Abstract
Introduction
Currently available treatments for chronic lower back pain (CLBP) do not adequately address both nociceptive and neuropathic components of pain. We evaluated efficacy and safety of fixed-dose combination (FDC) of low-dose pregabalin prolonged release 75 mg–etoricoxib 60 mg to address both pain components.
Methods
This randomized phase 3 trial conducted at 12 centres across India evaluated efficacy (based on mean change in numeric rating scale [NRS], Roland–Morris disability questionnaire [RDQ], visual analogue scale [VAS], patient global impression of improvement [PGI-I], clinical global impression of improvement [CGI-I] and rescue medication consumption) and safety of FDC in comparison to etoricoxib alone in adult patients with CLBP. Treatment duration was 8 weeks.
Results
Of the 371 patients screened, 319 were randomized and considered for efficacy and safety analysis. Both treatment groups had no significant difference in terms of demography and baseline disease characteristics. Significantly better outcomes with FDC compared to etoricoxib were observed at week 4 onwards. At week 8, both groups showed significant reduction in mean NRS score from baseline (− 4.00 ± 1.65 in FDC; − 2.92 ± 1.59 in etoricoxib) with mean NRS score being significantly less in the FDC group compared to etoricoxib group (3.26 ± 1.56 vs 4.31 ± 1.56; p < 0.0001). The FDC was more effective than etoricoxib in terms of significantly greater reduction in RDQ score (− 9.28 ± 4.48 vs − 6.78 ± 4.34; p < 0.0001) and VAS score (− 37.66 ± 18.7 vs − 28.50 ± 16.31; p < 0.0001) at week 8. The FDC was also better in terms of significantly more patients reporting their condition as ‘very much better’ (36.9% vs 5.0%; p < 0.0001) and clinicians reporting patient’s condition as ‘very much improved’ (36.3% vs 5.7%; p < 0.0001). Overall, study medications were well tolerated.
Conclusion
FDC of pregabalin and etoricoxib provided significant benefits in reducing pain and improving functional status compared with etoricoxib alone in patients with CLBP. Pregabalin prolonged release–etoricoxib FDC could be one of the treatment options for early and sustained pain relief and improvement in quality-of-life in treating CLBP as it addresses both neuropathic and nociceptive components of pain.
Trial Registration
CTRI/2018/10/015886.
Plain Language Summary
Low back pain is one of the most common causes of loss of productivity worldwide. About 60% of Indians suffer from low back pain at some point. Low back pain that persists for more than 3 months is classified as chronic low back pain which mostly includes both nociceptive and neuropathic components. Monotherapies, if prescribed, are not completely effective, as they generally only target either nociceptive or neuropathic components of pain. Multiple drugs are usually needed at multiple times a day, at higher doses for optimal effectiveness, and in most cases they have significant side effects if taken over prolonged periods and also add to the pill burden. To minimize treatment-associated adverse effects, and to increase treatment compliance, while addressing both the components of pain, we developed a fixed-dose combination of low-dose pregabalin prolonged release and etoricoxib. A phase 3 trial was designed to assess the efficacy and safety of the fixed-dose combination in comparison with etoricoxib alone in treating chronic low back pain. The combination demonstrated statistically and clinically significant improvement in patient-reported outcomes—pain, functionality and quality of life—as early as 4 weeks after starting the medication. No severe or serious adverse effects were reported. Thus, the combination of low-dose pregabalin prolonged release and etoricoxib could provide an option for optimal management of chronic low back pain. This would provide multiple benefits, such as addressing both nociceptive and neuropathic components of chronic low back pain, reducing drug-related adverse effects because of low dose, reducing pill burden and thereby increasing drug compliance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
With an annual prevalence of 15–45%, chronic low back pain (CLBP) is one of the leading causes of “years lived with disability”, “disability-adjusted life years” and loss of productivity globally. Optimal CLBP management requires multiple drugs for addressing both the nociceptive and neuropathic components of pain. Currently available drugs do not address both these components adequately. |
The purpose of this study was to assess a fixed dose combination (FDC) of pregabalin and etoricoxib that could adequately address both components of CLBP at low doses, thereby minimizing the side effects of higher doses of multiple drugs. |
What might be learnt from this study? |
This randomized, multicentre, phase 3 study has helped in understanding the potential clinical benefits of a fixed-dose combination of low-dose pregabalin with etoricoxib compared to etoricoxib alone. |
What were the study outcomes? |
The study showed that FDC of low-dose pregabalin prolonged release and etoricoxib was significantly more effective compared to etoricoxib alone in decreasing pain, increasing functionality and improving the overall quality of life (QoL) of patients with CLBP, without any serious adverse events. The changes observed within the two groups for multiple efficacy parameters measured were clinically and statistically significant and suggest superiority of the FDC over etoricoxib alone (p < 0.0001). |
How might this affect future treatment? |
The results of this study provide evidence for a new therapeutic option in the form of FDC of low-dose of pregabalin with etoricoxib for the optimal management of CLBP, especially when there is a need to address both neuropathic and nociceptive pain components. |
Introduction
Low back pain (LBP) is one of the most common major health problems in industrialized countries, and is ranked among the top ten conditions that account for the highest number of disability-adjusted life-years worldwide [1]. It is considered to be the most common cause of years lived with disability and the sixth leading cause of disability-adjusted life-years worldwide [2]. Approximately 60% of the Indian population suffer from LBP at some time during their lifespan [3]. The global years lived with disability for LBP were 42.5 million in 1990, and increased 52.7% to 64.9 million in 2017 [4]. LBP is generally associated with comorbid conditions such as depression, anxiety, and sleep disturbances [2].
Factors associated with increased prevalence of LBP include low socioeconomic status, physical factors such as lifting heavy loads, prolonged static posture and psychosocial factors such as anxiety, depression, mental stress, work–life balance, job dissatisfaction and obesity.
It has been reported that on an average, only 33% of patients seen in primary care settings recover from LBP in 3 months. LBP is considered chronic if it persists for over 3 months. Approximately 65% of patients seen in primary care settings experience pain even after 12 months. Recurring work absenteeism is due to chronic low back pain (CLBP) in 33% of patients [5]. The low rate of recovery could be attributed to the multiplicity of underlying causes of CLBP in an individual patient.
CLBP is a complex, heterogeneous condition, in which both nociceptive and neuropathic pain mechanisms may be involved. The nociceptive component of CLBP is pain arising from the vertebral column or its adnexa, evoked by noxious stimulation of structures in the lumbar spine, or from the deep soft tissue of the back, i.e. the muscles and thoracolumbar fascia. Moreover, in addition to back pain, noxious stimulation of parts of the lumbar spine can cause referred pain as well. The neuropathic component of CLBP may be caused by lesions of nociceptive sprouts within a degenerated disc (local neuropathic pain), by mechanical compression of the nerve root (mechanical neuropathic root pain), or by inflammatory mediators caused by degenerative discs, which causes inflammation and damage to the nerve roots [6]. Analysis of a US claims database found that 90% of patients with CLBP have a neuropathic component [2]. Neuropathic pain is generally associated with more severe pain symptoms. It is the result of multiple pathways at the peripheral, spinal, and supraspinal levels that trigger pain conduction pathway changes.
Because CLBP is often attributable to a combination of nociceptive and neuropathic pain elements, it is essential to identify subgroups within the heterogeneous CLBP population for long-term and effective pain management [7]. Current treatment options include pharmacotherapy, physical therapy and multidisciplinary approaches, which do not adequately distinguish between the nociceptive and neuropathic components of CLBP that may coexist in an individual patient.
On the basis of current evidence, combining agents with different mechanisms of action to target both nociceptive and neuropathic components of CLBP pathophysiology in a fixed-dose combination (FDC) represents a rational approach in pain management and may translate into improved patient outcomes with lower pill burden and better patient compliance.
Pregabalin is a specific ligand of the alpha2-delta protein auxiliary subunit of the voltage-gated calcium channel that is expressed at presynaptic neuron ends in the brain and spinal cord. Pregabalin binding modulates hyperexcited neurons, reducing calcium influx into presynaptic terminals, thus alleviating neuropathic pain. Apart from the analgesic function, this mechanism is also responsible for its anxiolytic and anticonvulsant properties. It is reported that low dose of pregabalin (50–75 mg/day) is prescribed by Indian clinicians to maintain a balance between efficacy and minimal side effects [8].
Etoricoxib, is a highly selective cyclooxygenase-2 (COX-2) inhibitor with anti-inflammatory, analgesic, and antipyretic properties, recommended in the relief of acute and chronic pain. It has approximately 106-fold higher selectivity for COX-2 inhibition over COX-1, reducing the generation of prostaglandins (PGs) from arachidonic acid.
The development of FDCs is becoming more common either to improve compliance or to get benefit from the added effects of two or more active drugs given together. They are being used in the treatment of a wide range of conditions and are particularly useful in the management of chronic conditions [9]. The FDCs are justified when they demonstrate clear benefits in terms of (a) potentiating the therapeutic efficacy, (b) reducing the incidence of adverse effect of drugs, (c) having pharmacokinetic advantage, (d) better compliance by reducing the pill burden, (e) reducing dose of individual drugs, (f) decreasing development of resistance and (g) cheaper than the individual drugs because of reduced costs from packaging to distribution [10].
The FDC used in this study was formulated on the basis of the effectiveness and safety profiles of pregabalin and etoricoxib to enhance the effects of each at lower doses. The FDC formulation used in the study (Fig. 1) is a bilayer tablet of a combination of low-dose pregabalin prolonged release 75 mg with etoricoxib 60 mg. The bottom etoricoxib layer disperses upon immediate contact with gastric fluid and is completely released within 20 min, allowing immediate relief. The top layer contains a hydrophilic polymer matrix that, upon contact with gastric fluid, swells to form a gel layer from which pregabalin continues to be released over a period of 8 h (Fig. 2). The formulation design aims to improve adherence to medication by using an FDC instead of multiple drugs and a prolonged release mechanism to reduce dose frequency, resulting in reduced pill burden for better therapeutic outcomes. We hypothesized that the prolonged release of pregabalin will address the neuropathic component of CLBP, whereas etoricoxib will combat the nociceptive component of CLBP, thereby providing better relief to patients with CLBP than any existing monotherapy.
This phase 3 clinical study was conducted to evaluate the efficacy and safety of a fixed-dose combination of low-dose pregabalin 75 mg prolonged release plus etoricoxib 60 mg in comparison with etoricoxib 60 mg alone for patients with CLBP.
Methods
Trial Design
This was a randomized, multicentre, open-label, comparative, phase 3 clinical trial conducted at 12 sites in India in patients with CLBP, to evaluate the efficacy and safety of an FDC of pregabalin prolonged release (75 mg) and etoricoxib (60 mg) in comparison to etoricoxib (60 mg) alone.
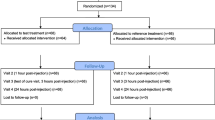
The study was scheduled to have four visits: visit 1, screening visit (day − 14 to day − 1); visit 2, enrolment visit (day 0); visit 3, follow-up visit (day 28 ± 4); visit 4, end of study visit (day 56 ± 4). All the efficacy and safety assessments were conducted as per the schedule of assessments. The trial design is illustrated in Fig. 3.
The clinical trial was registered with the clinical trials registry of India (CTRI; CTRI/2018/10/015886).
The trial was initiated after obtaining regulatory and ethics committee approval and was conducted in accordance with the ethical principles of the Declaration of Helsinki, approved study protocol and applicable regulatory requirements. All patients signed informed consent prior to any study-related procedure being performed.
Study Participants
Patients of either sex between 18 and 65 years of age suffering with CLBP and willing to comply with study procedures were included after obtaining written informed consent.
Male and non-pregnant, non-lactating female patients aged between 18 and 65 years (both inclusive) suffering from CLBP (symptoms duration at least 3 months) and having at least one of the following five features on the side corresponding to leg pain were considered eligible for participation in the study: (a) sharp and shooting pain below the knee; (b) pain evoked by straight leg raising to 60° or less; (c) decreased or absent ankle reflex; (d) weakness of muscles below the knee; (e) sensory loss in L5/S1 distribution, with a pain score of at least 4 on the numeric rating scale (NRS), without any critical illness or medical conditions .
A complete list of inclusion and exclusion criteria is given in the supplementary material.
Interventions
Enrolled patients were assigned randomly in a 1:1 ratio to receive either the FDC of low-dose pregabalin prolonged release 75 mg and etoricoxib 60 mg tablets (Emaxgalin, manufactured by Sun Pharma Laboratories Limited, Mumbai, India) (test) or etoricoxib 60 mg tablets (comparator) once a day with or without food. Randomization schedule was generated centre-wise by the sponsor using SAS Proc Plan (version 9.1.3 or higher). Permutated block size with undisclosed seed number was considered to maintain equal numbers of patients across centres. The randomization number was unique to each patient.
In case of intolerable CLBP, paracetamol 500 mg every 6–8 h was allowed to be used as rescue medication with total daily dose not exceeding 2 g.
Sample Size
The primary efficacy endpoint was used as the basis for sample size estimation. The sample size of 108 patients per group was required to detect a mean difference of 5 mm at 80% power and significance level set at 5%. Although the visual analogue scale (VAS) was used for estimation, given the high correlation between the VAS and NRS, it was assumed that the sample size calculated on the basis of VAS would be adequate to detect a difference in NRS as well. The common standard deviation was assumed to be 13 mm. In order to account for a 20% attrition in an 8-week study, 136 patients needed to be enrolled in each arm.
Outcome Measures and Endpoints
The primary efficacy outcome measure was assessment of mean change in the NRS from enrolment to 8 weeks.
The secondary efficacy outcome measures were (a) mean change in NRS from enrolment to 4 weeks, (b) mean change in Roland–Morris disability questionnaire (RDQ) from enrolment to 4 and 8 weeks, (c) mean change in VAS from enrolment to 4 and 8 weeks, (d) patient’s global impression of improvement (PGI-I) assessment at week 4 and 8, (e) clinical global impression of improvement (CGI-I) assessment at week 4 and 8 and (f) consumption of rescue medication (total dose of paracetamol tablets consumed) at week 4 and 8.
The safety outcome was the proportion of participants with adverse events (AEs) and serious adverse events throughout the study.
At the baseline visit, demographic information such as gender, age, height, weight, body mass index (BMI) and measurements pertaining to pain such as NRS, VAS, RDQ, PGI-I and CGI-I were recorded. All the efficacy outcome measures were recorded during the treatment period. Data pertaining to AEs and concomitant medications were recorded during the study.
The following scales were used to measure efficacy endpoints for the study.
Primary Outcome Measures
Numeric Rating Scale
NRS is an 11-point scale that is widely used clinically for the assessment of pain [11]. Patients were asked to rate their pain from 0 to 10 on the following 11-point scale (a lower rating suggests improvement in back pain): 0 = no pain, 1–3 = mild pain, 4–6 = moderate pain, 7–10 = worst/severe pain. For patients with CLBP, a minimum clinically important change (MCIC) score is considered as 2.5 [12]. Assessments were performed at enrolment, week 4 and week 8.
Secondary Outcome Measures
Roland–Morris Disability Questionnaire
RDQ is a commonly used patient-reported quality of life (QoL) questionnaire that assesses pain-related functional status. The RDQ consists of 24 statements relating to the person’s perceptions of their back pain and associated disability; 15 of these are related to physical ability/activity, three to sleep/rest, two to psychological aspects, two to household management, one to eating/diet and one to pain frequency [13]. Reduction in the RDQ score suggests improvement in functional status. It is recommended to consider a score of at least 3.5 as the MCIC [12]. Assessments were performed at enrolment, week 4 and week 8.
Visual Analogue Scale
VAS is the optimal tool for self-reporting of pain severity or intensity. It is usually presented as a 100-mm horizontal line on which the patient’s pain intensity is represented by a point between the extremes of ‘no pain at all’ (0 mm) and ‘worst pain imaginable’ (100 mm) [14]. The MCIC for VAS is 20 mm [12]. Assessments were performed at enrolment, week 4 and week 8.
Patient Global Impression of Improvement
As the name suggests, PGI-I is a global index used to rate the patients’ impression of their condition’s response to a particular therapy [15]. The assessment is based on the following ratings: 1 = very much better, 2 = much better, 3 = a little better, 4 = no change, 5 = a little worse, 6 = much worse, 7 = very much worse. Assessments were performed at week 4 and week 8.
Clinical Global Impression of Improvement
The CGI-I was developed to provide the clinicians’ assessment of patients’ global functioning before and after treatment [16]. The ratings are as follows: 1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change, 5 = minimally worse, 6 = much worse, 7 = very much worse. Assessments were performed at week 4 and week 8.
Safety
Safety assessment was based on monitoring and recording of all AEs and serious adverse events, if any, and regular monitoring of hematology, renal function tests, liver chemistry test, urine pregnancy test, urine analysis and electrocardiogram. Measurement of vital signs was performed on every scheduled visit.
Data Collection
All data relating to the study were documented in the case report form. Data collection and documentation were performed as per International Council for Harmonisation Good Clinical Practice guidelines.
Statistical Methods
Efficacy was assessed in the intention-to-treat (ITT) population. Descriptive statistics for continuous and ordinal variables were presented as mean ± standard deviation or percentage, as appropriate. Categorical variables like gender and AEs were summarized using count and percentage. Demographic characteristics (gender, age, height, weight and BMI) between the two treatment groups were analyzed using Fisher’s exact test and Wilcoxon Mann–Whitney test.
Change in the NRS, RDQ and VAS values at 4 and 8 weeks from baseline was summarized as mean ± SD. The mean change between groups was compared using the Wilcoxon Mann–Whitney test, whereas the mean change within groups was compared using Wilcoxon signed rank test.
All statistical tests were performed using SAS version 9.1.3, USA. Unless otherwise specified, all the statistical analyses were performed at a two-sided 5% level of significance.
Results
Patient Disposition
A total of 371 patients were screened for the study, of which 319 eligible patients were randomized to receive either the FDC (n = 160) or etoricoxib 60 mg (n = 159) once daily. The study was conducted between September 2018 and April 2019. Patient disposition is explained further in Fig. 4.
Baseline Characteristics
Both treatment groups were comparable with respect to demography and disease characteristics at baseline. Lumbosacral spine magnetic resonance imaging scans showed spine abnormalities in all the patients. Along with CLBP, more than 70% of patients in each treatment group had sharp shooting pain below the knee and 50% of patients felt pain when raising their legs up to 60° or less. Almost 80% of the patients from each treatment group reported pain of severe intensity. These baseline characteristics are reported in Table 1.
Efficacy Assessments
Primary Endpoint
NRS
The mean NRS score at baseline was comparable between the FDC and the etoricoxib groups (7.26 vs 7.22; p = 0.5941). Both groups showed significant reduction in pain intensity at week 4 and at week 8 from baseline (p < 0.0001). At week 8, mean reduction from baseline in NRS score was significantly greater in the FDC group when compared with etoricoxib (− 4.00 ± 1.65 vs − 2.92 ± 1.59; p < 0.0001) (mean percentage reduction was 55% and 40.1% in FDC and etoricoxib groups, respectively) (Table 2, Fig. 5).
Secondary Endpoints
NRS
After 4 weeks of treatment, the mean reduction in NRS score from baseline was significantly greater in the FDC group compared to the etoricoxib group (− 2.24 ± 1.36 vs − 1.57 ± 1.12; p < 0.0001) (mean percentage change was − 30.3% vs − 21.4% in FDC and etoricoxib group, respectively).
RDQ
At baseline, the mean RDQ score was not significantly different between FDC and etoricoxib groups (14.63 vs 14.57; p = 0.7225). At week 4 and 8, significant reduction in RDQ score was observed from baseline in both treatment groups (p < 0.0001). At week 4, the mean change from baseline was significantly greater in the FDC group compared with the etoricoxib group (− 5.42 ± 3.29 vs − 3.96 ± 3.11; p < 0.0001). At week 8 also, the mean change from baseline was significantly greater in the FDC group compared to the etoricoxib group (− 9.28 ± 4.48 vs − 6.78 ± 4.34; p < 0.0001) (Table 2, Fig. 6).
VAS
At baseline, the mean pain scores as measured on the VAS were not significantly different between treatment groups (71.25 and 70.82 in the FDC and etoricoxib arms, respectively, p > 0.05). At week 4 and 8, both treatments showed significant reduction in pain intensity from baseline (p < 0.0001). After 4 weeks of treatment, significantly greater reduction was observed in the FDC group than etoricoxib group (− 20.97 ± 14.56 vs − 15.52 ± 12.06; p < 0.0001) (mean score was 50.23 and 55.36 in FDC and etoricoxib groups, respectively). At 8 weeks also, there was significantly greater reduction in the FDC group compared to the etoricoxib group from baseline (− 37.66 ± 18.70 vs − 28.50 ± 16.31; p < 0.0001) (average pain score was 33.54 in the FDC group and 42.42 in the etoricoxib group; Table 2, Fig. 7).
Consumption of Rescue Medication
The median number of paracetamol tablets consumed in the FDC arm was four at week 4 and three at week 8 compared to five at both week 4 and 8 in the etoricoxib arm. The median dose of paracetamol in the FDC arm was 2000 mg at week 4 and 1500 mg at week 8, whereas it remained at 2500 mg at both week 4 and week 8 in the etoricoxib arm. The reduction in consumption of rescue medication in the FDC arm was statistically significant at week 8 (p = 0.0028) when compared with the etoricoxib arm.
PGI-I
Of the 158 patients in the FDC arm at week 4, one patient (0.6%) reported their disease condition as “very much better”, 84 patients (52.5%) reported it as “much better” and 63 patients (39.4%) assessed it as “a little better”. In the etoricoxib arm, of the 157 patients at week 4, the disease condition was observed to be “much better” for 26 patients (16.4%) and “a little better” for 106 patients (66.7%) (Fig. 8).
At week 8, of the 158 patients in the FDC arm, 59 patients (36.9%) reported their condition as “very much better”, 76 patients (47.5%) reported it as “much better” and 22 patients (13.8%) reported it as “a little better”. In the etoricoxib arm, of the 155 patients at week 8, the disease condition was assessed as “very much better” by 8 patients (5%), “much better” for 68 patients (42.8%) and “a little better” for 74 patients (46.5%) (Fig. 9).
The difference in PGI-I scores between the FDC and etoricoxib arms was statistically significant (p < 0.0001) in favour of FDC at both week 4 and 8.
CGI-I
Of the 158 patients in the FDC arm at week 4, clinicians assessed patients’ disease condition as “much improved” for 90 patients (56.3%) and “minimally improved” for 57 patients (35.6%). Of the 157 patients in the etoricoxib arm at week 4, clinicians assessed patients’ disease condition as “very much improved” for one patient (0.6%), “much improved” for 26 patients (16.4%) and “minimally improved” for 110 patients (69.2%) (Fig. 10).
Of the 158 patients in the FDC arm at week 8, patients’ disease condition was assessed as “very much improved” in 58 patients (36.3%), “much improved” in 75 patients (46.9%) and “minimally improved” in 24 patients (15%) by clinicians. Of the 155 patients in the etoricoxib arm at week 8, patients’ disease condition was assessed as “very much improved” in 9 patients (5.7%), “much improved” in 62 patients (39%) and “minimally improved” for 79 patients (49.7%) by clinicians (Fig. 11).
The difference in the CGI-I scores between the FDC and etoricoxib arms was statistically significant (p < 0.0001 in favour of FDC) at both week 4 and 8.
Treatment Compliance
A total of 154 patients (96.25%) in the FDC arm and 153 patients (96.23%) in the etoricoxib arm had at least 80% treatment compliance and completed the evaluations during the designated visits with no major protocol deviations.
Safety Outcomes
Overall, 22 treatment-emergent adverse events were reported (11 in the FDC group and 11 in the etoricoxib group) by 19 patients (11 in the FDC group and 8 in the etoricoxib group) (Table 3).
No serious adverse events were reported during the study. None of the patients were withdrawn from the study because of safety reasons. All the AEs were either mild or moderate in intensity. In the FDC group, five AEs were considered related to the study drug (one event each of localised oedema and oedema peripheral were probably related; two events of headache and one event of somnolence were possibly related). Involuntary muscle contractions and dysuria, one event each, were unlikely related, whereas two events each of pyrexia and rhinitis were unrelated to study medicine. In the etoricoxib group, three AEs were considered related to the study drug (two events of headache and one event of nausea were possibly related); one event each of face oedema, nasopharyngitis and cough were unlikely related, whereas two events each of pyrexia and nasopharyngitis and one event of peripheral swelling were unrelated to study medicine.
All the AEs were resolved/recovered during the study period.
There were no treatment-related significant changes in the biochemistry, hematology or urinalysis data following oral administration of study treatments. There was no clinically significant difference recorded from baseline in any of the patients for pulse rate, blood pressure, temperature, respiratory rate, electrocardiogram and vital sign parameters.
Discussion
CLBP is characterized by pain and disability leading to deterioration in mental wellbeing and reduced QoL over extended periods of time. Although the efficacy of gabapentinoids such as pregabalin and NSAIDs such as etoricoxib is well established as monotherapy, a combination targeting both nociceptive and neuropathic elements involved in the pathophysiology of CLBP has until now remained unavailable. In this phase 3 study, we compared the efficacy and safety of pregabalin prolonged release 75 mg and etoricoxib 60 mg FDC with etoricoxib alone in treating CLBP.
Patients’ perspectives are essential in making medical decisions and evaluating the results of treatment while treating painful medical conditions. Pain intensity is most frequently measured on the 11-point NRS, which ranges from no pain = 0 to the worst possible pain = 10 [17]. Along with NRS, VAS is also used for measuring pain intensity and both have good test–retest reliability in patients with chronic pain [18].
QoL is defined as the person’s evaluation of their well-being and functioning in different life domains. It is a subjective, phenomenological, multidimensional, dynamic, evaluative and yet quantifiable construct [19]. It is generally accepted that CLBP has a negative impact on health-related QoL [20] affecting physical and mental well-being, social relationships and functional ability [21]. In our study, RDQ and PGI-I were used to assess the QoL. RDQ is a commonly used patient-reported outcome measure that assesses pain-related functional status and a higher RDQ score indicates a lower QoL due to impairment by low back pain. PGI-I is a global index used to rate the patients’ impression of their condition’s response to a particular therapy.
In the current study, analysis of endpoints indicated significantly greater reductions in the NRS, RDQ and VAS scores and significantly better scores on PGI-I, and CGI-I scales with FDC than etoricoxib alone. Moreover, patients in the FDC group required lower dose of rescue medication compared to etoricoxib alone, thus confirming overall better pain management with the FDC.
The increased effectiveness of the combination of pregabalin and etoricoxib is in line with an emerging body of research recommending treatment with drugs having a multimodal mechanism of action for the management of neuropathies. The National Institute for Health and Care Excellence (NICE) guidance for pharmacological management of neuropathic pain suggests offering a choice of amitriptyline, duloxetine, gabapentin or pregabalin as initial treatment for neuropathic pain (with the exception of trigeminal neuralgia) [22]. The American Association of Neurology’s evidence-based report concluded that pregabalin is an effective (level A) treatment for painful diabetic neuropathy [23]. Multiple studies have also shown pregabalin emerging as the treatment of choice in managing severe diabetic neuropathy [24], both for alleviating pain and for improving overall QoL in patients [23, 25, 26].
Mishra et al. have shown maximum clinical benefits with pregabalin over amitriptyline and gabapentin in alleviating neuropathic cancer pain [27].
A study by Romanò et al. showed that combination therapy with pregabalin and celecoxib was more effective in alleviating CLBP than either pregabalin or celecoxib monotherapy, as seen by the VAS and Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) scores. No significant difference was observed in terms of reported AEs in combination therapy when compared with respective monotherapies [28].
While both FDC and etoricoxib alone showed statistically significant reduction in NRS, VAS and RDQ score, the effect size with FDC was higher in terms of statistically significant reduction in pain and better QoL when compared with etoricoxib monotherapy. At week 8, the mean change from baseline in NRS score was − 4, which is considerably more than the desired MCIC of 2.5. A similar trend was observed for the VAS score, where the change in the FDC arm from baseline at week 8 was − 37.6 mm, which is considerably higher than the desired MCIC of 20 mm. Findings observed for NRS and VAS validate the patient’s response on these two scales. The RDQ focuses on physical functions such as walking, bending over, sitting, lying down, dressing, sleeping, self-care and daily activities. The change in RDQ score at week 8 from baseline in the FDC arm was − 9.28 which is much higher than the desired MCIC of 3.5.
FDCs have come under intense scrutiny in India in recent years, mainly because of associated AEs [29]. Such AEs affect the patient’s QoL and/or drug compliance. Discontinuation rates owing to AEs in treatment groups across drug classes have been reported in the 5–20% range. Nonadherence to a drug regimen is another problem ranging from 8% to 53% in patients on medication for the management of chronic non-malignant pain [30].
The comparable safety profile of low, fixed-dose combination of pregabalin prolonged release and etoricoxib demonstrated in the current study supports other prevailing bodies of information. Bansal et al. compared efficacy of amitriptyline and pregabalin in alleviating pain associated with painful diabetic neuropathy. Both treatments showed comparable efficacy; however, pregabalin was considered as the more suitable treatment option for the Indian population as fewer AEs were observed with it [31]. Kamble et al. reported that a low dose of pregabalin (50–75 mg/day) is prescribed by Indian clinicians to maintain a balance between efficacy and minimal side effects [8].
Overall, study medications were well tolerated and no serious or severe AE was reported during the study. None of the patients had to be withdrawn from the study because of AEs.
Limitations
This phase 3 study was an open-label study conducted in controlled settings in a homogenous population. The double-blind design was not adapted as making a matching placebo of each drug was challenging. However, this was a randomized study and investigators did not know about treatment assignment in advance which ensured that selection/allocation bias was avoided. During the study, patients independently assessed/rated their pain on NRS and VAS, whereas patients assessed/rated their functional status on RDQ and PGI-I scale. Also, investigators did not interfere in the patients’ assessment of their own pain intensity and functional status which ensured that investigator-induced assessment bias was avoided.
Long-term studies in larger patient populations in real-world settings would help to establish the long-term efficacy and safety profile of FDC of pregabalin and etoricoxib in chronic painful conditions with a neuropathic component.
Conclusion
The FDC of low-dose pregabalin prolonged release 75 mg with etoricoxib 60 mg provided statistically and clinically significant benefits in reducing pain and improving functional status compared with etoricoxib alone in patients with CLBP. Significantly more benefits with FDC were evident from as early as week 4 and were sustained till week 8. The studied FDC could address both the neuropathic and nociceptive components of CLBP. Thus, considering the observed efficacy and safety profile of the pregabalin prolonged release 75 mg and etoricoxib 60 mg, it can be considered as a useful and viable therapeutic option for treating CLBP with neuropathic component. Further controlled, double-blind studies are necessary to confirm these findings.
References
Taguchi T, Igarashi A, Watt S, et al. Effectiveness of pregabalin for the treatment of chronic low back pain with accompanying lower limb pain (neuropathic component): a non-interventional study in Japan. J Pain Res. 2015;8:487–97.
Baron R, Binder A, Attal N, Casale R, Dickenson AH, Treede RD. Neuropathic low back pain in clinical practice. Eur J Pain. 2016;20(6):861–73.
Koley S, Sandhu NK. An association of body composition components with the menopausal status of patients with low back pain in Tarn Taran, Punjab, India. J Life Sci. 2009;1(2):129–32.
Wu A, March L, Zheng X, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann Transl Med. 2020;8(6):299.
Delitto A, Patterson CG, Stevans JM, et al. Stratified care to prevent chronic low back pain in high-risk patients: the TARGET trial. A multi-site pragmatic cluster randomized trial. EClinicalMedicine. 2021;34:100795.
Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13(3):185–90.
Spahr N, Hodkinson D, Jolly K, Williams S, Howard M, Thacker M. Distinguishing between nociceptive and neuropathic components in chronic low back pain using behavioural evaluation and sensory examination. Musculoskelet Sci Pract. 2017;27:40–8.
Kamble SV, Motlekar SA, D’souza LL, Kudrigikar VN, Rao SE. Low doses of amitriptyline, pregabalin, and gabapentin are preferred for management of neuropathic pain in India: is there a need for revisiting dosing recommendations? Korean J Pain. 2017;30(3):183–91.
Jayasheel BG. Regulatory requirements for marketing fixed dose combinations. Perspect Clin Res. 2010;1(4):120–3.
Gupta YK, Ramachandran SS. Fixed dose drug combinations: issues and challenges in India. Indian J Pharmacol. 2016;48(4):347–9.
Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure? Pain Pract. 2003;3(4):310–6.
Ostelo RW, de Vet HC. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol. 2005;19(4):593–607.
Stevens ML, Lin CC, Maher CG. The Roland Morris disability questionnaire. J Physiother. 2016;62(2):116.
Bodian CA, Freedman G, Hossain S, Eisenkraft JB, Beilin Y. The visual analog scale for pain: clinical significance in postoperative patients. Anesthesiology. 2001;95(6):1356–61.
Yue L, Wang J, Enomoto H, et al. The clinical relevance of pain severity changes: is there any difference between asian and caucasian patients with osteoarthritis pain? Pain Pract. 2020;20(2):129–37.
Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28–37.
Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–58.
Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S240–52.
Niv D, Kreitler S. Pain and quality of life. Pain Pract. 2001;1(2):150–61.
Montazeri A, Mousavi SJ. Quality of life and low back pain. In: Preedy VR, Watson RR, editors. Handbook of disease burdens and quality of life measures. New York: Springer; 2010.
Bonomi AE, Shikiar R, Legro MW. Quality-of-life assessment in acute, chronic, and cancer pain: a pharmacist’s guide. J Am Pharm Assoc (Wash). 2000;40(3):402–16.
Derry S, Bell RF, Straube S, Wiffen PJ, Aldington D, Moore RA. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1(1):CD007076.
Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758–65 (published correction appears in Neurology. 2011;77(6):603. Dosage error in article text).
Liampas A, Rekatsina M, Vadalouca A, Paladini A, Varrassi G, Zis P. Pharmacological management of painful peripheral neuropathies: a systematic review. Pain Ther. 2021;10(1):55–68.
Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–54.
Azmi S, ElHadd KT, Nelson A, et al. Pregabalin in the management of painful diabetic neuropathy: a narrative review. Diabetes Ther. 2019;10(1):35–56.
Mishra S, Bhatnagar S, Goyal GN, Rana SP, Upadhya SP. A comparative efficacy of amitriptyline, gabapentin, and pregabalin in neuropathic cancer pain: a prospective randomized double-blind placebo-controlled study. Am J Hosp Palliat Care. 2012;29(3):177–82.
Romanò CL, Romanò D, Bonora C, Mineo G. Pregabalin, celecoxib, and their combination for treatment of chronic low-back pain. J Orthop Traumatol. 2009;10(4):185–91.
McGettigan P, Roderick P, Mahajan R, Kadam A, Pollock AM. Use of fixed dose combination (FDC) drugs in India: central regulatory approval and sales of FDCs containing non-steroidal anti-inflammatory drugs (NSAIDs), metformin, or psychotropic drugs. PLoS Med. 2015;12(5):e1001826.
Markotic F, Cerni Obrdalj E, Zalihic A, et al. Adherence to pharmacological treatment of chronic nonmalignant pain in individuals aged 65 and older. Pain Med. 2013;14(2):247–56.
Bansal D, Bhansali A, Hota D, Chakrabarti A, Dutta P. Amitriptyline vs. pregabalin in painful diabetic neuropathy: a randomized double blind clinical trial. Diabet Med. 2009;26(10):1019–26.
Acknowledgements
We thank the participants of the study.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Sun Pharma Laboratories Limited, Mumbai, India.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Dr. Amit Yeole, Dr. G. Sree Lakshmi, Dr. Selvakumar CJ, Dr. Vijay Goni, Dr. CL Nawal, Dr. Bhanoth Valya, Dr. Brijesh Patel, Dr. Rohit Patel, Dr. Eknath Pawar, Dr. Ranajit Panigrahi, Dr. Ashish Kumar, and Dr. Shrinivas Shintre were involved in conduct of the study. Dr. Maulik Doshi was involved in designing the study. Dr. Prashant Devkare, Shruti Dharmadhikari, Dr. Sanjay Choudhari, Dr. Suyog Mehta, and Dr. Sadhna Joglekar were involved in data interpretation and manuscript writing and finalizing. All authors have read and approved the final manuscript.
Disclosures
Dr. Amit Yeole, Dr. G. Sree Lakshmi, Dr. Selvakumar CJ, Dr. Vijay Goni, Dr. CL Nawal, Dr. Bhanoth Valya, Dr. Brijesh Patel, Dr. Rohit Patel, Dr. Eknath Pawar, Dr. Ranajit Panigrahi, Dr. Ashish Kumar, and Dr. Shrinivas Shintre received research grant from the sponsor for conduct of the study. Dr. Sanjay Choudhari, Shruti Dharmadhikari, and Dr. Suyog Mehta are employees of Sun Pharma Laboratories Limited, India. Dr. Prashant Devkare is employee of Sun Pharmaceutical Industries Limited. Dr. Maulik Doshi was employee of Sun Pharma Laboratories Limited, India during conduct of the study. Dr. Sadhna Joglekar was employee of Sun Pharmaceutical Industries Limited, India during conduct of the study.
Compliance with Ethics Guidelines
The study was approved by the Drug Controller General of India and the ethics committee at each participating site (see supplementary material for details). The study was performed in accordance with the Declaration of Helsinki 1964 and its later amendments. All patients provided informed consent to participate in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yeole, A.B., Sree Ranga Lakshmi, G., Selvakumar, C.J. et al. Efficacy and Safety of Pregabalin Prolonged Release–Etoricoxib Combination Compared to Etoricoxib for Chronic Low Back Pain: Phase 3, Randomized Study. Pain Ther 11, 1451–1469 (2022). https://doi.org/10.1007/s40122-022-00437-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00437-2