigate the effect of cognitive load on anticipatory postural adjustment (APA) latency in patients with non-specific chronic low back pain (NCLBP) and its relationship with pain-related functional changes.
Methods
A cross-sectional study was conducted from December 15, 2022 to January 25, 2023. Participants were divided into a healthy control group (n = 29) and an NCLBP group (n = 29). Each group was assigned a single task of rapid arm raising and a dual task of rapid arm raising combined with a cognitive load. The cognitive load task was conducted using visual conflict. The APA latency for bilateral trunk muscles was observed using electromyography. The duration of electromyography recording in each task cycle was 28 s. Pain related-functional changes were evaluated using Roland–Morris Disability Questionnaire (RMDQ) before all tasks.
Results
The APA latency for the right multifidus was significantly delayed in the NCLBP group [25.38, 95% confidence interval (CI) 13.41–37.35] than in the healthy control group (− 5.80, 95% CI − 19.28 to 7.68) during dual task (p = 0.0416). The APA latency for the right multifidus (25.38, 95% CI 13.41–37.35) and transverse abdominis/internal oblique (29.15, 95% CI 18.81–39.50) were significantly delayed compared with on the left side in the NCLBP group during dual task (− 3.03, 95% CI − 15.18–9.13, p = 0.0220; 3.69, 95% CI − 6.81 to 14.18, p = 0.0363). The latency delay of the right and left multifidus APA in the NCLBP group under the dual-task was positively correlated with RMDQ scores (r = 0.5560, p = 0.0017; r = 0.4010, p = 0.0311).
Conclusions
Cognitive load could induce APA delay in the right trunk muscles and co-activation pattern changes in bilateral trunk muscle APA in patients with NCLBP. The APA onset delay in multifidus is positively related to pain-related daily dysfunction.
Trial Registration
ChiCTR2300068580 (retrospectively registered in February 23, 2023).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Non-specific chronic low back pain (NCLBP) is a major factor contributing to working efficiency loss and daily activity limitation. |
Anticipatory postural adjustment (APA) latency changes are characteristic of postural control changes in NCLBP; however, the changes in trunk muscle APA in NCLBP under cognitive load are not clear. |
This study aimed to explore the effects of cognitive load on changes in trunk muscle APA latency and the correlation between cognitive load-induced changes in APA and self-reported dysfunction |
What was learned from this study? |
Cognitive load could exacerbate the delay of right multifidus APA onset and bilateral trunk muscle APA co-activation pattern changes in patients with NCLBP when faced with postural disturbance. |
Delayed APA latency in multifidus induced by cognitive load was associated with self-reported dysfunction of daily life. |
Introduction
Non-specific chronic low back pain (NCLBP) accounts for 90–95% of chronic low back pain cases. It has become a major public health problem worldwide and is considered a serious burden on personal life and social economy [1, 2]. The pathogenesis of NCLBP remains largely unknown, and there is an urgent need to further explore the mechanisms of pain development in this pathological group.
Anticipatory postural adjustment (APA) is defined as a posture adjustment strategy in which the human body activates muscles to minimize instability during an expected postural perturbation through the feedforward mechanism [3, 4]. It occurs between − 150 and + 49 ms before postural disturbance and is a neuromuscular control process controlled by the central nervous system [5]. APA function primarily for stability and balance maintenance after predictable postural disturbances [6, 7]. Changes in APA have also been demonstrated to be associated with the development of NCLBP [8, 9]. For instance, pain induced by prolonged standing is associated with decreased regulatory capacity of transverse abdominis/internal oblique (TrA/IO) APA in patient with low back pain [10]. Compared with healthy subjects, the latency of multifidus (MF) activation in patients with chronic low back pain was significantly delayed in response to predictable postural interference [11]. A meta-analysis reports postural control abnormalities in patients with NCLBP is characterized by delayed activation of trunk muscle APA [12]. Especially, deep trunk muscles such as TrA/IO and MF play an important role in lumbar spine stabilization [13, 14]. However, whether the APA latency changes in TrA/IO and MF contribute to pain-related dysfunction in people with NCLBP is rarely reported. Further exploring the relationship between the APA latency changes in TrA/IO and MF and pain-related dysfunction may help in understanding the development of dysfunction in NCLBP.
Cognitive load has been reported to affect the feedforward control of APA [15]. Our previous research found that older patients with NCLBP had reduced APA control capacity when performing cognitive tasks simultaneously with postural control tasks, and reduced APA performance was associated with a greater risk of falling [16]. Another study showed that the activation latency of the tibialis anterior (agonist) was shortened while that of the gastrocnemius (antagonist) was prolonged in patients with NCLBP when performing postural perturbation combined with a backward digit span task, compared with that in healthy subjects [17]. These studies suggest that cognitive load have an impact on APA latency in patients with NCLBP. However, empirical evidence on the effect of cognitive load on the latency in trunk muscle APA activation and changes in pain-related functions is currently lacking. Based on the gaps in previous studies, the present study aimed to explore the effect of cognitive load on the latency of APA in the trunk muscles and the relationships between alterations of APA latency and pain-related functional changes in patients with NCLBP. This study would provide potential ideas for expanding clinicians' understanding of the dysfunction development in NCLBP, and optimizing the rehabilitation approach for patient with NCLBP. At the same time, this study suggested future researches aimed to exploring the APA changes may contribute to revealing the pathogenesis of NCLBP.
Methods
Participants and Grouping
This was a cross-sectional study performed in rehabilitation medicine laboratory of the First Affiliated Hospital, Sun Yat-sen University, from December 15, 2022 to January 25, 2023. A physician with experience in diagnosis and treatment of low back pain included the participants in the study. Sixty subjects (30 participants with NCLBP, 30 healthy participants) from medical and community centers were enrolled in this study. One participant with NCLBP and one healthy control participant dropped out due to personal reason. The participants were divided into a healthy control (HC) group (n = 29) and a NCLBP group (n = 29) and received rapid arm raising task (RAR) without and with cognitive load. The sample size calculation was based on our previous study [16] and an independent study [17]. All participants were informed of the experimental protocol and fully understood their role in the study. All subjects underwent a detailed medical history inquiry and physical examination to rule out specific low back pain. The inclusion and exclusion criteria for patients with NCLBP described previously [18, 19]. Briefly, inclusion criteria were: (1) age between 18 and 50 years; (2) pain located between the 12th rib and hip; (3) pain duration of > 3 months, visual analogue scale (VAS) scores of ≥ 3, and at least one recurrent episode of low back pain in the past 3–15 months; and (4) right-handed. Exclusion criteria were: (1) history of pelvic or spine surgery within the past 2 years; (2) presence of any specific lumbar pathological changes; (3) presence of radicular symptoms; (4) body mass index (BMI) of > 30 kg/m2; (5) low back pain treatment performed within the past 3 months; (6) preparing for pregnancy or currently pregnant; (7) severe dysfunctions of vital organs (such as the heart, lungs, and kidneys); and (8) presence of visual, auditory, or cognitive impairments. All participants signed an informed consent form after adequate communication regarding the experimental process and potential risks. Before the experiment, their personal information, such as name, age, sex, course of disease, weight, height, and BMI, was collected. The VAS for pain severity assessment and Roland–Morris Disability Questionnaire (RMDQ) for functional disability assessment [20] were applied before RAR rask in this study. The RMDQ and VAS questionnaires were reliable and valid approaches for function and pain assessment for chronic low back pain [21, 22]. This study was approved by the Human Subjects Ethics Subcommittee of the First Affiliated Hospital, Sun Yat-sen University (grant number: 2022.551) and retrospectively registered with the Chinese Clinical Trials Registry (number: ChiCTR2300068580, February 23, 2023). This study was conducted in accordance with the principles of the Declaration of Helsinki of 1964 and its amendments.
Surface Electromyography Acquisition (sEMG)
The sEMG is a reliable method for detecting muscle APA onset [23, 24]. Sixteen-channel wireless sEMG (Trigno™ Avanti Platform; Delsys Inc., Natick, MA, USA) was used to acquire sEMG signals of the target trunk muscles and the right upper limbs. Hair removal and cleaning of the target muscle regions with 75% anhydrous ethanol were performed before sEMG detection. The muscles included the dominant deltoid muscle, bilateral TrA/IO, and MF. Electrodes were placed in reference to previous studies [25, 26]. The sEMG onset in the anterior part of the right deltoid muscle was defined as the timepoint of sudden postural disturbances and recorded as T0. The APA signals in the right arm-raising test were collected, in which the APA onset timepoint (Tonset) for the target trunk muscle was defined as ± 2 standard deviation values higher than the electromyography acquisition (EMG) baseline, and APA latency (Tlatency) was calculated as Tonset − T0. A positive Tlatency indicated that the target trunk muscle is activated after the anterior deltoid muscle activation, or the target trunk muscle is activated before the anterior deltoid muscle activation. The duration of electromyography recording in each task cycle was 28 s.
Single Task of Postural Perturbation
The RAR task was used for single task of internal postural perturbation according to previous studies [27, 28] with slight modifications. To minimize muscle resting activity, subjects were required to remain relaxed and avoid coughing and speaking during the test. The participants stood on a platform with their feet shoulder width apart and their eyes staring at a monitor positioned straight ahead. The monitor was placed 2 m in front of each participant. An electrode pad was mounted on the participant’s dominant deltoid muscle to record the onset time (T0) of internal posture interference. For instance, the visual cue of “Prepare” was first displayed for 3 s, and then the visual cue of “right arrow” was displayed subsequently. When the participants noticed the “right arrow” prompt, they extended the dominant upper limb (right upper limb) as soon as possible within 10 s, with an extension angle of at least 90°. The participants then rested for 15 s for the next cycles. The participants performed two to three RAR tasks to familiarize themselves with the test path and adjust the direction and speed of arm raising before the formal test. The “right arrow” and “left arrow” in each single task were at random and balanced. The participants repeated the standard RAR task 20 times.
Dual Task of Postural Perturbation
Dual task was applied by RAR combined with a cognitive load task (visual conflict), as previously described [28] with slight modifications. The visual conflict task was demonstrated simultaneously with the RAR task. In the case of a visual conflict, there was an arrow indicating the opposite direction to the other arrows, and the participant was required to raise the arm in accordance with the inconsistent arrow direction. The arrow visual cues in dual task were at random and balanced. Dual task was performed after single task was completed. The participant repeated the dual task (RAR task combined with cognitive load task) 20 times.
Statistical Analysis
MATLAB R2019b (MathWorks, Natick, MA, USA) was used to process EMG signals offline. GraphPad Prism (version 8.0; GraphPad Software, USA) was used for statistical analysis. All data were tested for normality. The measurement data (age, height, weight, and BMI) of the two groups were tested for differences by an independent t test, while gender was tested using the chi-square test. APA latency was analyzed by three factors mixed-design ANOVA, in which the within-subject factors included time (before vs. after cognitive load) and muscle location (left vs. right), and the between-subject factor was group (NCLBP vs. HC). The correlation between APA latency and RMDQ score was analyzed using Spearman’s correlation analysis. Statistical significance was established at p < 0.05.
The Tukey test was performed for correcting the subsequent multiple comparisons when there was a statistical difference in the main effect or interaction.
Results
Basic Demographic Characteristics
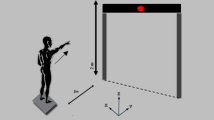
Sixty participants (30 participants with NCLBP and 30 healthy control participants) were considered for participation in this study. One participant with NCLBP and one healthy control participant dropped out due to personal reason. After removing anomalous data, 58 participants were finally included in the analysis. An illustration of the experiment and the test workflow for each group are shown in Fig. 1. The basic statistics of the participants in each group are shown in Table 1. There were no significant differences between the groups in terms of gender (p = 0.5990), age (p = 0.2269), weight (p = 0.9186), height (p = 0.3457), or BMI (p = 0.5599).
a Illustration of experiment. The participants executed the RAR task with or without visual conflict as indicated by the monitor. Each participant performed RAR task with visual conflict after RAR task without visual conflict was completed. The arrow visual cue in each task was at random and balanced. b Test workflow of each group. NCLBP non-specific chronic low back pain, HC health control, APA anticipatory postural adjustment, RAR rapid arm-raising, VAS visual analogue scale, RMDQ Roland–Morris Disability Questionnaire
APA Latency in Rapid Arm-raising Task with and Without Cognitive Load
The TrA/IO and MF play important roles in maintaining the stability of the lumbar spine [29, 30]. APA latency changes play an important role in postural control alteration in low back pain [31]. Therefore, we focused on APA latency in the TrA/IO and MF. As shown in Fig. 2a, time effects (F(1, 56) = 5.24, p = 0.0259), and location effects (F(1, 56) = 18.38, p < 0.0001) had significant effect on APA latency of the TrA/IO. The group effect (F(1, 56) = 1.02, p = 0.3159) had no significant effect on the latency of APA in the TrA/IO. There was no interaction effect between time and group (F(1, 56) = 0.43, p = 0.5140), between time and location (F(1, 56) = 1.20, p = 0.2789), or between group and location (F(1, 56) = 0.04, p = 0.8443). There were no interaction effects among group, time, and location (F(1, 56) = 1.36, p = 0.2487). In the case of cognitive load, the APA latency of the right TrA/IO was significantly delayed compared with that of the left TrA/IO in the NCLBP group [mean 29.15, 95% confidence interval (CI) 18.81 to 39.50 versus mean 3.69, 95% CI - 6.81 to 14.18, p = 0.0363]. As shown in Fig. 2b, time effects (F(1, 56) = 13.28, p = 0.0006), and the location effects (F(1, 56) = 23.81, p < 0.0001) had significant effect on the latency of APA in MF. There were significant interaction effects between time and group (F(1, 56) = 7.62, p = 0.0078). There were no interaction effects between time, group, and location (F(1, 56) = 0.88, p = 0.3523). The APA latency of the right MF under cognitive load was significantly delayed compared with that on the left side in patients with NCLBP (mean 25.38, 95% CI 13.41–37.35 versus means − 3.03, 95% CI − 15.18 to 9.13, p = 0.0220) and right side in patients with NCLBP without cognitive load (mean 25.38, 95% CI 13.41–37.35 versus means − 5.88, 95% CI − 22.56 to 10.80, p = 0.0092). During the dual task, the APA latency of right MF was significantly delayed than that on the right side in the HC group (mean 25.38, 95% CI 13.41–37.35 versus mean − 5.80, 95% CI − 19.28 to 7.68, p = 0.0416). Results of three-factors mixed-design ANOVA for APA latency of bilateral trunk muscles were present in Supplementary Table 1.
APA latency of bilateral TrA/IO and MF between NCLBP participants and healthy controls in the rapid arm-raising task with or without cognitive load. a The latency of the right TrA/IO was delayed significantly more than the left TrA/IO in participants with NCLBP during the dual task. * p < 0.05, right vs. left muscle in NCLBP in dual task. b The latency of the right MF during the dual task in participants with NCLBP was delayed significantly more than that of the left MF during the dual task and that of the right side during the single task. During the dual task, the APA latency of right MF was significantly delayed than that on the right side in the HC group. *p < 0.05, right vs. left muscle in NCLBP in dual task. ##p < 0.01, no cognitive load vs. cognitive load in right muscle in NCLBP. $p < 0.05, NCLBP vs. HC in right muscle during dual task. Data are presented as mean (standard deviation). TrA/IO transverse abdominis/internal oblique, MF multifidus, APA anticipatory postural adjustment, NCLBP non-specific chronic lower back pain, HC healthy control
Positive Correlation Between Delayed APA Onset and RMDQ Scores in Participants with NCLBP During the Dual Task
Several studies have reported a relationship between APA latency alterations and low back pain-related dysfunction. Therefore, we further analyzed the correlation between the changes in APA latency of the TrA/IO and MF with RMDQ scores. As shown in Fig. 3, the APA latency delay in the right MF (r = 0.5560, p = 0.0017) and left MF (r = 0.4010, p = 0.0311) during the dual task in the NCLBP group were positively correlated with RMDQ scores. However, there was no significant positive correlation between APA delay of the bilateral TrA/IO during the dual task with RMDQ scores. Spearman correlation analysis for APA latency of bilateral trunk muscles with RMDQ score during the dual task was showed in Supplementary Table 2.
Correlation of APA latency of bilateral TrA/IO and MF with RMDQ score during the dual task. A, B, C, and D represent the correlation of the APA latency of the right TrA/IO, left TrA/IO, right MF, and left MF with the RMDQ score, respectively. As shown in C and D, the APA latency in the right MF (r = 0.5560, p = 0.0017) and left MF (r = 0.4010, p = 0.0311) in participants with NCLBP during dual task were positively correlated with the RMDQ score. There was no significant positive correlation between the APA latency of bilateral TrA/IO during the dual task with the RMDQ score. APA anticipatory postural adjustment, TrA/IO transverse abdominis/internal oblique, MF multifidus, RMDQ Roland–Morris Disability Questionnaire, NCLBP non-specific chronic lower back pain
Discussion
The study showed that cognitive load can prolong the latency of right MF APA activation and change the trunk muscle APA co-activation pattern in young people with NCLBP. The cognitive load-induced APA onset delay in MF showed a positive relationship with pain-related dysfunction.
APA Latency Changes in a Single Task of Postural Disturbance
The APA latency changes could be affected by many factors such as age [32], task type [33], and muscle fatigue [34]. Compared with healthy young people, the muscle APA activation and displacement of pressure center were significantly delayed in elderly people when performing the object push task [35]. For the elderly population, the activations of the rectus femoris, semitendinosus and soleus and the displacement of pressure center were also significantly delayed in patients with low back pain compared to the healthy participants when performing arm lifting task [32]. However, there were no significant changes in APA latencies of the TrA/IO, external oblique, rectus abdominis, MF, or erector spinae between young participants with NCLBP and the matched healthy participants when perform ball catching task with or without eyes open [36]. Therefore, young people with NCLBP may show no significant differences in trunk muscle APA latency when performing single task of postural disturbance compared with healthy participants. In this study, we also found no significant differences between bilateral APA latencies of the trunk muscles in young patients with NCLBP and young healthy participants when performing a single task of rapid arm-raising task. Meanwhile, no significant differences were found between the APA latency of the right and left trunk muscles in young patients with NCLBP or young healthy participants when performing a rapid arm-raising task.
APA Latency Changes in Dual Task of Postural Disturbance with Cognitive Load
In recent years, researchers have gradually realized the important role of cognitive load in postural control. The “U-shaped” relationship [37], limited resource hypothesis [38], and task prioritization model [39] have received extensive attention in understanding the interaction mechanism between cognitive load and postural control. To date, there has been no convincing model to explain the effect and mechanism of cognitive load on postural control. Several studies found that cognitive load could have an impact on altered postural control in people with NCLBP, which may be linked to the risk of falls [40] and trunk coordination [41]. However, the effect of cognitive load on the APA latency of trunk muscles in patients has rarely been reported.
For healthy people, it has been reported that during the task of bilateral hands receiving a sudden dropped ball as an external postural interference, different levels of cognitive load (minus 3 calculation task, time-limited minus 3 calculation task) showed no significant effect on the APA latency of the erector spinae or biceps brachii [42]. Similarly, we found that visual conflict cognitive load did not have a significant effect on APA latency in the TrA/IO or MF in healthy participants. Considering the “U-shaped” model relationship, the results may be related to the fact that the cognitive load difficulty applied in our study was not strong enough to affect APA latency in healthy participants.
For patients with low back pain, study has shown later activation of the tibialis anterior muscle (agonist) and earlier activation of the gastrocnemius muscle (antagonist) in people with NCLBP under dual tasks (postural interference task combined with back digit span task) than in healthy participants [17]. Besides, people with chronic low back pain showed less trunk flexibility when facing posture disturbance combined with cognitive load than that under the condition only facing a single task of posture disturbance [43]. Cognitive load could increase the Stroop reaction time and affect the initial velocity adaptation in patients with low back pain when facing posture disturbance [44].When faced with posture disturbance, patients with NCLBP were more likely to show delayed APA activation in trunk muscle[12]. However, whether cognitive load could induce trunk muscle APA latency change in patients with NCLBP is seldom reported. Previous study has reported that cognitive load could induce longer interval to peak force at the stepping preparation phase [45]. In this study, we found that participants with NCLBP showed APA latency delay in the right MF compared with healthy participants under cognitive load combined with a postural control task. Furthermore, participants with NCLBP showed later APA activation in the right MF and TrA/IO than on the left side while performing the rapid arm-raising task combined with cognitive load. The results suggested that cognitive load could induce the APA co-activation pattern change in bilateral TrA/IO and MF. In addition, we found there were no statistical difference in APA latencies of left-sided trunk muscles between NCLBP and HCs with or without cognitive load. Since previous studies have found ipsilateral inhibition of trunk muscle APA onset during arm-raising tasks [46, 47], which may have contributed to the inconsistent onset of the left and right trunk muscles in this study.
APA latency and daily functional changes
Abnormal trunk neuromuscular control alterations, such as decreased muscular endurance, muscle strength, and lumbar proprioception, are positively correlated with pain severity and pain-related dysfunction in patients with NCLBP [48]. APA, an important strategy of posture control, have also been reported to be related with the development of non-specific chronic low pain [49, 50]. It has been demonstrated that an increasing delay in the latency of external oblique muscle activation is accompanied by an increased Oswestry Disability Index score in patients with chronic low back pain, which occurs when performing a rapid arm-raising task [51]. Pain-related disabilities in patients with NCLBP may be related to APA under endogenous and exogenous postural disturbances [52]. All these studies suggest that dysregulated APA onset in trunk muscles may be associated with pain-related dysfunction, but the relationship remains unclear. In this study, we found that young people with NCLBP showed abnormal changes in APA latency of the MF and TrA/IO when performing rapid arm raises combined with cognitive load posture interference, and there was a positive correlation between these delays in cognitive load-induced APA onset of MF and pain-related dysfunction. All these findings further contribute to the pivotal role of the MF in lumbar spine stability and its close relationship with functional change in low back pain [53,54,55]. This study suggests that young people with NCLBP in a complex environment may be more vulnerable to APA onset delay in deep lumbar muscles in daily life, which may be one of the reasons for self-reported pain-related dysfunction in daily life.
This study has some limitations. Firstly, motor-related cortex functional changes may be the central mechanism of APA changes in chronic low back pain [56, 57]. Future studies should apply approaches to explore functional changes in the central nervous system. Secondly, the effects of cognitive loads with different difficulty levels on APA in people with NCLBP should be investigated in future studies, which would provide an experimental basis for understanding the interaction between different levels of cognitive load on APA in people with NCLBP. Finally, this study did not further explore whether improving APA onset under cognitive load could be beneficial for the dysfunction of chronic low back pain.
Conclusions
This study shows that cognitive load could induce right MF APA onset delay and bilateral trunk muscle APA co-activation pattern change in participants with NCLBP. The MF APA onset delay induced by cognitive load is closely related to pain-related dysfunction. Further research to explore the approach to improve trunk muscle APA latency under cognitive load and its therapeutic effects on pain-related dysfunction in NCLBP is worthwhile.
Change history
21 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40122-023-00515-z
References
Bardin LD, King P, Maher CG. Diagnostic triage for low back pain: a practical approach for primary care. Med J Aust. 2017;206(6):268–73.
Koes BW, van Tulder MW, Thomas S. Diagnosis and treatment of low back pain. BMJ. 2006;332(7555):1430–4.
Olafsdottir H, Yoshida N, Zatsiorsky VM, Latash ML. Elderly show decreased adjustments of motor synergies in preparation to action. Clin Biomech (Bristol, Avon). 2007;22(1):44–51.
Masse-Alarie H, Schneider C. Revisiting the corticomotor plasticity in low back pain: challenges and perspectives. Healthcare (Basel). 2016;4(3):67.
Chen B, Lee YJ, Aruin AS. Anticipatory and compensatory postural adjustments in conditions of body asymmetry induced by holding an object. Exp Brain Res. 2015;233(11):3087–96.
Xie L, Wang J. Anticipatory and compensatory postural adjustments in response to loading perturbation of unknown magnitude. Exp Brain Res. 2019;237(1):173–80.
Wang Y, Watanabe K, Asaka T. Anticipatory and compensatory postural adjustments in response to dynamic platform perturbation during a forward step. J Mot Behav. 2022(1940–1027 (Electronic)):1–8.
van Dieen JH, Reeves NP, Kawchuk G, van Dillen LR, Hodges PW. Motor control changes in low back pain: Divergence in presentations and mechanisms. J Orthop Sports Phys Ther. 2019;49(6):370–9.
Cholewicki J, Silfies SP, Shah RA, et al. Delayed trunk muscle reflex responses increase the risk of low back injuries. Spine (Phila Pa 1976). 2005;30(23):2614–20.
Marshall PW, Romero R, Brooks C. Pain reported during prolonged standing is associated with reduced anticipatory postural adjustments of the deep abdominals. Exp Brain Res. 2014;232(11):3515–24.
Suehiro T, Mizutani M, Ishida H, Kobara K, Osaka H, Watanabe S. Individuals with chronic low back pain demonstrate delayed onset of the back muscle activity during prone hip extension. J Electromyogr Kinesiol. 2015;25(4):675–80.
Knox MF, Chipchase LS, Schabrun SM, Romero RJ, Marshall PWM. Anticipatory and compensatory postural adjustments in people with low back pain: a systematic review and meta-analysis. Spine J. 2018;18(10):1934–49.
Jull GA, Richardson CA. Motor control problems in patients with spinal pain: a new direction for therapeutic exercise. J Manipulative Physiol Ther. 2000;23(2):115–7.
Freeman MD, Woodham MA, Woodham AW. The role of the lumbar multifidus in chronic low back pain: a review. PM R. 2010;2(2):142–67.
Song YH, Cho SN, Nam SM. Asymmetric influence of dual-task interference on anticipatory postural adjustments in one-leg stance. Int J Environ Res Public Health. 2022;19(18):11289.
Ge L, Yu Q, Wang C, et al. How cognitive loads modulate the postural control of older women with low back pain? BMC Geriatr. 2021;21(1):82.
Hemmati L, Piroozi S, Rojhani-Shirazi Z. Effect of dual tasking on anticipatory and compensatory postural adjustments in response to external perturbations in individuals with nonspecific chronic low back pain: Electromyographic analysis. J Back Musculoskelet Rehabil. 2018;31(3):489–97.
Saragiotto BT, Maher CG, Yamato TP, et al. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst Rev. 2016(1):CD012004.
Goubert D, Oosterwijck JV, Meeus M, Danneels L. Structural changes of lumbar muscles in non-specific low back pain: a systematic review. Pain Physician. 2016;19(7):E985–1000.
Saper RB, Lemaster C, Delitto A, et al. Yoga, physical therapy, or education for chronic low back pain: a randomized noninferiority trial. Ann Intern Med. 2017;167(2):85–94.
Yi H, Ji X, Wei X, et al. Reliability and validity of simplified Chinese version of Roland–Morris questionnaire in evaluating rural and urban patients with low back pain. PLoS ONE. 2012;7(1): e30807.
Boonstra AM, Schiphorst Preuper HR, Reneman MF, Posthumus JB, Stewart RE. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int J Rehabil Res. 2008;31(2):165–9.
Matsumoto A, Liang N, Ueda H, Irie K. Corticospinal excitability of the lower limb muscles during the anticipatory postural adjustments: a TMS study during dart throwing. Front Hum Neurosci. 2021;15: 703377.
Shiozawa S, Hirata RP, Jeppesen JB, Graven-Nielsen T. Impaired anticipatory postural adjustments due to experimental infrapatellar fat pad pain. Eur J Pain. 2015;19(9):1362–71.
Li X, Liu H, Lin KY, et al. Effects of Different Sling Settings on Electromyographic Activities of Selected Trunk Muscles: A Preliminary Research. Biomed Res Int. 2020;2020:2945952.
Mehta R, Cannella M, Henry SM, Smith S, Giszter S, Silfies SP. Trunk postural muscle timing is not compromised in low back pain patients clinically diagnosed with movement coordination impairments. Mot Control. 2017;21(2):133–57.
Smith JA, Ignasiak NK, Jacobs JV. Task-invariance and reliability of anticipatory postural adjustments in healthy young adults. Gait Posture. 2020;76:396–402.
Coelho DB, Bazan PR, Zimeo Morais GA, et al. Frontal hemodynamic response during step initiation under cognitive conflict in older and young healthy people. J Gerontol A Biol Sci Med Sci. 2021;76(2):216–23.
Mitchell UH, Owen PJ, Rantalainen T, Belavy DL. Increased joint mobility is associated with impaired transversus abdominis contraction. J Strength Cond Res. 2022;36(9):2472–8.
Russo M, Deckers K, Eldabe S, et al. Muscle control and non-specific chronic low back pain. Neuromodulation. 2018;21(1):1–9.
Silfies SP, Mehta R, Smith SS, Karduna AR. Differences in feedforward trunk muscle activity in subgroups of patients with mechanical low back pain. Arch Phys Med Rehabil. 2009;90(7):1159–69.
Garcez DR, da Silva Almeida GC, Silva CFO, et al. Author Correction: Postural adjustments impairments in elderly people with chronic low back pain. Sci Rep. 2021;11(1):16837.
Masse-Alarie H, Beaulieu LD, Preuss R, Schneider C. Task-specificity of bilateral anticipatory activation of the deep abdominal muscles in healthy and chronic low back pain populations. Gait Posture. 2015;41(2):440–7.
Allison GT, Henry SM. The influence of fatigue on trunk muscle responses to sudden arm movements, a pilot study. Clin Biomech (Bristol, Avon). 2002;17(5):414–7.
Lee YJ, Chen B, Aruin AS. Older adults utilize less efficient postural control when performing pushing task. J Electromyogr Kinesiol. 2015;25(6):966–72.
Akbari M, Sarrafzadeh J, Maroufi N, Haghani H. Changes in postural and trunk muscles responses in patients with chronic nonspecific low back pain during sudden upper limb loading. Med J Islam Repub Iran. 2015;29:265.
Decker LM, Cignetti F, Hunt N, Potter JF, Stergiou N, Studenski SA. Effects of aging on the relationship between cognitive demand and step variability during dual-task walking. Age (Dordr). 2016;38(4):363–75.
Wollesen B, Voelcker-Rehage C, Regenbrecht T, Mattes K. Influence of a visual-verbal Stroop test on standing and walking performance of older adults. Neuroscience. 2016;318:166–77.
Yogev-Seligmann G, Hausdorff JM, Giladi N. Do we always prioritize balance when walking? Towards an integrated model of task prioritization. Mov Disord. 2012;27(6):765–70.
Hamacher D, Hamacher D, Schega L. A cognitive dual task affects gait variability in patients suffering from chronic low back pain. Exp Brain Res. 2014;232(11):3509–13.
Rowley KM, Winstein CJ, Kulig K. Persons in remission from recurrent low back pain alter trunk coupling under dual-task interference during a dynamic balance task. Exp Brain Res. 2020;238(4):957–68.
Zhang Z, Gao Y, Wang J. Effects of vision and cognitive load on anticipatory and compensatory postural control. Hum Mov Sci. 2019;64:398–408.
Shanbehzadeh S, Salavati M, Talebian S, Khademi-Kalantari K, Tavahomi M. Attention demands of postural control in non-specific chronic low back pain subjects with low and high pain-related anxiety. Exp Brain Res. 2018;236(7):1927–38.
Etemadi Y, Salavati M, Arab AM, Ghanavati T. Balance recovery reactions in individuals with recurrent nonspecific low back pain: Effect of attention. Gait Posture. 2016;44:123–7.
Melzer I, Liebermann DG, Krasovsky T, Oddsson LI. Cognitive load affects lower limb force-time relations during voluntary rapid stepping in healthy old and young adults. J Gerontol A Biol Sci Med Sci. 2010;65(4):400–6.
Masse-Alarie H, Flamand VH, Moffet H, Schneider C. Corticomotor control of deep abdominal muscles in chronic low back pain and anticipatory postural adjustments. Exp Brain Res. 2012;218(1):99–109.
Davarian S, Maroufi N, Ebrahimi E, Parnianpour M, Farahmand F. Normal postural responses preceding shoulder flexion: co-activation or asymmetric activation of transverse abdominis? J Back Musculoskelet Rehabil. 2014;27(4):545–51.
Hu H, Zheng Y, Wang X, et al. Correlations between lumbar neuromuscular function and pain, lumbar disability in patients with nonspecific low back pain: a cross-sectional study. Medicine (Baltimore). 2017;96(36): e7991.
Boucher JA, Preuss R, Henry SM, Nugent M, Lariviere C. Trunk postural adjustments: medium-term reliability and correlation with changes of clinical outcomes following an 8-week lumbar stabilization exercise program. J Electromyogr Kinesiol. 2018;41:66–76.
Zheng YL, Hu HY, Liu XC, Su X, Chen PJ, Wang XQ. The effects of whole-body vibration exercise on anticipatory delay of core muscles in patients with nonspecific low back pain. Pain Res Manag. 2021;2021:9274964.
Jacobs JV, Lyman CA, Hitt JR, Henry SM. Task-related and person-related variables influence the effect of low back pain on anticipatory postural adjustments. Hum Mov Sci. 2017;54(1872–7646 (Electronic)):210–9.
Yu Q, Huo Y, Chen M, et al. A study on the relationship between postural control and pain-related clinical outcomes in patients with chronic nonspecific low back pain. Pain Res Manag. 2021;2021:9054152.
Macdonald DA, Dawson AP, Hodges PW. Behavior of the lumbar multifidus during lower extremity movements in people with recurrent low back pain during symptom remission. J Orthop Sports Phys Ther. 2011;41(3):155–64.
Wilke HJ, Wolf S, Claes LE, Arand M, Wiesend A. Stability increase of the lumbar spine with different muscle groups. A biomechanical in vitro study. Spine (Phila Pa 1976). 1995;20(2):192–8.
Masse-Alarie H, Beaulieu LD, Preuss R, Schneider C. Repetitive peripheral magnetic neurostimulation of multifidus muscles combined with motor training influences spine motor control and chronic low back pain. Clin Neurophysiol. 2017;128(3):442–53.
Masse-Alarie H, Beaulieu LD, Preuss R, Schneider C. Corticomotor control of lumbar multifidus muscles is impaired in chronic low back pain: concurrent evidence from ultrasound imaging and double-pulse transcranial magnetic stimulation. Exp Brain Res. 2016;234(4):1033–45.
Jafarzadeh A, Ehsani F, Yosephi MH, Zoghi M, Jaberzadeh S. Concurrent postural training and M1 anodal transcranial direct current stimulation improve postural impairment in patients with chronic low back pain. J Clin Neurosci. 2019;68(1532–2653 (Electronic)):224–34.
Acknowledgements
The authors wish to thank Si-yun Zhang and Ting-ni Li for their assistance with the statistical analysis. The authors thank the participants of the study.
Funding
This study was supported by the National Natural Science Foundation of China (82172532) and Development Center for Medical Science and Technology National Health Commission of the People’s Republic of China (DCMST-NHC-2019-AHT-01). The Rapid Service Fee was funded by the corresponding authors.
Authorship
All authors have reviewed the manuscript and approved it for publication. Huai-chun Yang and Wen-wu Xiao contributed equally to the study. The corresponding authors were Chu-huai Wang and Zeng-ming Hao.
Author Contributions
All the authors contributed to the conception and design of the study. Chu-huai Wang and Zeng-ming Hao designed the study and revised the main manuscript. Huai-chun Yang and Wen-wu Xiao collected and analyzed the data. Huai-chun Yang wrote the majority of this manuscript. Wen-wu Xiao helped in writing the methods section of the manuscript. Hai-an Mao and Ye-xiao Guan helped collect data. All authors read and approved the final manuscript.
Disclosures
All authors declare that they have no competing interests.
Compliance with Ethics Guidelines
This study was approved by the Human Subjects Ethics Subcommittee of the First Affiliated Hospital, Sun Yat-sen University (grant number 2022.551). This study was conducted in accordance with the principles of the Declaration of Helsinki of 1964 and its amendments. All participants were informed of the experimental protocol and signed the informed consent in the study.
Data Availability
The data generated during and/or analyzed during the current study are available from the corresponding author on reasonable requests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yang, Hc., Xiao, Ww., Guan, Yx. et al. Effect of Cognitive Load on Anticipatory Postural Adjustment Latency and its Relationship with Pain-Related Dysfunction in Non-specific Chronic Low Back Pain: A Cross-Sectional Study. Pain Ther 12, 723–735 (2023). https://doi.org/10.1007/s40122-023-00495-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00495-0