Abstract
The mechanisms underlying neuropathic pain remain unclear. Lysophosphatidic acid (LPA) is a bioactive phospholipid derived mainly from lysophosphatidylcholine (LPC) by extracellular autotaxin (ATX), and has attracted attention as a candidate biomarker of neuropathic pain. We aimed to investigate the levels of LPA, LPC, and ATX in patients with lumbar spinal canal stenosis (LSCS) or other neuropathic pain diseases, and to distinguish the underlying mechanism of LSCS from other neuropathic pain conditions. Furthermore, the levels of phosphorylated neurofilament heavy chain (pNF-H), an objective surrogate marker of axonal damage, were also measured. Cerebrospinal fluid (CSF) samples were obtained from 56 patients with LSCS (n = 31) and various etiologies other than LSCS (n = 25). Patients with LSCS complained of pain intensity comparable to that of patients without LSCS. The LPA levels were significantly higher in patients with LSCS than in non-LSCS patients, while the ATX levels were significantly lower. However, the differences in LPC and pNF-H levels between the two patient groups were not significant. The LPA/LPC ratio was significantly higher in the LSCS group. Notably, the difference in LPA between the two groups diminished in the analysis of covariance (ANCOVA) with ATX as a covariate. Thus, it helped to reveal that LPA synthesis in patients with LSCS depends more efficiently on ATX than in non-LSCS neuropathic pain patients with other etiologies. Our findings further suggest that the triad of LPA, LPC, and ATX in LSCS may contribute to the development and maintenance of neuropathic pain in a manner different from non-LSCS neuropathic conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lysophosphatidic acid (LPA) levels of cerebrospinal fluids were significantly higher in patients with lumbar spinal canal stenosis (LSCS) than in non-LSCS patients, while autotaxin (ATX) levels were significantly lower. The triad of LPA, ATX, and lysophosphatidylcholine (LPC), the substrate of LPA, of LSCS might contribute to the development and maintenance of neuropathic pain in a different way than non-LSCS neuropathic conditions |
Introduction
Pain affects one in every five people globally, and is considered a public health crisis [1]. It is one of the most common reasons people seek medical attention, and it is costly for both healthcare systems and patients. Pain has a substantial effect on health-related quality of life (QOL) and imposes a significant economic burden on both individuals and society. Among chronic pain conditions, neuropathic pain is especially debilitating, as it consistently demonstrates more severity, longer disease duration, poorer sleep quality, and more impaired physical and mental QOL than other pain conditions without neuropathic characteristics [2, 3].
Spinal disorders are primary musculoskeletal pain conditions and are highly prevalent in all adult age groups. In particular, lower back pain is the leading cause of years lost to disability, and its burden is growing alongside the increasing and aging population [4]. Spinal disorders are a diverse set of conditions affecting bones, joints, muscles, connective tissues, and the peripheral and central nervous systems. Pain that radiates to the lower extremities in a characteristic distribution (i.e., radicular pain, sometimes called “sciatica” or “radiculopathy”) and intermittent claudication are typical neuropathic symptoms in patients with lumbar spinal canal stenosis (LSCS). Although LSCS is a spinal disorder, it remains controversial whether the neuropathic pain caused by LSCS is homologous to other neuropathic pain conditions. Radicular neuropathic pain due to LSCS is reportedly less likely to be classified correctly based on pain descriptions than other neuropathic pain conditions [5]. The analgesic effect of pregabalin on lumbar radicular pain [6] seems to differ from that on other neuropathic pain diseases such as post-herpetic neuralgia, diabetic polyneuropathy, post-spinal cord injury pain, and persistent postsurgical radiculopathy after surgery for lumbar disc herniation [7,8,9].
Lysophospholipids such as lysophosphatidic acid (LPA), have been identified as important candidates for the development and maintenance of neuropathic pain [10]. LPA is a bioactive phospholipid that is primarily synthesized extracellularly from lysophosphatidylcholine (LPC) by autotaxin (ATX). LPA binds to six G-coupled protein receptors (GPCRs): LPA1-LPA6, coded by LPAR1–LPAR6 (human) or Lpar1–Lpar6 (mouse) genes. Notably, LPAR1 is highly expressed in the nervous system. We previously reported that LPAR1 is strongly expressed in the spinal cord of a neuropathic pain animal model [11] and that LPAR1 is associated with chemotherapy-induced peripheral neuropathy [12] through its gene polymorphism. Furthermore, we found that LSCS patients with severe intermittent claudication demonstrated increased levels of LPA in cerebrospinal fluids [13] and that, in the LSCS animal model, both LPA and LPC increased transiently after epidural compression procedures, and LPA was linearly associated with LPC [11]. Furthermore, we revealed that inhibition of ATX activity ameliorates neuropathic pain in a lumbar radicular neuropathic pain animal model [14].
Separately from the LSCS, we revealed a linear interaction between LPA levels and pain intensity in neuropathic pain patients with varied etiologies [15]. Thus, LPA, LPC, and ATX interact mutually and are involved in the development and maintenance of neuropathic pain in patients with LSCS and other neuropathic pain conditions. Several LPA species correspond to LPC species, and these activate several types of LPA receptors differently [16]. One LPA species, 18:1 LPA, is converted from 18:1 LPC by autotaxin, which is involved in the development of neuropathic pain via LPA1 and LPA3 receptors [17]. Considering clinical findings [6,7,8,9] and our findings about profiles of the triad of LPA, LPC, and ATX of either LSCS or other neuropathic pain conditions, the aim of this study is to elucidate and distinguish the underlying mechanism of LSCS from other neuropathic pain conditions by investigating, not only LPA species but also LPC species and ATX in patients with LSCS or other neuropathic pain diseases.
Methods
Subject Recruitment
The study protocols (study subjects, procedures under clinical settings, and laboratory and statistical analyses) were approved by the Institutional Review Board of the University of Tokyo Ethics Committee (approval number: 10516). We compared participants from both our previous reports of LSCS patients [13] and neuropathic pain patients with various etiologies [15], with new participants. All participants were the patients in our departments of orthopedic surgery and anesthesiology, who received cerebrospinal fluid (CSF) access procedures (i.e., CSF examination, spinal myelography, or neural blockade) in clinical practice. All the patients at our institute provided written informed consent before participating in the study. The investigation was conducted according to the principles expressed in the Declaration of Helsinki. Our inclusion and exclusion criteria are described in detail elsewhere [13, 15].
Briefly, the inclusion criteria were as follows: complaints of pain in any part of the body with a maximum and average numerical rating scale (NRS) > 1, and the presence of at least possible neuropathic pain as diagnosed by a certified orthopedic or pain physician based on the International Association for the Study of Pain [18]. The diagnosis of LSCS was based on a documented history, magnetic resonance imaging findings of the lumbar root nerve and/or cauda equina compression, typical clinical symptoms, and signs (i.e., pain radiating into the lower limb and evoked pain by specific procedures such as straight leg raising, impairment of motor reflexes, and intermittent claudication), and sometimes additional tests (e.g., electromyogram and lumbar myelography). We did not distinguish among nerve root type, cauda equine type, and mixed type of LSCS. LSCS included both of bone and disc deformation, and we did not distinguish types of lumbar disc herniation causing LSCS (e.g., protrusion or extrusion). Disease names and etiologies of neuropathic pain conditions other than LSCS were not considered as inclusion criteria. Patients with concomitant neurological (e.g., cognitive impairments such as dementia and hydrocephalus) or psychiatric conditions (e.g., major depressive disorders, general anxiety disorder, or delirium) that could affect and complicate neuropathic pain complaints were excluded from the study. Disease duration was not included as an inclusion criterion because the time courses of LPA, LPC, and ATX in CSF were not fully elucidated. This study included 56 patients. Thirty-one patients suffered from LSCS, while the remaining 25 had other neuropathic diseases (five suffered from adhesive arachnoiditis; three, post-herpetic neuralgia; three, noninfectious peripheral polyneuropathy; and 13, partial injury of the spinal cord; and one, lesions of multiple lumbosacral nerve roots neurinomas).
Laboratory Analysis
All participants provided CSF samples, which were collected by lumbar puncture of the L3/L4 or L4/L5 interspace. The details of our measurement methodology have been described elsewhere [13, 15]. CSF samples were sonicated and centrifuged, and the supernatant was transferred to a chromatography–tandem mass spectrometer (LC–MS) [19, 20]. After ionizing elution using an ESI probe, the parent ion (m/z 380.2) and fragment ion (m/z 264.2) were observed in the positive mode of the Quantum Ultra Triple Quadrupole Mass Spectrometer (Thermo Fisher Scientific). For each LPA and LPC molecular family, we monitored and analyzed 14 species (LPA 16:0, 18:0, 18:1, 18:2, and 20:4; and LPC 14:0, 16:0, 16:1, 18:0, 18:1, 18:2, 20:4, 20:5, and 22:5) of lipid acyl chains, and the total levels of either LPA or LPC species were analyzed. Autotaxin antigen levels were measured using a two-site immunoenzymatic assay with an autotaxin assay reagent and the TOSOH AIA system (TOSOH, Tokyo). In addition to LPA, LPC, and autotaxin, we measured the concentration of phosphorylated neurofilament heavy subunit (pNF-H), a known biomarker of the severity of neuronal damage [21]. However, its role as a biomarker of neuropathic pain remains controversial [22, 23]. Several studies have suggested that pNF-H concentration in the CSF is affected by lumbar canal stenosis, which correlates with the severity of claudication and pain [24].
Statistical Analysis
The levels of LPA, LPC, ATX, and pNF-H in the CSF and other demographic and clinical variables were compared between LSCS and other neuropathic pain patients using the Mann–Whitney U test. Since LPA is synthesized from LPC by ATX, we compared the ratio of the respective LPA species to the corresponding LPC species between the two patient groups using the Mann–Whitney Utest. Fisher’s exact test was used to compare the sex ratios of these groups. In both patient groups, we performed Pearson’s correlation analyses among CSF measurements, and rated maximum and average pain intensities on an 11-point numerical rating scale. Furthermore, we performed forced-entry multiple regression analyses using sex, age, ATX, pNF-H, total LPC, and five corresponding LPC species as independent variables, and total LPA and five LPA species as respective dependent variables. Since LPC is the substrate and ATX is the mediator for LPA synthesis, either or both total and respective LPC species and ATX would directly affect total and respective LPA species. Therefore, we entered total and respective LPC species and ATX into the multiple regression analyses as independent variables. Since previous reports have found that older age and female sex are potentially independent predictors of higher LPA, we also entered these as independent variables [25]. All statistical analyses were carried out using R version 3.4.0 software (The R Foundation for Statistical Computing, Vienna, Austria) and the Statistical Package for Social Sciences (SPSS) version 22.0 (IBM Inc., Chicago, IL, USA). The statistical significance level was set at p < 0.05.
Results
Participant Characteristics
Table 1 presents the background information of the participants. Sex and age had no statistically significant differences. The maximum and average pain intensities of the LSCS and non-LSCS patients were compared (Table 1).
Correlations Between Biomarkers
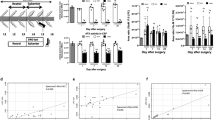
The total LPC levels of patients with LSCS (7.578 ± 6.062 nM, mean ± standard deviation) were not significantly different from those of non-LSCS neuropathic pain patients (8.40 ± 13.30, p = 0.92). The total LPA levels of patients with LSCS (123.47 ± 103.38) were significantly higher than those of non-LSCS neuropathic pain patients (60.95 ± 76.82, p < 0.01). Several LPA and LPC species differed significantly between the two patient groups (Figs. 1 and 2). The LPA 16:0 and 18:1 ratios to the corresponding LPC species were significantly different, but ratios of other LPA species (18:0, 18:2, and 20:4) were not. Patients with LSCS demonstrated significantly lower ATX levels of CSF (1.05 ± 0.11 µM) than non-LSCS patients (1.18 ± 0.11, p < 0.01). The significant difference between LPA levels in LSCS and non-LSCS neuropathic conditions was diminished by ANCOVA with ATX as a covariate (p = 0.076). The candidate biomarker of axonal damage, pNF-H, was compared between the patient groups (LSCS: 1089.2 ± 1833.1; non-LSCS: 850.0 ± 1225.7; p = 0.27).
CSF concentrations of nine LPC species between LSCS and non-LSCS patients. Gray (LSCS) and white (non-LSCS) boxes represent the 25th–75th percentiles, and the horizontal line shows the median values of the respective LPC species in the cerebrospinal fluid. The extended bars represent the 10th–90th percentiles. *p < 0.05, Mann–Whitney U test. CSF cerebrospinal fluid, LPC lysophosphatidylcholine, LSCS lumbar spinal canal stenosis
CSF concentrations of six LPA species between LSCS and non-LSCS patients. Gray (LSCS) and white (non-LSCS) boxes represent the 25th–75th percentiles, and the horizontal line shows the median values of the respective LPA species in the cerebrospinal fluid. The extended bars represent the 10th–90th percentiles. *p < 0.05, Mann–Whitney U test. CSF cerebrospinal fluid, LPA lysophosphatidic acid, LSCS lumbar spinal canal stenosis
Pain Dependency on Lysophospholipids
In patients with LSCS, the total LPC levels were significantly correlated with the maximum pain intensity (r = 0.43, p < 0.05). Furthermore, the maximum or average pain intensity was found to be significantly related to some LPA and LPC species. However, the correlations observed in LSCS were not the same between LSCS and non-LSCS patients (Table 2). The ratios of LPA species to corresponding LPC species were significantly correlated with pain intensity (Table 2); in particular, the ratio of LPA 16:0 to LPC 16:0 and pain intensity were significant in both LSCS and non-LSCS patients.
Regression Analysis with LPA
Using multiple regression analyses, we revealed that most LPA species were associated with the corresponding LPC species, except for LPA 18:0 in LSCS patients, but only LPA 18:2 in non-LSCS patients was associated with LPC 18:2 (Table 2). Furthermore, ATX was differentially associated with LSCS patients (the total LPA levels, LPA 16:0 and LPA 18:1) and non-LSCS patients (LPA 18:0) (Table 3). The axonal damage biomarker pNF-H was associated with total LPA levels and some LPA species in the LSCS.
Discussion
This study investigates the differences between LSCS and non-LSCS neuropathic pain conditions from the perspective of LPA, LPC, and the associated regulator ATX. Although our previous reports [13, 15] demonstrated that LPA levels in the CSF were associated with pain severity, a direct comparison of these patient groups clearly indicated that patients with LSCS demonstrated higher LPA levels but lower pain intensity than non-LSCS patients. Furthermore, LPC and ATX levels were significantly lower in LSCS patients than in non-LSCS patients. These results indicate that the triad of LPA, LPC, and ATX in LSCS might contribute to the development and maintenance of neuropathic pain in a manner different from non-LSCS neuropathic conditions.
Considering the relationships where LPA is synthesized extracellularly from LPC by ATX, LPA seems to be produced more efficiently by LPC and ATX in LSCS patients. Patients with non-LSCS neuropathic pain, on the other hand, appeared to inefficiently synthesize LPA from LPC and ATX. Multiple regression analyses revealed that LPA synthesis in the LSCS may be more dependent on ATX than in non-LSCS neuropathic pain conditions.
ATX is considered an essential mediator of neuropathic pain because partial sciatic nerve injury reduces hyperalgesia in ATX heterozygous-null mice [26]. The total amount of ATX in the current study was not associated with pain intensity under either LSCS or non-LSCS conditions, implying that ATX mediates the development of neuropathic pain rather than directly inducing neuropathic pain. A previous study found that intrathecal injection of LPC results in significant potent dorsal root demyelination and neuropathic pain, which were reduced or even eliminated in LPA receptor 1 gene (lpar1) null or ATX heterozygous-null mice [10]. As a result, several lines of evidence implicate the ATX–LPA–LPA receptor axis as a key regulator of microglia signaling, demyelination, and neuropathic pain [27, 28]. Our present findings demonstrate that, while LPA species are possibly associated with pain intensity, LPC species are more profoundly associated with pain intensity, especially in the LSCS. Taking these factors into account, the mechanisms that mediate the conversion of LPC to LPA by ATX are necessary to induce nerve lesions associated with neuropathic pain in patients with LSCS. In our previous study using an animal model of LSCS, inhibition of ATX activity ameliorated neuropathic pain [14]. Thus, ATX appears to be a promising key regulator in the development and maintenance of neuropathic pain, especially in the LSCS. Furthermore, extracellular LPA signaling is regulated by the balance of LPA formation by ATX and LPA degradation by lipid phosphate phosphatases (LPP) [29]. LPP are found in neural tissue membranes and are considered to regulate neuronal function [30, 31]. Although the pNF-H surrogate for neural damage was comparable in LSCS and non-LSCS patients, neural damage might affect LPP function differently, resulting in different triads of LPA, LPC, ATX dynamics, and pain phenotypes.
Based on the length of the methylene chain and the number of unsaturated bonds, LPA consists of several species, including stearoyl (18:0), palmitoyl (16:0), oleoyl (18:1), linoleoyl (18:2), and arachidonoyl (20:4). Among these, LPA 18:1 is considered the major causative molecule responsible for the development and maintenance of neuropathic pain. LPA 18:1 is involved in the amplification of LPA production feed-forward via microglial activation and demyelination [32].
Our present findings in non-LSCS patients tended to support our previous finding that LPA 18:1 is responsible for pain severity in neuropathic pain patients with various etiologies [15], but not in current LSCS patients. Rather, patients with LSCS showed a link between most LPC species and total LPC levels in the CSF, and pain intensity. One study directly focused on LPC-induced demyelination and neuropathic pain [33]. LPC may have a greater impact on neuronal damage and neuropathic pain in LSCS patients than LPA. LPA 20:4 was found to be associated with pain intensity in both LSCS and non-LSCS patients, and the CSF levels were comparable. In an animal model of neuropathic pain, LPA 20:4 was reported to have a relatively high agonist potency on LPA1 and LPA3 receptors, secondary to LPA 18:1 [32]. Furthermore, supporting evidence has been derived from other disease conditions, such as fibrosis and cancer, in which the pathogenicity of the LPA1 receptor signaling is potent, with LPA 20:4 being second behind LPA 18:1 [34]. LPA20:4 and its LPA1 receptor signaling could be potential targets for the development of medicines to treat neuropathic pain with broad etiologies.
It is worth focusing on our direct comparison finding, which shows that non-LSCS neuropathic pain patients demonstrate higher ATX levels but lower LPA levels, and their pain intensity is comparable to that of patients with LSCS. Furthermore, the ratios of LPA 16:0 to LPC 16:0 were correlated with pain intensity in both patient groups, but a significant difference was found between them. In conjunction with LPC 16:0, ATX contributed to the production of LPA 18:1 in the patients with LSCS but not in the non-LSCS patients. Such different profiles of LPA species, LPC species, and ATX might influence the clinical manifestations of LSCS and non-LSCS neuropathic pain [5].
Some limitations should be noted. This study was a preliminary finding with a very limited numbers of participants. Because this was preliminarily conducted to compare LSCS and other neuropathic pain conditions, the study sample size might not still provide adequate statistical power. The present findings might help to provide further studies with larger sample sizes with adequate statistical power. Furthermore, the patients in the present non-LSCS study were heterogeneous. Future research should look at homogeneous patients with different etiologies.
Conclusion
We revealed that LPA is produced more efficiently by LPC and ATX in patients with LSCS, suggesting that the triad of LPA, LPC, and ATX of LSCS may contribute to the development and maintenance of neuropathic pain in a different way than non-LSCS neuropathic conditions. Our findings would help to understand more detailed underlying pathophysiologic mechanisms of LSCS and other neuropathic pain conditions, and might provide differential diagnoses for unspecified leg and low back pain.
References
Hoeger Bement M, St Marie B, Nordstrom T, Christensen N, Mongoven J, Koebner I, Fishman S, Sluka K. An Interprofessional consensus of core competencies for prelicensure education in pain management: curriculum application for physical therapy. Phys Ther. 2014;94:451–64.
Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–7.
Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira B. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152:2836–43.
Buchbinder R, Tulder M von, Öberg B, Menezes Costa L, Woolf A, Schoene M, Croft P, Lancet Low Back Pain Series WG. Low back pain: a call for action. Lancet. 2018;391: 2384–2388.
Behrman M, Linder R, Assadi AH, Stacey BR, Backonja M-M. Classification of patients with pain based on neuropathic pain symptoms: comparison of an artificial neural network against an established scoring system. Eur J Pain. 2007;11:370–6.
Mathieson S, Maher CG, McLachlan AJ, Latimer J, Koes BW, Hancock MJ, Harris I, Day RO, Billot L, Pik K, Jan S, Lin C-WC. Trial of pregabalin for acute and chronic sciatica. N Engl J Med. 2017;376:1111–20.
Derry S, Bell R F, Straube S, Wiffen P J, Aldington D, Moore R A. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1: CD007–076.
Yu X, Liu T, Zhao D, Yang K, Zhang X, Zhang M, Zhang S, Huang W, Wu B, Li J. Efficacy and safety of pregabalin in neuropathic pain followed spinal cord injury: a review and meta-analysis of randomized controlled trials. Clin J Pain. 2019;35:272–8.
Canos A, Cort L, Fernández Y, Rovira V, Pallarés J, Barberá M, Morales-Suárez-Varela M. Preventive analgesia with pregabalin in neuropathic pain from “failed back surgery syndrome”: assessment of sleep quality and disability. Pain Med. 2016;17:344–52.
Ueda H. Lysophosphatidic acid signaling is the definitive mechanism underlying neuropathic pain. Pain. 2017;158:S55–65.
Uranbileg B, Ito N, Kurano M, Saigusa D, Saito R, Uruno A, Kano K, Ikeda H, Yamada Y, Sumitani M, Sekiguchi M, Aoki J, Yatomi Y. Alteration of the lysophosphatidic acid and its precursor lysophosphatidylcholine levels in spinal cord stenosis: a study using a rat cauda equina compression model. Sci Rep. 2019;9:16578.
Tsuchida R, Tanabe Y, Nishizawa D, Ikeda K, Abe H, Inoue R, Kurano M, Yatomi Y, Tamura K, Takano T, Shimizu C, Uchida K, Sumitani M. Genetic polymorphisms of lysophosphatidic acid receptor 1 are associated with the onset of taxane-induced peripheral neuropathy. Br J Anaesth. 2021;127:e43–6.
Hayakawa K, Kurano M, Ohya J, Oichi T, Kano K, Nishikawa M, Uranbileg B, Kuwajima K, Sumitani M, Tanaka S, Aoki J, Yatomi Y, Chikuda H. Lysophosphatidic acids and their substrate lysophospholipids in cerebrospinal fluid as objective biomarkers for evaluating the severity of lumbar spinal stenosis. Sci Rep. 2019;9:9144.
Uranbileg B, Ito N, Kurano M, Kano K, Uchida K, Sumitani M, Aoki K, Yatomi Y. Inhibition of autotaxin activity ameliorates neuropathic pain derived from lumbar spinal canal stenosis. Sci Rep. 2021;11:3984.
Kuwajima K, Sumitani M, Kurano M, Kano K, Nishikawa M, Uranbileg B, Tsuchida R, Ogata R, Aoki J, Yatomi Y, Yamada Y. Lysophosphatidic acid is associated with neuropathic pain intensity in humans: An exploratory study. PLoS ONE. 2018;13: e0207310.
Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure-activity relationship of cloned LPA receptors. FEBS Lett. 2000; 478: 159–65
Ma L, Nagai J, Chun J, Ueda H. An LPA species (18:1 LPA) plays key roles in the self-amplification of spinal LPA production in the peripheral neuropathic pain model. Mol Pain. 2013;17(9):29.
Treede R-D, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–5.
Sakai E, Kurano M, Morita Y, Aoki J, Yatomi Y. Establishment of a measurement system for sphingolipids in the cerebrospinal fluid based on liquid chromatography-tandem mass spectrometry, and its application in the diagnosis of carcinomatous meningitis. J Appl Lab Med. 2020;5:656–70.
Morita Y, Kurano M, Sakai E, Nishikawa M, Sawabe M, Aoki J, Yatomi Y. Evaluation of lysophospholipid measurement in cerebrospinal fluid samples using liquid chromatography-tandem mass spectrometry. Lipids. 2019;54:487–500.
Inoue R, Sumitani M, Ogata T, Chikuda H, Matsubara T, Kato S, Shimojo N, Uchida K, Yamada Y. Direct evidence of central nervous system axonal damage in patients with postoperative delirium: a preliminary study of pNF-H as a promising serum biomarker. Neurosci Lett. 2017;653:39–44.
Natori A, Ogata T, Sumitani M, Kogure T, Yamauchi T, Yamauchi H. Potential role of pNF-H, a biomarker of axonal damage in the central nervous system, as a predictive marker of chemotherapy-induced cognitive impairment. Clin Cancer Res. 2015;21:1348–52.
Sumitani M, Ogata T, Natori A, Hozumi J, Shimojo N, Kida K, Yamauchi H, Yamauchi T. Poor efficacy of the phosphorylated high-molecular-weight neurofilament heavy subunit serum level, a biomarker of axonal damage, as a marker of chemotherapy-induced peripheral neuropathy. Biomed Rep. 2016;4:758–60.
Ohya J, Chikuda H, Kato S, Hayakawa K, Oka H, Takeshita K, Tanaka S, Ogata T. Elevated levels of phosphorylated neurofilament heavy subunit in the cerebrospinal fluid of patients with lumbar spinal stenosis: preliminary findings. Spine J. 2015;15:1587–92.
Michalcczyk A, Budkowska M, Dolegowska B, Chlubek D, Safranow K. Lysophosphatidic acid plasma concentrations in healthy subjects: circadian rhythm and associations with demographic, anthropometric and biochemical parameters. Lipids Health Dis. 2017;16:140.
Inoue M, Ma L, Aoki J, Chun J, Ueda H. Autotaxin, a synthetic enzyme of lysophosphatidic acid (LPA), mediates the induction of nerve-injured neuropathic pain. Mol Pain. 2008;4:6.
Inoue M, Rashid MH, Fujita R, Contos JJA, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004;10:712–8.
Ueda H. Molecular mechanisms of neuropathic pain-phenotypic switch and initiation mechanisms. Pharmacol Ther. 2006;109:57–77.
Hemmings DG, Brindley DN. Signalling by lysophosphatidate and its health implications. Essays Biochem. 2020;64:547–63.
Yu P, Agbaegbu C, Malide DA, Wu X, Katagiri Y, Hammer JA, Geller HM. Cooperative interactions of LPPR family members in membrane localization and alteration of cellular morphology. J Cell Sci. 2015;128:3210–22.
Strauss U, Bräuer AU. Current views on regulation and function of plasticity-related genes (PRGs/LPPRs) in the brain. Biochim Biophys Acta. 2013;1831:133–8.
Ma L, Nagai J, Chun J, Ueda H. An LPA species (18:1 LPA) plays key roles in the self-amplification of spinal LPA production in the peripheral neuropathic pain model. Mol Pain. 2013;9:29.
Matsuoka H, Tanaka H, Sayanagi J, Iwahashi T, Suzuki K, Nishimoto S, Okada K, Murase T, Yoshikawa H. Neurotropin ® accelerates the differentiation of schwann cells and remyelination in a rat lysophosphatidylcholine-induced demyelination model. Int J Mol Sci. 2018;19:516.
Sattikar A, Dowling MR, Rosethorne EM. Endogenous lysophosphatidic acid (LPA1) receptor agonists demonstrate ligand bias between calcium and ERK signaling pathways in human lung fibrosis. Br J Pharmacol. 2017;174:227–37.
Acknowledgements
The authors would like to thank all the participants of the study.
Funding
This work was supported by Core Research for Evolutional Science and Technology (CREST) from the Japan Science and Technology Agency (JST)/Japan Agency for Medical Research and Development (AMED) (Grant number: 17gm0710001h0105; Recipient: Junken Aoki), Leading Advanced Projects for Medical Innovation (LEAP) from the Japan Agency for Medical Research and Development (AMED) (Grant number: 17gm0010004s0101; Recipient: Junken Aoki), the Research Project on Elucidation of Chronic Pain from the Japan Agency for Medical Research and Development (AMED) (Grant number: 22ek0610028h0001; Recipient: Makoto Kurano), Grant-in-Aid for Scientific Research on Innovative Areas from the Japan Society for the Promotion of Science (JSPS) (Grant number: 15H05906; Recipient: Yutaka Yatomi). The funding organizations had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All authors critically revised the draft, commented on the manuscript, and approved the final version of the manuscript.
Author Contributions
Tatsuma Edamura wrote the manuscript and analyzed the whole data. Kentaro Hayakawa, Reo Inoue, Hiroaki Abe, Rikuhei Tsuchida and Hirotaka Chikuda collected clinical CSF samples. Masahiko Sumitani directed the study and supervised the analysis. Junken Aoki, Toru Ogata, Makoto Kurano and Yutaka Yatomi, measured the biomarkers. Hirotaka Chikuda, Toru Ogata, Makoto Kurano, Junken Aoki, Yutaka Yatomi and Kanji Uchida supported analysis and advised from the standpoints of orthopedic surgery, rehabilitation medicine, laboratory medicine, anesthesiology and pain medicine.
List of Investigators
Tatsuma Edamura, Masahiko Sumitani, Kentaro Hayakawa, Reo Inoue, Hiroaki Abe, Rikuhei Tsuchida, Hirotaka Chikuda, Toru Ogata, Makoto Kurano, Junken Aoki, Yutaka Yatomi, Kanji Uchida.
Prior Presentation
Participants were recruited from both our previous reports of LSCS patients [13] and neuropathic pain patients with various etiologies [15], with new participants.
Disclosures
Tatsuma Edamura, Masahiko Sumitani, Kentaro Hayakawa, Reo Inoue, Hiroaki Abe, Rikuhei Tsuchida, Hirotaka Chikuda, Toru Ogata, Makoto Kurano, Junken Aoki, Yutaka Yatomi, Kanji Uchida have nothing to disclose.
Compliance with Ethics Guidelines
The study protocols were approved by the Institutional Review Board of the University of Tokyo Ethics Committee (approval number: 10516). All the patients at our institute were provided for written informed consent before participating in the study. The investigation was conducted according to the principles expressed in the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Edamura, T., Sumitani, M., Hayakawa, K. et al. Different Profiles of the Triad of Lysophosphatidylcholine, Lysophosphatidic Acid, and Autotaxin in Patients with Neuropathic Pain Diseases: a Preliminary Observational Study. Pain Ther 11, 1439–1449 (2022). https://doi.org/10.1007/s40122-022-00445-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00445-2