Abstract
Introduction
The administration of methylprednisolone (MP) is a component of perioperative multimodal analgesia that mitigates the potentially deleterious effects of postoperative pain and opioid consumption. However, a systematic evaluation of the efficacy and safety of MP is lacking. The present systematic review and meta-analysis was performed to quantify the potential clinical benefits and risks of perioperative MP in lung surgery.
Methods
We searched seven electronic databases for randomized controlled trials (RCTs) comparing MP with placebo. Coprimary outcomes were rest pain scores, dynamic pain scores, and cumulative morphine equivalent consumption within 24 h postoperatively.
Results
A total of 11 trials including 643 participants were selected for our meta-analysis. The results demonstrated that the MP group had a significant difference in coprimary outcomes (rest pain scores, dynamic pain scores, and cumulative morphine equivalent consumption) compared with the placebo group; nevertheless, the improvement was not clinically meaningful based on minimum clinically important differences (MCID). Notably, MP administration reduced serum levels of interleukin (IL)-6 at 6 h (weighted mean difference −20.49 pg/mL; 95% CI −29.94 to −11.04), and decreased the incidence rate of acute lung injury (rate ratio 0.18; 95% CI 0.03–0.98) and cognitive dysfunction (rate ratio 0.43; 95% CI 0.21–0.88) compared with the placebo group.
Conclusions
Our findings suggest that the administration of MP contributed to an insignificant relief in acute postoperative pain for lung surgery in a clinical setting. Future studies should focus on exploring the role of MP in reducing pulmonary and surgical-related complications after lung surgery.
Clinical Trial Number
PROSPERO registration number CRD42022314224.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Although the additional benefits of perioperative MP in lung surgeries are supported by several randomized controlled trials (RCTs), the utility of perioperative MP remains controversial for postoperative analgesia. |
Therefore, summary of evidence is needed regarding the clinical effects and safety of perioperative methylprednisolone in lung surgeries. |
What was learned from the study? |
A systematic review and meta-analysis of the effects of perioperative MP on lung surgery are presented in the article. |
The results indicate that the MP has little effect on relieving acute postoperative pain after lung surgery clinically. |
Further evidence is needed regarding the role of MP in reducing complications after lung surgery. |
Introduction
Acute postoperative pain is a common complication after thoracic surgery, and is the result of surgical trauma and inflammatory reactions [1,2,3]. Poor management of acute pain in lung surgeries can contribute to short- and long-term negative consequences such as a decrease in pulmonary function, chronic postsurgical pain, and delayed rehabilitation [4,5,6]. However, the postoperative use of analgesics (such as opioids and nonsteroidal antiinflammatory drugs) may increase the risk of adverse effects, including gastrointestinal symptoms and respiratory depression, which significantly limits their clinical application [7]. Consequently, more attention has been paid to the wide range of multimodal perioperative pain management for lung surgeries.
Glucocorticoids such as dexamethasone and methylprednisolone (MP), characterized by potent inflammatory suppressive properties, are a component of perioperative multimodal analgesia that mitigate the potentially deleterious effects of postoperative pain and opioid consumption [3, 8]. Of particular interest is that MP has more frequent and long-term records and studies of safety and effectiveness in analgesia after lung surgery compared with the administration of dexamethasone [8, 9, 11, 12]. It has been reported previously that the administration of MP during the perioperative period decreased the cytokine response and thus improved surgical outcomes [10]. Of note, the additional benefits of perioperative MP in lung surgeries are supported by several randomized controlled trials (RCTs), which have explored whether MP results in decreased levels of inflammation, prolonged pain relief, and fewer pulmonary complications [11,12,13,14,15,16,17]. Nevertheless, the utility of perioperative MP remains controversial for postoperative analgesia. Moreover, the possible side effects of MP, including an increased risk of infection, delayed wound healing, and transient hyperglycemia, should be considered [10, 18,19,20,21]. Therefore, further high-quality evidence is needed regarding the clinical effects and safety of perioperative methylprednisolone in lung surgeries.
To build a robust evidence base, a systematic review and meta-analysis of the literature was performed to quantify the potential clinical efficacy of perioperative methylprednisolone in acute pain after lung surgeries. Pain scores and cumulative analgesic consumption 24 h postoperatively were designated as the coprimary outcomes in this meta-analysis. Moreover, the safety of methylprednisolone was explored through a comprehensive review of complications and adverse events.
Materials and Methods
Search Strategy
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), Assessing the Methodological Quality of Systematic Reviews (AMSTAR), and the Cochrane Collaboration [22]. This meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022314224). This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. A comprehensive search was conducted from seven electronic databases, including PubMed, Web of Science, Embase, the Cochrane Library, Google Scholar, China National Knowledge Infrastructure, and the Wan-Fang database. Completed and ongoing trials were also searched from two trial registries (ClinicalTrials.gov and the International Clinical Trial Registry Platform). The date of the last search was 20 February 2022.
The search process was conducted after consultation with an information specialist (Q.L.W.). Structured literature searches were applied by using subject words and free words such as “methylprednisolone,” “thoracic surgical procedures,” “lung surgery,” and “randomized controlled trial.” More details on the retrieval strategy are shown in the supplementary materials (eMethods 1–5). No restrictions on the language, publication year, journal, or region were considered.
Eligibility Criteria and Study Selection
Inclusion criteria:
Participants Patients who underwent lung surgery were included.
Interventions MP was administered perioperatively. Notably, we considered the number of interventions (including single and multiple) for the diversity of clinical practice.
Comparator The control interventions were placebo or no treatment.
Outcomes Studies that reported at least one of the following outcomes were eligible for inclusion: pain-related outcomes, inflammatory indicators, other clinical indicators (such as duration of hospitalization and time to extubation), and safety indicators.
Study design RCTs were included in our review.
Exclusion criteria: (1) Methylprednisolone was outside the surgical setting or not used for lung surgery; (2) Non-peer-reviewed publications, certain study designs (such as retrospective observational studies, case reports, case series, review articles, letters to the editor), and nonhuman trials.
The title and abstract were independently screened by two investigators (X.Y. and L.N.A.). Then, two investigators evaluated each study by reviewing the full text for the selection of eligible studies based on the inclusion criteria. Any disagreement was resolved by a third arbitrator (Y.F.R.).
Data Extraction
Data were extracted independently by two investigators with a standardized data extraction form (W.B.H. and J.D.). The following information and data were extracted: the name of the first author, year of publication, surgical procedures, anesthesia methods, sample size, intervention details, comparison group, time of intervention, number of interventions, and outcomes. When the results were expressed as figures, we extracted the required data using the software GetData Graph Digitizer version 2.26 (http://getdata-graph-digitizer.com/). Corresponding authors were consulted to obtain missing information. All data were presented in an electronic extraction form (Microsoft Excel 2019, Microsoft, Redmond, USA).
Assessment of Methodological Quality and Risk of Bias
The modified Cochrane Collaboration tool (ROB 2) was used to assess the methodological quality of the included studies [23]. Each study was evaluated for the risk of bias from six aspects: randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, selection of the reported result, and overall bias. ROB 2 evaluated risk with three ranks: “low,” “some concerns,” and “high.” The detailed score of each study is available in the supplementary material (eTable 1).
We also assessed the certainty of the summary finding with the Grades of Recommendation, Assessment, Development and Evaluation Profiler software (GRADEpro, version 3.6.1, McMaster University, Hamilton, ON, Canada). The strength of evidence was rated as high (⊕ ⊕ ⊕ ⊕), moderate (⊕ ⊕ ⊕\(\bigcirc\)), low (⊕ ⊕\(\bigcirc \bigcirc\)) or very low ( ⊕\(\bigcirc \bigcirc \bigcirc\)) [24, 25].
All quality assessments of RCTs were assessed by two investigators independently (Y.H. and H.J.). Disagreements were resolved by an independent reviewer (X.Y.).
Primary and Secondary Outcomes
The coprimary outcomes included (1) rest pain scores (cm) at 24 h postoperatively; (2) dynamic pain scores (cm) at 24 h postoperatively; and (3) rescue analgesic consumption: intravenous morphine equivalent consumption (mg) within 24 h postoperatively. This time point was selected because it was reported frequently and is representative of acute postoperative pain [11, 21].
The secondary outcomes included (1) rest pain scores (cm) at 48 h postoperatively; (2) dynamic pain scores (cm) at 48 h postoperatively; (3) rescue analgesic consumption: intravenous morphine equivalent consumption (mg) within 48 h postoperatively; (4) inflammatory indicators including interleukin-6 (IL-6) (pg/mL), interleukin-10 (IL-10) (pg/mL), tumor necrosis factor-α (TNF-α) (pg/mL), and C-reactive protein (CRP) (mg/mL); (5) duration of hospitalization (days); and (6) time to extubation (days).
The safety indicators included (1) pulmonary complications (such as pulmonary infection, atelectasis, acute lung injury, and respiratory failure); (2) surgical-related complications (such as wound infection, bleeding, and cognitive dysfunction); (3) methylprednisolone-related side effects such as elevated blood glucose (mg/dl); and (4) opioid-related side effects (such as nausea and vomiting).
Analysis of Outcome Data
All pain scores were standardized by transformation to a straight line of 0–10 cm (0, no pain; 10, worst pain). Furthermore, cumulative analgesic consumption was converted to intravenous morphine equivalents (intravenous butorphanol 7 mg = intravenous morphine 1 mg) [26]. For outcomes measured at different time points, we used the pooled estimates of the weighted mean difference (WMD) for our interval of interest (Fig. 3).
Statistical Analysis
All continuous variables were presented as the mean ± standard deviation (SD). If the included studies provided only the median, range, and/or interquartile range, the sample mean and SD were obtained using methods reported by Luo et al. and Wan et al. [27, 28]. According to the Cochrane Collaboration tool, the mean and confidence interval (CI) were converted to the mean and SD [29]. All categorical data were summarized using proportions. Where necessary, continuous data were translated into categorical variables. We converted the nausea and vomiting scores from the trial by Shi et al. into dichotomous variable of “yes” and “no” according to the score interval [21].
Meta-analysis
We analyzed all data using Review Manager software (version 5.4, The Cochrane Collaboration) and STATA statistical software (version 16, STATA Corp). The analysis of continuous variables used inverse variance weighted estimators, while the Mantel–Haenszel method was employed for dichotomous variables [30]. For our coprimary outcomes, the weighted mean difference (WMD) with a 99% CI was calculated to account for multiple comparisons [31, 32]. The statistical significance level was set at two-sided p < 0.01 to reduce type I error.
The risk ratio (RR) or the WMD with 95% CI (two-sided p < 0.05) were used for dichotomous and continuous secondary outcomes.
A meta-analysis was conducted if two or more studies reported the same measurable parameters. Otherwise, qualitative analysis was performed for all prespecified primary, secondary, and safety outcomes.
Interpretation of Outcome Results
The minimal clinically important difference (MCID) was set to determine the clinical significance of the outcomes. For the pain scores, an absolute change of 1.1 cm at a single time point was considered the MCID [32]. For the intravenous morphine equivalent consumption, an MCID threshold of 10 mg was defined as clinically significant [33, 34].
Heterogeneity, Subgroup, and Sensitivity Analyses
Heterogeneity was evaluated by the Chi-squared (Chi2) test with significance set at a p-value of 0.10, and the I-square (I2) statistic was used to quantify heterogeneity. According to the Cochrane Handbook for Systematic Reviews of Interventions, the following values of I2 were used for the interpretation of heterogeneity levels: I2 of 0–40%: might not be important; I2 of 30–60%: may represent moderate heterogeneity; I2 of 50–90%: may represent substantial heterogeneity; I2 of 75–100%: considerable heterogeneity [35, 36]. Predefined covariates were as follows: (1) intervention time (before surgery versus after surgery); (2) the number of interventions (single versus multiple); (3) surgical approach (open thoracotomy versus video-assisted thoracoscopic surgery); and (4) age (> 18 years versus ≤ 18 years). Of note, prespecified subgroup analyses were not performed due to the limited number of studies. In addition, by excluding trials of non-preoperative interventions, multiple administration, non-thoracoscopic surgery, and patients aged 0–18 years, a posthoc sensitivity analysis was conducted only for outcomes where enough data were available to assess the robustness of our findings.
Assessment of Publication Biases
Publication bias in all outcomes was explored by an Egger’s regression test using Stata software V. 16.0 (StataCorp, College Station, USA) [37]. And p < 0.05 was considered suggestive of statistically significant publication bias.
Results
Study selection
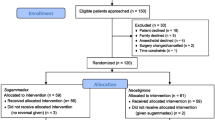
A total of 707 potentially relevant studies were identified by the systematic literature search, of which 89 were duplicate studies. We screened 618 records based on the title and abstract. Among these, 604 records were excluded because of unrelated studies (n = 411), irrelevant comparator (n = 23), and not an RCT (n = 170). After reviewing the full text of eligible articles, one clinical protocol was excluded, and the full text of two studies were not available. Finally, 11 RCTs including 643 participants were selected for our meta-analysis [10,11,12,13,14,15,16,17, 19,20,21]. The flow diagram for the literature search, screening, and eligibility evaluation is shown in Fig. 1.
Characteristics of Included Studies
The general characteristics and results of interest in the included studies are detailed in Table 1. The sample size of the included studies ranged from 21 to 200 patients. Thoracotomy was performed in 4 of 11 included trials [10, 13, 19, 20], while video-assisted thoracoscopic surgery (VATS) was carried out in six studies [11, 12, 14,15,16, 21], and only one study did not report the surgical approach [17]. The procedure was performed under general anesthesia (GA) in five trials [12,13,14, 16, 17], GA plus epidural anesthesia in three trials [10, 19, 20], GA plus paravertebral block in one trial [11], GA plus intercostal nerve block in one trial [21], and one study did not specify anesthesia methods [15]. In addition, 10 RCTs enrolled patients who were adults [10,11,12,13, 15,16,17, 19,20,21], whereas one RCT enrolled children aged 3–18 years [14].
MP was administered intravenously in 10 trials [10,11,12,13,14,15,16,17, 19, 21], and epidural MP infusion was performed in one trial [20]. Furthermore, the time of MP administration was before surgery in six trials [10,11,12,13, 19, 21], during surgery in three trials [14, 16, 17], and both before and after surgery in two trials [15, 20]. The total volume of MP ranged from 40 to 120 mg in three trials, and the dose of MP ranged from 1 to 30 mg/kg in eight trials. All included RCTs used placebo saline as a control.
Risk of Bias
Regarding the risk assessment for the 11 included RCTs, 4 studies were rated as having a low risk of bias [11, 12, 14, 21], while 7 studies were deemed unclear risk [10, 13, 15,16,17, 19, 20]. The complete assessment for separate studies is provided in the supplementary material (eTable 1). The risk of bias summary and risk of bias graph can also be found in the supplementary materials (Fig. 2 and eFig. 1).
Coprimary Outcomes
Rest Pain Scores at 24 h Postoperatively
Two studies (n = 248) utilized an 11-point scale, including a numeric rating scale (NRS) or VAS, to assess rest pain scores at 24 h postoperatively [12, 21]. The results indicated that the MP group made a significant difference in this outcome compared with the placebo group (WMD –0.50 cm; 99% CI −0.82 to −0.18; p < 0.0001; I2 = 0%) (Table 2 and Fig. 3). However, the difference was minimal and did not reach the MCID threshold (1.1 cm). The quality of evidence was “high” (eTable 2). No publication bias was detected (p = 1.00).
A Band plot for weighted mean difference (WMD) of the change in rest pain scores from 24 to 48 h postoperatively between methylprednisolone versus control. B Band plot for weighted mean difference (WMD) of the change in dynamic pain scores from 24 to 48 h postoperatively between methylprednisolone versus control; Pooled estimates of the WMD for each time point are represented by a dark line and 95% confidence intervals are represented by the surrounding shaded region
Dynamic Pain Scores at 24 h Postoperatively
This outcome was also evaluated by two studies (n = 248) with an 11-point NRS or VAS [12, 21]. The dynamic pain scores at 24 h postoperatively were significantly reduced in the MP group compared with the placebo group (WMD –0.55 cm; 99% CI –0.97 to –0.13; p = 0.0007; I2 = 0%) (Table 2 and Fig. 3). Nevertheless, the improvement was not clinically meaningful based on MCID. The quality of evidence for this outcome was rated as “high” (eTable 2). Egger’s test showed that no publication bias existed (p = 0.71).
Rescue Analgesic Consumption Within 24 h Postoperatively
Three studies (n = 195) reported rescue analgesic consumption within 24 h postoperatively [11, 12, 20]. There was a statistically significant but clinically nonsignificant difference in this outcome between the MP and the placebo groups (WMD –2.65 mg; 99% CI −4.63 to −0.66; p = 0.0006; I2 = 0%) (Table 2). Sensitivity analysis revealed the favorable robustness of the result by excluding trials of non-preoperative interventions, multiple administration, and non-thoracoscopic surgery (eTable 3) [20]. The quality of the results was assessed as “moderate” owing to the small sample size (eTable 2). The evaluation by Egger’s test indicated no publication bias for this outcome (p = 0.82).
Secondary Outcomes
Rest Pain Scores at 48 h Postoperatively
Rest pain scores at 48 h postoperatively were evaluated on an 11-point scale (VAS or NRS) by two studies (n = 262) [11, 21]. The pooled result showed no difference in this outcome between the MP and placebo groups (p = 0.07) (Table 2 and Fig. 3). According to the GRADE system, the quality of evidence was “high” (eTable 2). We did not find publication bias for this outcome (p = 0.60).
Dynamic Pain Scores at 48 h Postoperatively
Two studies (n = 262) examined dynamic pain scores at 48 h postoperatively by an 11-point scale (VAS or NRS) [11, 21]. Administration of MP in lung surgery did not significantly relieve dynamic pain at 48 h postoperatively compared with the placebo group (p = 0.05) (Table 2 and Fig. 3). The quality of evidence was “high” (eTable 2). The result did not exhibit publication bias (p = 0.41).
Rescue of Analgesic Consumption Within 48 h Postoperatively
Two studies (n = 120) reported this outcome [11, 20]. The combined results showed that MP did not significantly reduce rescue analgesic consumption within 48 h postoperatively compared with the placebo group (p = 0.59) (Table 2). The quality of evidence was “moderate” because of the small sample size (eTable 2). Egger’s test for publication bias was nonsignificant (p = 0.54).
Inflammatory Indicators
IL-6 at 6 h
The serum IL-6 level at 6 h postoperatively was reported in two studies (n = 86) [14, 17]. There was a significant difference in the outcome between the MP and placebo groups (WMD –20.49 pg/mL; 95% CI −29.94 to −11.04; p < 0.0001; I2 = 0%) (Table 2). The evidence exhibited a “low” quality owing to allocation concealment, performance bias, detection bias, and small sample size (eTable 2). There was no publication bias (p = 0.46).
IL-6 at 24 h
Three studies (n = 254) detected IL-6 levels at 24 h postoperatively [10, 17, 21]. The results showed that MP did not decrease this indicator compared with the placebo group (p = 0.13) (Table 2). Sensitivity analysis excluding trials of non-preoperative interventions showed a significant difference for this outcome in the MP group (WMD −5.62 pg/mL; 95% CI −10.98 to −0.26; p = 0.04; I2 = 0%) (eTable 3) [17]. The quality of this evidence was “low” because of sequence generation, allocation concealment, performance bias, detection bias, and high heterogeneity (eTable 2). No publication bias was found for the result (p = 0.55).
IL-10 at 6 h
Two studies (n = 86) measured serum IL-10 levels at 6 h postoperatively [14, 17]. The MP group was not different from the placebo group for this indicator (p = 0.11) (Table 2). The quality of evidence was rated as “very low” because of allocation concealment, performance bias, detection bias, small sample size, and high heterogeneity (eTable 2). The outcome showed no publication bias (p = 0.23).
TNF-α at 6 h
This outcome was reported by two studies (n = 86) [14, 17]. The serum TNF-α level at 6 h postoperatively was not different between the MP group and the placebo group after lung surgery (p = 0.28) (Table 2). The quality of evidence was evaluated as “very low” owing to allocation concealment, performance bias, detection bias, high heterogeneity, and publication bias (eTable 2). Egger’s test showed publication bias (p < 0.001).
CRP at 24 h
Two studies (n = 373) detected CRP at 24 h [15, 21]. There was no significant difference in the level of CRP at 24 h postoperatively between the MP and placebo groups (p = 0.06) (Table 2). The quality of this evidence was “low” for sequence generation, allocation concealment, performance bias, detection bias, and high heterogeneity (eTable 2). There was no publication bias (p = 0.19).
Duration of hospitalization
Duration of hospitalization was reported in five studies (n = 565) [10,11,12, 15, 21]. Administration of MP did not significantly reduce the length of hospital stay compared with the placebo group (p = 0.38) (Table 2). Posthoc sensitivity analyses revealed that the result was credible and stable after excluding trials of non-preoperative interventions, multiple administration, and non-thoracoscopic surgery (eTable 3) [10, 15]. The quality of evidence was assessed as “low” using the GRADE system because of sequence generation, allocation concealment, performance bias, detection bias, and high heterogeneity (eTable 2). The publication bias was not significant (p = 0.30).
Time to Extubation
Two studies (n = 296) reported the time to extubation results [11, 15]. The MP group could not decrease the time to extubation when compared with the placebo group (p = 0.16) (Table 2). The quality of evidence was “moderate” owing to sequence generation, allocation concealment, performance bias, and detection bias (eTable 2). Analysis showed no significant publication bias (p = 0.78).
Safety outcomes
Pulmonary complications
Pulmonary infection
Four studies (n = 348) reported the incidence of pulmonary infection [13, 15,16,17]. The rate of this outcome was similar between the MP and placebo groups (p = 1.00) (Table 2). No significant difference was observed in posthoc sensitivity analysis excluding trials of multiple administration and non-thoracoscopic surgery (eTable 3) [13, 15, 17]. The evidence quality of the pulmonary infection rate was assessed as “moderate” for uncertainty about the magnitude of the effect, sequence generation, allocation concealment, and performance bias (eTable 2). Publication bias was not found by Egger’s test (p = 0.81).
Atelectasis
Atelectasis was reported by four RCTs (n = 346) [14,15,16,17]. Our combined result demonstrated that there was no difference in this indicator between the MP and placebo groups (p = 0.40) (Table 2). Sensitivity analysis did not significantly change the pooled effects by excluding trials of multiple administration, non-thoracoscopic surgery, and patients aged 0–18 years (eTable 3) [14, 15, 17]. The evidence quality of this outcome was rated as “moderate” because of uncertainty about the magnitude of the effect, sequence generation, allocation concealment, and performance bias (eTable 2). No publication bias was detected (p = 0.70).
Acute Lung Injury
The occurrence of acute lung injury was reported by two studies (n = 260) [15, 17]. The acute lung injury rate in the placebo group was 0.06% (8/130), whereas it was 0.008% (1/130) in the MP group (Table 2). There was a slight decrease in the risk of acute lung injury for the MP group compared with the placebo group (RR 0.18; 95% CI 0.03–0.98; p = 0.05; I2 = 7%) (Table 2). The quality of evidence was “moderate” owing to sequence generation, allocation concealment, performance bias, and detection bias (eTable 2). There was no publication bias (p = 0.32).
Respiratory Failure
Respiratory failure was reported by three studies (n = 247) [10, 14, 15]. There was no difference in the incidence of respiratory failure after lung surgery between the two groups (p = 0.89) (Table 2). The posthoc sensitivity analysis implied the main result to be robust when trials of multiple administration, non-thoracoscopic surgery, and patients aged 0–18 years were excluded (eTable 3) [10, 14, 15]. The quality of the results was “moderate” because of sequence generation, allocation concealment, performance bias, and detection bias (eTable 2). Egger’s test showed insignificant publication bias (p = 0.56).
Surgical-Related Complications
Wound Infection
Three studies (n = 344) reported this outcome [11, 12, 21]. The results of two trials indicated no wound infection [12, 21]; thus, quantitative analysis was not performed for this indicator. Another trial reported two cases in 49 patients for the MP group and two cases in 47 patients for the placebo group.
Bleeding
Two studies (n = 96) reported on bleeding [10, 12]. The incidence of bleeding was 4% in both the MP and placebo groups. The difference in bleeding was not significant between the two groups (p = 0.98) (Table 2). The quality assessment showed that the outcome was of “low” quality overall owing to uncertainty about magnitude of effect, sequence generation, allocation concealment, performance bias, and small sample size (eTable 2). No publication bias was assessed (p = 0.34).
Cognitive Dysfunction
This outcome was reported by two studies (n = 156) [11, 16]. The cognitive dysfunction rate (8.86%) in the MP group was significantly less than the occurrence (22.07%) in the placebo group (RR 0.43; 95% CI 0.21–0.88; p = 0.02; I2 = 0%) (Table 2). The level of evidence was “moderate” for sequence generation, allocation concealment, and performance bias (eTable 2). No publication bias existed in the results (p = 0.57).
Methylprednisolone-Related Side Effects
Elevated Blood Glucose at 24 h
Two studies (n = 233) reported elevated blood glucose at 24 h [16, 21]. The MP likely results in a certain increase in blood glucose at 24 h compared with the placebo group after lung surgery (WMD 0.38 mg/dl; 95% CI 0.13–0.64; p = 0.003; I2 = 0%) (Table 2). The quality of the evidence was assessed as “moderate” because of allocation concealment, performance bias, and detection bias (eTable 2). Egger’s test did not suggest publication bias (p = 0.86).
Opioid-Related Side Effects
Nausea and Vomiting
Nausea and vomiting was reported by three studies (n = 272) [12, 20, 21]. The results showed a certain decrease in the MP group versus the placebo group for nausea and vomiting (RR 0.30; 95% CI 0.18–0.50; p < 0.0001; I2 = 13%) (Table 2). Sensitivity analysis obtained robust results when trials of non-preoperative interventions, multiple administration, and non-thoracoscopic surgery were included (eTable 3). The quality of the evidence was “high” (eTable 2). The pooled analysis showed no evidence of publication bias (p = 0.17).
Discussion
We conducted a comprehensive systematic review and meta-analysis, which synthesized new quantitative and qualitative evidence on the clinical role of MP in lung surgery. The primary analysis demonstrated that the administration of MP relieved postoperative pain severity and analgesic consumption in the first 24 h after lung surgery. However, the observed discrepancies in mean values were tiny and not clinically important. Meanwhile, the absence of a significant difference in secondary pain-related outcomes measured at 48 h also supported the marginal effect of the MP. Moreover, the results showed no significant difference in the length of hospital stay or time to extubation between the two groups. Posthoc sensitivity analyses revealed that most predefined covariates (including the number of interventions, surgical approach, and age) showed no influence on the results. In general, our findings undermine the rationality of MP application in lung surgery analgesia.
Notably, although our research yielded similar results as those described by previous studies [11, 12, 21], we came to a completely different conclusion, which was interpreted based on the clinical context. For instance, a difference of 0.55 cm in dynamic pain scores at 24 h between the MP and placebo groups was insufficient to reach the minimum clinical threshold of 1.1 cm. Beyond this point, more robust statistical methods could be another reason for the different interpretations. To ensure sufficient statistical power to detect true differences between both groups, adjusted p-values (two‐tailed p < 0.01) were applied to the primary outcomes after multiple comparisons.
It is surprising that the use of MP reduced serum levels of IL-6 at 6 h without any significant decrease in TNF-α or CRP, or an increase in IL-10 at other time points. As a potent glucocorticoid, MP is widely known for its antiinflammatory effects. Although MP has been proven to reduce proinflammatory mediators (such as TNF-α, IL-6, and CRP) in surgeries such as lung surgery [14, 15, 17, 21] and cardiac surgery with cardiopulmonary bypass (CPB) [39], a review conducted by Kathrine et al. resulted in findings similar to ours that showed little effect of MP administration on proinflammatory cytokines in lung surgery [8]. One possible reason is that serum level alterations of inflammation-related factors vary with the type of operation performed and the infected condition [10, 40]. In parallel, the few cases of infection reported by the included RCTs after lung surgery in our meta-analysis may be a plausible source of discrepancy between our findings and those of other studies. In addition, sensitivity analysis showed that preoperative administration of the MP was a source of heterogeneity and significantly attenuated the systemic IL-6 response to lung surgery at 24 h. Apparently, the timing of MP administration is crucial for perioperative management. The onset of effect for the MP occurred within approximately 1 h, and preoperative administration could provide full benefit and antiinflammatory effects when the inflammatory cascade is activated after the surgical incision [41]. Moreover, the decreased incidence rate of acute lung injury and cognitive dysfunction in the MP group also offered supporting evidence for the antiinflammatory effects [42, 43].
Of note, although this meta-analysis revealed a clinically insignificant effect of the MP in acute postoperative pain after lung surgery, the potential advantages of the administration of MP in postoperative complications attracted our attention. Despite the fact that MP may slightly increase postoperative serum glucose levels, its relief of acute lung injury, cognitive impairment, and nausea and vomiting makes it attractive for the prevention of complications after pulmonary surgery. Additionally, the strength of the recommendation for glucocorticoids was “strong” in lung surgery according to guidelines for enhanced recovery after lung surgery [38]. Therefore, considering the potential benefits and negligible risks, we believe that future multicenter studies with larger sample sizes should focus on exploring the reduction in postoperative complications when MP is administered in lung surgery.
There are several noticeable strengths in our systematic review and meta-analysis. First, as far as we are aware, this is the first integrated evidence for the efficacy and safety of MP in lung surgery, and this review presents several important findings. Second, our study was prospectively registered in PROSPERO and adhered to PRISMA guidelines to guarantee systematicness and integrity. Third, a comprehensive literature search without language restrictions was performed to minimize potential selection bias. Fourth, rigorous evaluation of literature quality and grade of evidence were performed to ensure the authenticity of the data and objectivity of the results based on the modified Cochrane Collaboration RoB and GRADE tool. Fifth, our findings have direct clinical applicability because they were interpreted in a clinical context with MCID. Moreover, correcting p-values in primary outcomes with conservative multiple comparisons results in a decreased risk of false positives. Finally, low levels of heterogeneity in primary outcomes increase the external validity of our study.
Our study also has some limitations. First, the doses of MP among the included studies were not consistent, which restricted the exploration of dose–response effects. Qualitative analysis showed that only the study reported by Shi et al. demonstrated that high-dose MP (120 mg) were superior to those of low-dose methylprednisolone (40 mg) on postoperative pain relief in lung surgery [21]. Second, we failed to perform subgroup analysis owing to the insufficient number of RCTs; nonetheless, posthoc sensitivity analysis explained partial heterogeneity. Third, there was a high heterogeneity among studies that downgraded the quality of evidence. The small number of included studies for secondary outcomes also limited the exploration of heterogeneity. Therefore, such unexplained heterogeneity may lead to potential unreliability of the results. Fourth, although Egger’s regression test was conducted to explore bias, the validity of publication bias may be restrained by the number of included studies. Finally, the lack of data restricted the assessment of long-term effects such as chronic postoperative pain and quality of life.
Conclusion
Our findings demonstrated that the administration of MP contributed to an insignificant relief in acute postoperative pain for lung surgery within the clinical setting. “Moderate-to-high” quality evidence suggested the lack of clinical difference between the MP and placebo groups in pain improvement within 24 h after lung surgery. However, MP had potential advantages in postoperative complications such as acute lung injury, cognitive impairment, and nausea and vomiting. Thus, future multicenter studies with larger sample sizes should focus on exploring the reduction in postoperative complications when MP is administered in lung surgery.
References
Jin J, Min S, Chen Q, Zhang D. Patient-controlled intravenous analgesia with tramadol and lornoxicam after thoracotomy: A comparison with patient-controlled epidural analgesia. Medicine (Baltimore). 2019;98: e14538.
Prabhakar A, Mancuso KF, Owen CP, et al. Perioperative analgesia outcomes and strategies. Best Pract Res Clin Anaesthesiol. 2014;28:105–15.
Piccioni F, Segat M, Falini S, et al. Enhanced recovery pathways in thoracic surgery from Italian VATS Group: perioperative analgesia protocols. J Thorac Dis. 2018;10:S555–63.
Argoff CE. Recent management advances in acute postoperative pain. Pain Pract. 2014;14:477–87.
Follin SL, Charland SL. Acute pain management: operative or medical procedures and trauma. Ann Pharmacother. 1997;31:1068–76.
Miaskowski C, Crews J, Ready LB, Paul SM, Ginsberg B. Anesthesia-based pain services improve the quality of postoperative pain management. Pain. 1999;80:23–9.
Shafi S, Collinsworth AW, Copeland LA, et al. Association of opioid-related adverse drug events with clinical and cost outcomes among surgical patients in a large integrated health care delivery system. JAMA Surg. 2018;153:757–63.
Holte K, Kehlet H. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg. 2002;195:694–712.
Sauerland S, Nagelschmidt M, Mallmann P, Neugebauer EA. Risks and benefits of preoperative high dose methylprednisolone in surgical patients: a systematic review. Drug Saf. 2000;23:449–61.
Tønnesen E, Wanscher M, Höhndorf K, et al. Effect of methylprednisolone on the cytokine response in patients undergoing lung surgery. Acta Anaesthesiol Scand. 1993;37:410–4.
Bjerregaard LS, Jensen PF, Bigler DR, et al. High-dose methylprednisolone in video-assisted thoracoscopic surgery lobectomy: a randomized controlled trial. Eur J Cardiothorac Surg. 2018;53:209–15.
Li X, Song B, Teng X, et al. Low dose of methylprednisolone for pain and immune function after thoracic surgery. Ann Thorac Surg. 2022;113:1325–32.
Mikawa K, Ikegaki J, Maekawa N, et al. Perioperative effect of methylprednisolone given during lung surgery on plasma concentrations of C3a and C5a. Scand J Thorac Cardiovasc Surg. 1990;24:229–33.
Theroux MC, Fisher AO, Rodriguez ME, et al. Prophylactic methylprednisolone to reduce inflammation and improve outcomes from one lung ventilation in children: a randomized clinical trial. Paediatr Anaesth. 2015;25:587–94.
Ye J, Gu W, Yang S, Wang F, Xiao Y, Zhang X, Luo L, Zhao N, Wu L. Effect of perioperative application of methylprednisolone in reducing postoperative respiratory complications after video-assisted thoracoscopic surgery for lung cancer. China Pract Med. 2018;13(32):1–3.
Nan Zhao XW, Wang L, Zhao S, Duan W, Wang L, Guo P. Effect of methylprednisolone on cognitive dysfunction after thoracoscopic assisted pulmonary lobectomy in elderly patients. J Clin Anesthesiol. 2018;7:14.
Hui Xu SS, Wang D, Chai X, Pan J. Effects of methylprednisolone on lung function and inflammation during one-lung ventilation in patients undergoing pulmonary lobectomy. J Clin Anesthesiol. 2017;33(7):647–65.
Li Y, Zhang Y, Zhou Y, Li Y. The effect of prophylactic methylprednisolone need more evidences on postoperative outcomes. Paediatr Anaesth. 2015;25:649–50.
Bigler D, Jonsson T, Olsen J, Brenøe J, Sander-Jensen K. The effect of preoperative methylprednisolone on pulmonary function and pain after lung operations. J Thorac Cardiovasc Surg. 1996;112:142–5.
Blanloeil Y, Bizouarn P, Le Teurnier Y, et al. Postoperative analgesia by epidural methylprednisolone after posterolateral thoracotomy. Br J Anaesth. 2001;87:635–8.
Shi W, Chen Y, Zhang MQ, Che GW, Yu H. Effects of methylprednisolone on early postoperative pain and recovery in patients undergoing thoracoscopic lung surgery: A randomized controlled trial. J Clin Anesth. 2021;75: 110526.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94.
Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ. 2008;336:1049–51.
Tavakoli M, Corssen G, Caruso FS. Butorphanol and morphine: a double-blind comparison of their parenteral analgesic activity. Anesth Analg. 1976;55:394–401.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Hoboken NJ. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. The Cochrane Collaboration. 2011.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70.
Ye X, Ren YF, Hu YC, et al. Dexamethasone does not provide additional clinical analgesia effect to local wound infiltration: a comprehensive systematic review and meta-analysis. Adv Wound Care (New Rochelle). 2022. https://doi.org/10.1089/wound.2021.0163.
Hussain N, Brull R, Noble J, et al. Statistically significant but clinically unimportant: a systematic review and meta-analysis of the analgesic benefits of erector spinae plane block following breast cancer surgery. Reg Anesth Pain Med. 2021;46:3–12.
Ren Y, Shi W, Chen C, et al. Efficacy of dexmedetomidine as an adjuvant to local wound infiltration anaesthesia in abdominal surgery: a meta-analysis of randomised controlled trials. Int Wound J. 2019;16:1206–13.
https://training.cochrane.org/handbook/current/. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. The Cochrane Collaboration. 2021; Accessed 24 Nov 2021.
Findlay JM, Middleton MR, Tomlinson I. A systematic review and meta-analysis of somatic and germline DNA sequence biomarkers of esophageal cancer survival, therapy response and stage. Ann Oncol. 2015;26:624–44.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2019;55:91–115.
Danielson M, Reinsfelt B, Westerlind A, et al. Effects of methylprednisolone on blood-brain barrier and cerebral inflammation in cardiac surgery-a randomized trial. J Neuroinflammation. 2018;15:283.
Sugasawa Y, Yamaguchi K, Kumakura S, et al. The effect of one-lung ventilation upon pulmonary inflammatory responses during lung resection. J Anesth. 2011;25:170–7.
Al-Habet SM, Rogers HJ. Methylprednisolone pharmacokinetics after intravenous and oral administration. Br J Clin Pharmacol. 1989;27:285–90.
Safavynia SA, Goldstein PA. The role of neuroinflammation in postoperative cognitive dysfunction: moving from hypothesis to treatment. Front Psychiatry. 2018;9:752.
Qiu Y, Tang Z. Dexmedetomidine attenuates LPS-induced acute lung injury in rats by activating the Nrf2/ARE pathway. J Healthc Eng. 2022;2022:4185195.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Xi Fu: Conceptualization, Formal Analysis, Writing-Original Draft, Software, Visualization, Validation. Xin Ye: Conceptualization, Writing-Review and Editing, Visualization, Validation, Critical revision of the article. Li-Na An: Investigation, Data Curation, Software, Validation. Hua Jiang: Investigation, Data Curation, Validation. Wen-Bo Huang: Writing-Review and Editing, Validation. Ya Huang: Writing-Review and Editing, Validation. Jing Dong: Investigation, Data Curation, Software, Writing-Review and Editing, Validation. Yi-Feng Ren: Conceptualization, Writing-Review and Editing, Visualization, Validation, Critical revision of the article.
Disclosures
All authors (Xi Fu, Xin Ye, Li-Na An, Hua Jiang, Wen-Bo Huang, Ya Huang, Jing Dong, Yi-Feng Ren) declare no conflicts of interest related to this work.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fu, X., Ye, X., An, LN. et al. Efficacy and Safety of Methylprednisolone for Lung Surgery: a Systematic Review and Meta-analysis of Randomized Controlled Trials. Pain Ther 12, 165–186 (2023). https://doi.org/10.1007/s40122-022-00443-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00443-4