Abstract
Introduction
Chronic pelvic pain (CPP) is a symptom that derives from a complex group of heterogeneous pathologies of the pelvic organs. The aim of this study was to review the available evidence on efficacy of neuromodulatory modalities including sacral neuromodulation, dorsal root ganglion stimulation, dorsal column neuromodulation, and pudendal nerve stimulation.
Methods
This narrative review focuses on updated information on neuromodulation for management of chronic pelvic pain. In 2022, we searched English-language studies on neuromodulation, pelvic pain, and chronic pain in a comprehensive search. We searched the following databases: PubMed, Medline, SciHub, Cochrane Database of Systematic Reviews, and Google Scholar. We used the following combinations of keywords: neuromodulation, pelvic pain, chronic pain, chronic pelvic pain, pelvic pain treatment. We tried to include as many recent manuscripts as possible (within the last 3 years) but also included papers older than 3 years if they were particularly relevant to our topic. We also attempted to search for, use, and cite primary manuscripts whenever possible.
Results
CPP is a challenging entity to treat because of diagnostic inconsistencies and limited evidence for therapeutic modalities. Our review found evidence suggestive of benefit for all modalities reviewed but the data was of overall low quality with numerous limitations. The literature highlights a lack of randomized controlled trials for neuromodulatory therapies but suggests a growing role for such techniques in treating refractory chronic pelvic pain syndrome (CPPS).
Conclusions
This review explores the available evidence on efficacy of neuromodulatory modalities for CPPS and contextualizes the results with information about the type of neuromodulation, lead location and waveform, pain outcomes and assessment timepoints, and reported adverse effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chronic pelvic pain (CPP) is a sign of various pelvic organ diseases. Chronic pelvic pain syndrome (CPPS) has several clinical manifestations, making a unified definition difficult |
CPP is noncyclical pain with a 6-month duration that might affect physical performance and quality of life |
This narrative review illustrates the variety of neuromodulatory methods that are available for the treatment of CPP, including sacral neuromodulation, conus medullaris stimulation, dorsal root ganglion (DRG) stimulation, dorsal column spinal cord stimulation (SCS), and pudendal nerve stimulation |
All of the modalities we examined had evidence that appeared to be beneficial, but the data generally was of poor quality and had several flaws, such as varied research circumstances and small sample numbers |
Our review draws attention to the dearth of randomized controlled studies for neuromodulatory treatments while also recognizing the expanding importance of these approaches for refractory CPPS |
Introduction
Chronic pelvic pain (CPP) is a symptom that represents a complex group of heterogeneous pathologies in specific pelvic organs. A consistent consensus definition continues to be elusive and perhaps appropriately so, considering the widely different clinical phenotypes that comprise chronic pelvic pain syndrome (CPPS). Broadly speaking, CPP is often defined as noncyclical pain that has a duration of at least 6 months with the potential to manifest with lower physical performance and quality of life [1].
CPPS has the potential to affect both men and women. A systematic review conducted in 2014 suggested that the lifetime prevalence of CPP ranged from 5.7% to 26.6%. The authors identified a scarcity of population-based prevalence studies and observed a number of obstacles to accurate estimation including lack of multidisciplinary studies, lack of statistical data and registration systems, lack of common definitions and consensus about CPP, inappropriate health system performance, and lack of education for both patients and clinicians [1]. An accurate estimate of the incidence or prevalence of CPPS does not exist currently. Frustratingly, for patients in particular, inconsistent diagnosis and classification often manifest with self-management and inadequate referrals to specialists or multidisciplinary care teams [2].
Various groups and guidelines have advocated for individual treatment strategies aimed at identifying and targeting important characteristics of CPPS including predisposing factors and causes, chronic pain mechanisms for ongoing pain, associated visceral dysfunctions, associated musculoskeletal dysfunctions, emotional consequences, behavioral consequences, sexual consequences, and social consequences [3]. To support a multidimensional and multidisciplinary approach to management of CPPS, Shoskes et al. proposed a phenotype-based classification that comprised six domains including urinary, psychosocial, organ specific, infection, neurologic/systemic, and tenderness (UPOINT) [4]. This approach has been supported by a systematic series of domains outlined by the International Continence Society Standardization for Terminology in CPP Syndromes. The domains detailed for CPPS include four domains involving the pelvic organs: lower urinary tract domain, female genital domain, male genital domain, and gastrointestinal domain. Two domains include sources that may be perceived in the pelvis: musculoskeletal domain and neurological domain. The final three domains detail factors that may influence the response to and impact of pain on the individual: psychological domain, sexual domain, and comorbidities [5]. Both classification structures are designed as clinical aids to ensure a logical and comprehensive mode of evaluation for a heterogenous syndrome.
In light of these expansive diagnostic challenges, interventional therapies including spinal cord stimulation have gained traction and popularity as a potential option for refractory CPPS. Novel targets for neuromodulation have continued to be identified under the original premise that the pathophysiology of CPPS may parallel some centralized, neuropathic and sympathetically driven pain models. In spite of the unique presentations and etiologies of CPPS, neuromodulation has demonstrated some efficacy with adequate and appropriate coverage to affected regions. Further research has continued to identify new targets and applications of neuromodulation in CPPS but identification and coverage of the pain transmission targets continue to be challenging [6].
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Pelvic Pain Mechanisms
Understanding the anatomy and neural pathways innervating the pelvic viscera is critical to identify appropriate neuromodulatory targets. The pelvis is an anatomic region located between the floor of the pelvic cavity and the pelvic brim. This area comprises both visceral and somatic structures with complex and varied innervations [7]. Visceral structures include the bladder, terminal ureters, urethra, ovaries, fallopian tubes, uterus, vagina, sigmoid colon, rectum, and associated vasculature and lymphatics. Somatic structures include the pelvic bones, ligaments, muscles, fascia, and body wall cutaneous dermatomes. Both somatic and visceral structures have the potential to cause CPP [8].
The somatic innervation of the pelvis derives from efferent motors fibers that originate from the spinal cord to innervate the skeletal muscle of the pelvic walls, pelvic floor, and perineum. Sensory afferent fibers transmit sensations from musculoskeletal organs and parietal peritoneum in addition to corresponding dermatomes and myotomes. The sacral plexus, formed by the lumbosacral trunk (L4 and L5 of lumbar plexus and anterior branches of S1–S4), provides somatic innervation of the pelvis including the levator ani, obturator internus, coccygeus, piriformis, gemellus, and quadratus femoris muscles. Innervation of the anterior and lateral abdominal wall originates from the intercostal nerves (T7–T11), subcostal nerve (T12), and iliohypogastric and ilioinguinal nerves (L1) [8].
The visceral innervation of the pelvis consists of efferent autonomic branches and afferent branches [9]. The efferent autonomic system consists of sympathetic and parasympathetic fibers that supply the urethra, bladder, ureters, vagina, cervix, uterus, fallopian tubes, ovaries, sigmoid colon, rectum, anal canal, and visceral peritoneum in women. The afferent system transmits signals from abdominal wall, pelvic viscera, and visceral peritoneum [8, 10, 11]. A minimum of five pathways are believed to transmit nociceptive stimuli from the pelvis with three transmitting through the inferior hypogastric plexus [12]. The inferior hypogastric plexus is a major relay center and innervates the genital and reproductive tract, bladder, urethra, distal ureter, internal anal sphincter, and rectum. It also contributes to three other plexuses: rectal plexus, uterovaginal plexus, and vesical plexus. The superior hypogastric plexus supplies the ureteral, ovarian, common iliac, and inferior hypogastric plexuses [8]. Visceral afferents synapse to second-order neurons in the dorsal horn of the spinal cord with subsequent transmission to supraspinal regions for processing [11].
The efferent sympathetic neurons originate predominantly from the thoracolumbar spinal cord from T12 to L2 nerve roots and are transmitted via the superior hypogastric plexus. The efferent parasympathetic neurons originate from the S2 to S4 nerve roots and are transmitted in the splanchnic nerves [6, 10]. The phenomenon of vague or overlapping CPP symptoms is possibly explained by the idea that visceral nociceptors are through to be poorly myelinated or unmyelinated nerve endings that mediate both somatic and visceral stimuli. The input to the spinal cord is therefore influenced by somatic input and may participate in a “cross-talk” phenomenon in which afferent activation of a pelvic structure influences efferent output to another [8, 12].
Neuromodulation
Summary
Electrotherapy neuromodulation (referred to as “neuromodulation” henceforth) has been used for pain management since ancient times. Scrobonus Largus, court physician to emperor Claudius, reported treatment of headaches with electric eels (4–100 V, 100 Hz) in 46–47 AD [13]. The era of modern neuromodulation is thought to have started in the 1960s with deep brain stimulation, followed by spinal cord stimulation, for intractable pain. Over the next 50 years, technological advances have significantly broadened the field of neuromodulation and impact on patient outcomes [6].
Current neuromodulation techniques for CPP target the sacral roots, pudendal nerve, mid-thoracic spinal cord, conus medullaris, and dorsal root ganglion (DRG) [6, 14, 15]. Unfortunately, given the complex nature of diagnosing CPP, it is increasingly common that patients are not evaluated by pain management specialists until unsuccessful treatment by two or more specialists from other fields (e.g., gynecology). By this point, CPP has often evolved into a chronic phase, rendering techniques including physical therapy, nerve blocks, radiofrequency lesions, and pharmacologic options increasingly ineffective. In such patients, neuromodulation may be the last bastion of potentially effective therapy. While the efficacy of neuromodulation for CPP often depends on adequate lead positioning, a lack of consensus exists with respect to the optimal target and location of leads [6].
Sacral Neuromodulation
Sacral neuromodulation (SNM) is an emerging minimally invasive treatment option for refractory CPP. SNM was first described by Tanagho and Schmidt in 1982 [16] and applied to human patients in 1988 [17]. Originally approved in 1997 by the US Food and Drug Administration (FDA) for urinary urge incontinence, urinary urgency-frequency, and non-obstructive urinary retention, SNM was eventually adopted for off-label usage in recalcitrant CPP [18]. SNM is an attractive treatment target in CPP given the role of the sacral nerve roots in relaying sensory information from the pelvic floor [19,20,21]. These sensory fibers may theoretically be subject to neuromodulation at any portion of the anatomical trajectory. In reality, caudal neuroanatomy is less mobile, less packed, and has a thinner insulating dorsal cerebrospinal fluid (CSF) layer relative to the conus medullaris and cauda equina. In addition, spatial representation of the distal sacral fibers diminishes in the cephalad direction; a stimulus may therefore preferentially recruit cephalad fibers. At the level of the thoracic spinal cord, sacral fibers are also smaller than lumbar fibers entering the dorsal column. The size discrepancy necessitates more energy to stimulate sacral fibers at the cost of indiscriminately stimulating thoracic fibers and inducing extraneous paresthesias. Thus, sacral nerve root stimulation may provide more selective pain modulation and stable delivery of electric pulses relative to cephalad structures [6, 19].
In 1997, a staged protocol involving a peripheral nerve evaluation (PNE) trial prior to permanent device placement was developed to identify favorable responders [22, 23]. Leads are typically inserted with local anesthesia and connected to an external temporary stimulator to allow for patient sensory responses. Trial duration generally lasts between 1 and 4 weeks and necessitates at least 50% symptomatic improvement to justify permanent SNM implant; a minimum 2-week trial period is recommended [22, 24, 25]. Success rates of PNE trials are typically around 50% with a reported range of 40–100% [24, 26]. Our review suggests a PNE success rate of 41–100% (Table 1). In studies that described lead arrangements, quadripolar leads were more utilized than octopolar leads and unilateral nerve roots were targeted more than bilateral nerve roots. Any roots from S1 to S4 were subject to neuromodulation with the most common target being unilateral S3 (Table 1).
Of the multitude of etiologies that underlie CPP, interstitial cystitis/bladder pain syndrome (IC/BPS) is the most well-documented indication for SNM (Table 1). In sum, 15 of 35 studies exclusively treated for this indication [21, 24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Several studies did not characterize CPP by diagnosed etiology [13, 38,39,40,41,42]. Perineal or anorectal pain was reported in four studies [43,44,45,46].
Martellucci et al. reported pain outcomes in a population of patients with CPP and prior pelvic surgeries [13]. Falletto et al. documented outcomes of SNM in patients with chronic anal and perianal pain [46] Sokal et al. applied SNM to patients with idiopathic CRPS and failed-back surgery syndrome [47]. Less common indications included vulvodynia [20, 48], coccydynia [48], severe endometriosis [49], postsurgical neuropathic pain [48], actinic proctitis [48], sacroiliac joint dysfunction [50], dyspareunia [50, 51], clitoral pain after abdominal surgery [52], and cauda equina syndrome [51] (Table 1).
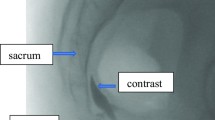
Lead placement strategies are broadly stratified into percutaneous (retrograde, anterograde, and transforaminal) and open approaches. The retrograde, also known as cephalocaudal, approach has been described as the standard technique for SNM implantation [6, 19] and entails lumbar epidural puncture with caudal advancement of electrodes. Unfortunately, this approach has a relatively high technical failure rate as advancement in the sacral promontory is frequently impossible [48]. Additionally, the retrograde approach lends itself to an increased risk of dural puncture, intrathecal lead placement, and cerebrospinal fluid leak. Reported stimulation parameter ranges include amplitude of 0.8–1.6 V, frequency of 30–50 Hz, and pulse width of 350–450 μs [53]. In our review, five studies described the retrograde approach [13, 20, 21, 27, 51]. Stimulation parameters were described in three of these studies and were variable (Table 1) [13, 20, 51].
In contrast, the anterograde, also known as the caudal or trans-hiatal, approach is technically easier and entails needle advancement under fluoroscopic guidance through the sacral hiatus. The risk of dural puncture is decreased compared to the retrograde approach. Leads emerging from the needle often need to be advanced a short distance to the nerve roots. However, the thin subcutaneous layer overlying the sacral hiatus often makes lead anchoring difficult and increases the risk of skin erosion. The anterograde approach has raised concerns regarding implant sterility and risk of infection [6, 48, 53]. In total, six studies described the anterograde approach [20, 38, 45, 47, 48, 50]. Stimulation parameters were described in two cases reports, of which only one mentioned voltage (Table 2). Parameters included amplitude of 1–10.5 V, frequency of 60–1200 Hz, and pulse width of 20–300 μs [20, 50].
The third method is the transforaminal approach, usually targeting the S3 nerve root, which is technically easier and has been widely adopted in staged SNM. The approach has a reduced risk of dural puncture and skin erosion but at the cost of a higher incidence of reprogramming and lead migration due to challenges with anchoring. Anterior lead positions also tend to stimulate motor fibers and may generate uncomfortable paresthesias. Tined leads have improved fixation success [54, 55]. Previously reported stimulation parameters include amplitudes of 0.8–1.0 V, frequencies of 25–30 Hz, and pulse width of 180–210 μs [53, p. 200]. In total, 17 studies employed the transforaminal approach [24, p. 2; 25, 28,29,30,31,32,33,34,35, 40, 41, 46, 49, 51, 53, 56]. Stimulation parameters were described in eight studies (Table 1) with amplitude of 1–9 V, frequency of 14–54 Hz, and pulse width of 200–300 μs. Notably, Marinkovic et al. compared long-term pain outcomes in patients who underwent neuromodulation with low voltage (less than or equal to 3 V) to high voltage (at least 4 V) [35].
The final approach is open surgery, which is often a last resort and has been largely replaced by the aforementioned minimally invasive alternatives. Open surgery has previously been described as placing paddle leads unilaterally or bilaterally following a partial L5–S1 laminectomy. Leads are advanced caudally beneath the dorsal sacrum to overlay the S2–S4 roots. Paddle leads may provide broader paresthesia coverage. Stimulation parameters are typically set to lower amplitudes and higher frequencies [28, 31]. Overall complication rates have previously been reported as 5% and consist primarily of infection, subdural implantation, and CSF leak [53]. Our review identified two studies describing open surgery in patients with CPP though stimulation parameters were not included [28, 31].
Our literature review of SNM for the treatment of CPP identified 35 manuscripts published from 1999 to 2018, of which eight were case reports. A total of 786 patients with CPP were SNM candidates, 542 of whom had documented long-term pain outcomes after permanent implantation [20, 29, 32, 34] (Tables 1, 2). Long-term pain outcomes were most commonly quantified with the visual analogue scale (VAS) and occasionally with 4- to 7-point ordinal pain scales. Other metrics related to pain included pain medication usage, 36-Item Short Form Health Survey (SF-36) scores, subjective symptom improvement, Pain Urgency and Frequency questionnaire (PUF), O’Leary/Sant IC Symptom Problem Index (ICSPI), and Global Response Assessment (GRA).
All but one study reported improvement in long-term pain outcomes in the majority of patients who had at least one follow-up based on aforementioned metrics. Of the four patients who underwent permanent SNM in a study by Elhilali et al., only one (25%) experienced subjective mean pain improvement on follow-up at 6.5 years [56]. Analysis for statistical significance was included in 15 studies [13, 24, 25, 29, 30, 32, 34,35,36, 38,39,40, 45,46,47], though only 12 studies provided analysis for the permanent implantation stage [13, 24, 25, 30, 35, 36, 38, 39, 45,46,47]. Of the 12 studies, six of which were prospective, recalcitrant chronic pelvic pain and interstitial cystitis/painful bladder syndrome were the most common indications for SNM (Table 1). Four studies were conducted in specific populations including CPP with prior pelvic surgery [13], idiopathic CRPS and failed-back surgery syndrome [47], and chronic anal and/or perineal pain [45, 46]. Mean and median duration of CPP, if specified, spanned between 3 and 6 years. Approaches were not described consistently but the most common target of neuromodulation was the S3 root unilaterally (Table 1). Progression to permanent implant ranged from 44% to 100%. VAS was observed to improve by at least 2.6 points minimum but by at least 3 points on follow-up of at least 6 months [13, 24, 25, 35, 36, 38, 39, 45,46,47] (Table 1).
Marinkovic et al. retrospectively studied the largest CPP population with the longest follow-up to date [35]. Long-term pain outcomes in 100 patients with IC/BPS with low voltage (less than or equal to 3 V) were compared to 48 patients with IC/BPS with high voltage (at least 4 V) S3 root stimulation. Conversation rates to permanent implant were superior in the low voltage group (95.4% vs. 73.8%; p < 0.001) with a higher subsequent success rate (87.6% vs. 66.2%, p < 0.002). On 10-year follow-up, the low voltage group (mean voltage 3.35 V) reported a VAS improvement of 5 points versus 2.6 points in the high voltage group (mean voltage 6.06 V). Furthermore, the low voltage group demonstrated superior ICSPI and PUF scores. Complication rates were similar (6.7% vs. 9.2%, p > 0.18). Two other studies demonstrated statistically significant decreases in use of pain medications. Peters et al. observed a decrease in narcotic usage by 36% following permanent implant, from 81.6 mg per day before implantation to 52.0 mg per day after implantation. Notably, 22% of patients (4 of 18) discontinued narcotics altogether and 95% of patients reported moderate to marked improvement in pain at 15 months mean follow-up [36]. Ghazwani et al. also observed a decrease from 4.9 to 1.9 (p ≤ 0.001) in the number of unique pain medications used at a mean follow-up of 71.5 months in 11 patients who underwent permanent implant [24] (Table 1).
Of particular interest, 4 of 12 studies reported non-sustained pain control or conflicting improvement in pain during follow-up [13, 38, 40, 47]. Martellucci et al. prospectively studied 16 patients with CPP and prior pelvic surgery who underwent implantation with a retrograde and primarily unilateral S3 approach. Reported VAS improved from 8.1 to 2.1 at 6 months but the improvement was not sustained at 12–60 months as a result in part of patients lost to follow-up [13]. Sokal et al. prospectively studied nine patients with idiopathic CRPS/failed back surgery syndrome who were implanted with an anterograde mixed laterality approach, spanning S2–S4. VAS improved from 9 pre-operatively to 3 postoperatively at 6 months (p = 0.043) but again, statistical significance was not sustained at 12 months [47]. In 2001, Aboseif et al. conducted a prospective study of 41 patients with CPP who underwent implantation with an anterograde, unilateral S3 approach. Reported VAS was not significantly improved at mean follow-up of 24 months [40]. Separately, Zahibi et al. retrospectively studied 23 patients with bilateral S2–S4 permanent implants inserted via anterograde approaches. In spite of a 40% overall improvement in VAS (p = 0.04) and the pain components of the ICSI (44%, p < 0.05) and ICPI (33%, p < 0.05), SF-36 bodily pain score was not significantly improved on follow-up at 6 months.
Complications with SNM implant included explant from infection, pain at implantation site, poor analgesic efficacy, intolerable paresthesias, lead migration or displacement, lead breakage, or device failure (Tables 1, 2). Device failures generally consisted of malfunction or battery failure. CSF leak was reported in one study and was unique to the retrograde approach [27]. Overall, adverse effects were inconsistently reported.
Conus Medullaris Stimulation
Conus medullaris stimulation (CMS) was first described in 1970 to improve the function of a paralyzed bladder in a paraplegic patient [57]. To our knowledge, isolated CMS for CPP has only been described in one study. The conus medullaris is the tapered distal end of the spinal cord and transitions into the cauda equina, a bundle of lumbar and sacral nerve roots that innervates the pelvic anatomy. It typically aligns with the lower third of the L1 vertebral body but may span anywhere between the middle of T12 to the upper third of L3. As a result of anatomic variation, definitive localization of the conus medullaris with imaging is essential. Similar to the PNE trial described for SNM, a trial period lasting 1–3 weeks is generally conducted prior to permanent CMS [58].
CMS for management of CPP has only been described in a prospective multicenter case series of 27 patients with refractory unilateral or bilateral pudendal neuralgia (PN). Refractory PN was diagnosed by the Nantes criteria. Patients had to meet multiple requirements including failed response to standard pain management, failed pudendal nerve decompression surgery via Robert’s technique using a transgluteal approach, chronic neuropathic pain per the Neuropathic Pain Diagnostic Questionnaire, and maximum pain VAS of at least 50/100. A 50% reduction in maximum pain and/or average pain and/or greater than 50% increase in sitting constituted a successful trial [58].
Specific technical approaches were guided by patient anatomy. In the trial phase, a stimulating electrode was implanted under fluoroscopic visualization. The preferred approach was the transcutaneous technique with a Lamitrode S8 electrode. In cases of complex spinal anatomy including spinal deformity and prior lumbar surgery, a direct surgical approach with two- or three-column electrodes was used. Intraoperative stimulation tests were performed under local anesthesia and electrode placement was confirmed with fluoroscopy prior to discharge regardless of technique. Permanent CMS candidates underwent subsequent implantation of a subcutaneous generator. Reported CMS settings included intensity of 1.4–8.7 mA, pulse width of 60–325 ms, and frequency of 50–200 Hz [58].
Twenty of 27 patients (74%) progressed to permanent CMS implantation with a mean follow-up of 15 months. In this cohort, mean age was 60 years with pain characteristics that included bilateral pain in 90%, mean pain duration of 72 months, and mean follow-up after pudendal nerve decompression surgery of 29 months; 75% of the cohort preferred an intensity between 1.5 and 3 mA with roughly 50% needing a pulse width of less than 100 ms. A frequency greater than 100 Hz was necessary in 85% and 40% needed 200 Hz. Stimulation parameters did not require significant adjustments. On follow-up, maximum VAS was reduced by 53.5% with a concomitant reduction in average VAS of 51.4% and tripling of sitting time. In addition, the estimated percentage of improvement was 55.5%. All patients reported a preference for undergoing the procedure again. Complications were isolated to one electrode displacement and one superficial surgical site infection [58].
Dorsal Root Ganglion Stimulation
Dorsal root ganglion (DRG) stimulation has emerged in a handful of small studies as a potentially promising target for chronic pelvic pain. The DRG is a bilateral structure at each vertebral level that houses the cell bodies of primary sensory neurons and is intimately involved in the transmission of noxious stimuli including pain. Studies conducted in rats demonstrated reduced neuronal excitability and action potential propagation with electrode-mediated stimulation of the DRG, thus suggesting that artificial stimulation of the DRG may modulate the transmission of pain signals in chronic pain syndrome. The DRG is accessible via fluoroscopically guided electrode placement into the epidural space. A trial of stimulation is typically performed prior to permanent implantation of an implantable pulse generator (IPG). Up to four DRGs may be stimulated with conventional DRG devices. Purported advantages of DRG stimulation include the precise ability to target subdermatomal pain and insensitivity of lead placement to posture and patient movement.
In our review, we identified four studies evaluating DRG stimulation for treatment of chronic pelvic pain (Table 3). The studies ranged in design from case reports to prospective randomized controlled trials. Of particular interest to our discussion, patients in the studies presented with chronic groin and/or buttock pain related to multiple etiologies including complex regional pain syndrome (CRPS), causalgia/neuropathy, and/or pelvic girdle pain. For the majority of the cases, DRG stimulation was pursued as an interventional option for refractory pain syndromes often in the context of failed medical management with chronic oral analgesics. Due in part to the heterogeneous indications for DRG stimulation, technical parameters varied considerably. Leads were inserted at levels ranging from lower thoracic to sacral vertebrae [59]. Only two of the four studies included waveform parameters for stimulation; parameters included frequencies ranging from 20 to 40 Hz with pulse width between 200 and 500 μs [60, 61].
Overall, DRG stimulation was observed to provide significant pain relief for the study participants. Average decrease in VAS and numerical rating scale (NRS) scores exceeded 50% in all multipatient studies [59, 60, 62]. Patients reported increased quality of life in addition to improved function and mobility [60, 61]. The most longitudinal study followed patients for at least 12 months post-implantation and observed sustained pain relief [60]. Compared to traditional spinal cord stimulation (SCS), one randomized controlled trial noted significantly improved reduction in pain scores with DRG stimulation [60]. Of note, the DRG implants were also observed to be more resistant to postural variation with respect to adequacy of pain coverage [60, 62]. Unfortunately, the remaining studies examining DRG stimulation were not designed with a control group. Across all studies, DRG stimulation was observed to have no difference in adverse event rates compared to traditional SCS therapy. The most common adverse events reported included incisional site pain, IPG pocket pain, and overstimulation [60].
DRG stimulation therapy appears to be a promising treatment modality for chronic pelvic pain, albeit with a limited and heterogeneous evidence base. Patient outcomes with respect to pain relief and improvement of function in the single randomized control trial to date observed superiority compared to traditional SCS therapy. Additional high-quality research with standardized patient populations is needed to understand the long-term efficacy and potential role of DRG stimulation for chronic pelvic pain.
Dorsal Column Stimulation
Dorsal column SCS is a mainstay of interventional treatment for chronic pain syndromes and has been posited as a potential option for chronic pelvic pain syndromes. Dorsal column (DC) lesions in particular have been shown to attenuate the pain associated with pelvic cancer, thus implying a potential role for spinal cord neuromodulation in managing non-malignant etiologies of pelvic pain [63]. Interestingly, significant overlap has been observed between chronic pelvic pain syndromes and CRPS, especially with respect to hypersensitization of pain-sensing neurons in response to non-painful stimuli. For chronic pelvic pain, this allodynia-like phenomenon often manifests with urination, bladder distension, sexual activity, ovulation, or even prolonged sitting [6, 64]. The overlap of potential pain pathways and clinical presentation has raised the profile of SCS as a potential management option for chronic pelvic pain [65]. Similar to DRG stimulation, SCS is initiated with placement of electrodes in the epidural space with permanent implantation considered after a successful trial period. Notably, SCS stimulation is focused on ascending nerve tracts in the spinal cord and has been demonstrated to offer a less targeted region of analgesia compared to DRG stimulation.
In our review, we identified ten studies evaluating SCS for management of chronic pelvic pain. Nine studies were either case reports or case series for a cumulative of 56 patients (Table 4) and one study was a randomized controlled trial comparing SCS to DRG stimulation [60]. The designated etiology of patient’s chronic pelvic pain was heterogeneous and included irritable bowel syndrome (IBS), pudendal neuralgia, post-herniorrhaphy pain, Bannayan-Riley-Ruvacalba syndrome, and non-specific pelvic pain. As with patients undergoing DRG stimulation, patients had generally failed conservative management prior to being offered SCS therapy.
One case series of three patients involved high-frequency SCS whereas the others involved conventional SCS therapy [66]. The studies displayed heterogeneity with respect to description of SCS implantation technique. In general, lead implantation was in the mid- to lower-thoracic spine with the highest reported lead at T5 and lowest at L2. Only two studies reported on specifics of the waveform parameters used; amplitude ranged from 1.8 to 3.8 V with a pulse width range from 300 to 450 μs. Frequency ranged from 40 to 65 Hz with the exception of the high-frequency stimulation patients who were stimulated at 10 kHz [66,67,68].
Heterogeneity in reporting of patient outcomes was also evident with only a fraction of the studies reporting quantitative VAS scores. Follow-up times varied from 3 months to upwards of 3 years. With these limitations in mind, outcomes appeared to be positive with reported VAS scores decreasing by more than 50% across multiple case reports [6, 58, 66, 67, 69,70,71; 72, p. 20]. Notably, analgesic requirements were also markedly reduced following SCS implantation though one case series of six patients observed no significant reduction in opioid use [6, 67, 69,70,71,72]. Quality of life, including patient mobility and sitting tolerance, was also noted to be improved [58, 66; 67, p. 200; 68, 69]. Two case studies, however, reported on the eventual return of pain and opioid requirements [67, 71]. A total of five revisions were reported, all for lead migration, which represented slightly less than 10% of all patients studied.
Spinal cord stimulation therapy appears to be a viable option for chronic pelvic pain as reported through case reports and case series. To our knowledge, no randomized controlled trials are available comparing SCS to placebo in this population. The purported efficacy should be tempered with an appreciation of the limitations of case reports and series and potential for significant bias. Nevertheless, given the difficult-to-treat and refractory nature of chronic pelvic pain, SCS holds promise as a potential strategy for pain relief.
Pudendal Nerve Stimulation
Targeted neuromodulation of the peripheral nervous system via pudendal nerve stimulation has also been investigated as a treatment modality for chronic pelvic pain. Interestingly, relief of pain with a pudendal nerve block is often used as diagnostic criterion for pudendal neuralgia [73]. Perhaps it is unsurprising that a natural corollary has been targeting of the pudendal nerve with permanent implantation of electrodes for refractory chronic pain. Prior research has demonstrated that pudendal nerve stimulation may be effective for treatment of neurogenic bladder [22]. Electrode implantation has been demonstrated with minimally invasive needle techniques and neurophysiologic guidance under local anesthesia [33, 74]. As with dorsal column and dorsal root ganglion stimulation, a successful trial generally precedes permanent generator implantation.
In our review, we identified five studies comprising a total of 129 patients who underwent pudendal nerve stimulation for chronic pelvic pain (Table 5). The study designs included two retrospective studies, a case series, a case report, and a prospective double-blind crossover trial comparing pudendal nerve stimulation to sacral stimulation. The most common identified etiologies of pain were interstitial cystitis and pudendal neuralgia. All patients underwent pudendal nerve stimulation therapy with electrode placement along the course of the pudendal nerve; one case report discussed a patient who underwent concomitant pudendal nerve decompression. The majority of procedures involved a posterior ischial-rectal approach. Only one study discussed waveform parameters: pulse width of 200 μs and frequency of 16 Hz [33].
Patients were followed longitudinally from 6 months to 6 years with evaluation of mixed pain outcomes. Three studies reported promising results with greater than 80% pain relief and overall high patient satisfaction [74,75,76]. The largest study, however, comprising an 84-patient case series, did not identify a statistically significant change in self-reported pain scores at the 12-month follow-up, though more than half of the patients reported improvement in pain [74]. When compared with sacral neuromodulation, pudendal nerve stimulation appeared to offer no significant short-term advantage. However, a majority of blinded patients in the crossover trial elected for pudendal nerve stimulation over sacral stimulation and long-term VAS score reduction was greater in the pudendal nerve stimulation group [33]. Reported complications of implantation included lead migration, paresthesias, infection, and seroma formation [33, 77].
Of the limited data available, pudendal nerve stimulation appears to have a positive impact on pelvic pain outcomes. Notably, the spectrum of pathologies investigated was smaller in scale than for either DRG or SCS therapy. The peripheral nature of the technique may ultimately limit the indications of the therapy in comparison to a more centrally acting therapy. Conversely, in an appropriately selected subset of patients, pudendal nerve stimulation may offer selectively targeted analgesia.
Conclusion
This narrative review highlights the array of neuromodulatory modalities such as sacral neuromodulation, conus medullaris stimulation, DRG stimulation, dorsal column SCS, and pudendal nerve stimulation available for the treatment of CPP. We found evidence suggestive of benefit for all modalities reviewed but the data was of overall low quality with numerous limitations including heterogeneous study conditions and sample sizes. Our review highlights the lack of randomized controlled trials for neuromodulatory therapies but acknowledges the growing role of such techniques for refractory CPPS.
References
Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Physician. 2014;17(2):E141–147.
Baranowski AP, Lee J, Price C, Hughes J. Pelvic pain: a pathway for care developed for both men and women by the British Pain Society. Br J Anaesth. 2014;112(3):452–9. https://doi.org/10.1093/bja/aet421.
Engeler DS, Baranowski AP, Dinis-Oliveira P, Elneil S, et al. The 2013 EAU guidelines on chronic pelvic pain: is management of chronic pelvic pain a habit, a philosophy, or a science? 10 years of development. Eur Urol. 2013;64(3):431–9. https://doi.org/10.1016/j.eururo.2013.04.035.
Shoskes DA, Nickel JC, Dolinga R, Prots D. Clinical phenotyping of patients with chronic prostatitis/chronic pelvic pain syndrome and correlation with symptom severity. Urology. 2009;73(3):538–42. https://doi.org/10.1016/j.urology.2008.09.074.
Rana N, Drake MJ, Rinko R, Dawson M, Whitmore KE. The fundamentals of chronic pelvic pain assessment, based on International Continence Society recommendations. Neurourol Urodynam. 2018. https://doi.org/10.1002/nau.23776.
Hunter C, Davé N, Diwan S, Deer T. Neuromodulation of pelvic visceral pain: review of the literature and case series of potential novel targets for treatment. Pain Pract. 2013;13(1):3–17. https://doi.org/10.1111/j.1533-2500.2012.00558.x.
Elkins N, Hunt J, Scott KM. Neurogenic pelvic pain. Phys Med Rehabil Clin N Am. 2017;28(3):551–69. https://doi.org/10.1016/j.pmr.2017.03.007.
Lamvu G, Steege JF. The anatomy and neurophysiology of pelvic pain. J Minim Invasive Gynecol. 2006;13(6):516–22. https://doi.org/10.1016/j.jmig.2006.06.021.
Gebhart GF, Bielefeldt K. Physiology of visceral pain. Compr Physiol. 2016;6(4):1609–33. https://doi.org/10.1002/cphy.c150049.
Kanai A, Andersson K-E. Bladder afferent signaling: recent findings. J Urol. 2010;183(4):1288–95. https://doi.org/10.1016/j.juro.2009.12.060.
Roy H, Offiah I, Dua A. Neuromodulation for pelvic and urogenital pain. Brain Sci. 2018. https://doi.org/10.3390/brainsci8100180.
Toner BB, Tang TN. Chronic pelvic pain. In: Drutz HP, Herschorn S, Diamant NE, editors. Female pelvic medicine and reconstructive pelvic surgery. London: Springer; 2003. p. 235–42. https://doi.org/10.1007/1-84628-238-1_22.
Martellucci J, Naldini G, Carriero A. Sacral nerve modulation in the treatment of chronic pelvic pain. Int J Colorect Dis. 2012. https://doi.org/10.1007/s00384-011-1394-2.
Tam J, Loeb C, Grajower D, Kim J, Weissbart S. Neuromodulation for chronic pelvic pain. Curr Urol Rep. 2018;19(5):32. https://doi.org/10.1007/s11934-018-0783-2.
Mahran A, Baaklini G, Hassani D, et al. Sacral neuromodulation treating chronic pelvic pain: a meta-analysis and systematic review of the literature. Int Urogynecol J. 2019;30(7):1023–35. https://doi.org/10.1007/s00192-019-03898-w.
Tanagho EA, Schmidt RA. Bladder pacemaker: scientific basis and clinical future. Urology. 1982;20(6):614–9. https://doi.org/10.1016/0090-4295(82)90312-0.
Tanagho EA. Neural stimulation for bladder control. Semin Neurol. 1988;8(2):170–3. https://doi.org/10.1055/s-2008-1041373.
Giannantoni A, Bini V, Dmochowski R, et al. Contemporary management of the painful bladder: a systematic review. Eur Urol. 2012;61(1):29–53. https://doi.org/10.1016/j.eururo.2011.07.069.
Richter EO, Abramova MV, Aló KM. Percutaneous cephalocaudal implantation of epidural stimulation electrodes over sacral nerve roots—a technical note on the importance of the lateral approach. Neuromodulation. 2011. https://doi.org/10.1111/j.1525-1403.2010.00293.x.
Alo KM, Yland MJ, Redko V, Feler C, Naumann C. Lumbar and sacral nerve root stimulation (NRS) in the treatment of chronic pain: a novel anatomic approach and neuro stimulation technique. Neuromodulation. 1999;2(1):23–31. https://doi.org/10.1046/j.1525-1403.1999.00023.x.
Aló KM, Mckay E. Selective nerve root stimulation (SNRS) for the treatment of intractable pelvic pain and motor dysfunction: a case report: SNRS for treatment of intractable pelvic pain and motor dysfunction. Neuromodulation. 2001. https://doi.org/10.1046/j.1525-1403.2001.00019.x.
Spinelli M, Malaguti S, Giardiello G, Lazzeri M, Tarantola J, Van Den Hombergh U. A new minimally invasive procedure for pudendal nerve stimulation to treat neurogenic bladder: description of the method and preliminary data. Neurourol Urodyn. 2005;24(4):305–9. https://doi.org/10.1002/nau.20118.
Janknegt RA, Weil EH, Eerdmans PH. Improving neuromodulation technique for refractory voiding dysfunctions: two-stage implant. Urology. 1997;49(3):358–62. https://doi.org/10.1016/S0090-4295(96)00506-7.
Ghazwani YQ, Elkelini MS, Hassouna MM. Efficacy of sacral neuromodulation in treatment of bladder pain syndrome: long-term follow-up. Neurourol Urodyn. 2011. https://doi.org/10.1002/nau.21037.
Marinkovic SP, Gillen LM, Marinkovic CM. Minimum 6-year outcomes for interstitial cystitis treated with sacral neuromodulation. Int Urogynecol J. 2011;22(4):407–12. https://doi.org/10.1007/s00192-010-1235-9.
Powell CR, Kreder KJ. Long-term outcomes of urgency-frequency syndrome due to painful bladder syndrome treated with sacral neuromodulation and analysis of failures. J Urol. 2010;183(1):173–6. https://doi.org/10.1016/j.juro.2009.08.142.
Feler CA, Whitworth LA, Brookoff D, Powell R. Recent advances: sacral nerve root stimulation using a retrograde method of lead insertion for the treatment of pelvic pain due to interstitial cystitis. Neuromodulation. 1999. https://doi.org/10.1046/j.1525-1403.1999.00211.x.
Gajewski JB, Al-Zahrani AA. The long-term efficacy of sacral neuromodulation in the management of intractable cases of bladder pain syndrome: 14 years of experience in one centre. BJU Int. 2011. https://doi.org/10.1111/j.1464-410X.2010.09697.x.
Chai TC, Zhang C, Warren JW, Keay S. Percutaneous sacral third nerve root neurostimulation improves symptoms and normalizes urinary HB-EGF levels and antiproliferative activity in patients with interstitial cystitis. Urology. 2000;55(5):643–6. https://doi.org/10.1016/s0090-4295(00)00476-3.
Comiter CV. Sacral neuromodulation for the symptomatic treatment of refractory interstitial cystitis: a prospective study. J Urol. 2003;169(4):1369–73. https://doi.org/10.1097/01.ju.0000053863.96967.5a.
Peters KM, Carey JM, Konstandt DB. Sacral neuromodulation for the treatment of refractory interstitial cystitis: outcomes based on technique. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(4):223–8. https://doi.org/10.1007/s00192-003-1070-3 (discussion 228).
Whitmore KE, Payne CK, Diokno AC, Lukban JC. Sacral neuromodulation in patients with interstitial cystitis: a multicenter clinical trial. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(5):305–8. https://doi.org/10.1007/s00192-003-1080-1 (discussion 308–309).
Peters KM, Feber KM, Bennett RC. A prospective, single-blind, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystitis. BJU Int. 2007. https://doi.org/10.1111/j.1464-410X.2007.07082.x.
Maher CF, Carey MP, Dwyer PL, Schluter PL. Percutaneous sacral nerve root neuromodulation for intractable interstitial cystitis. J Urol. 2001;165(3):884–6.
Marinkovic SP. Improving clinical outcomes with lower motor voltage (≤3 V) during stage 1 sacral neuromodulation for interstitial cystitis or bladder pain syndrome. Neurourol Urodyn. 2019;38(8):2233–41. https://doi.org/10.1002/nau.24123.
Peters KM, Konstandt D. Sacral neuromodulation decreases narcotic requirements in refractory interstitial cystitis. BJU Int. 2004;93(6):777–9. https://doi.org/10.1111/j.1464-410X.2003.04745.x.
Zermann DH, Weirich T, Wunderlich H, Reichelt O, Schubert J. Sacral nerve stimulation for pain relief in interstitial cystitis. Urol Int. 2000;65(2):120–1. https://doi.org/10.1159/000064852.
Zabihi N, Mourtzinos A, Maher MG, Raz S, Rodríguez LV. Short-term results of bilateral S2–S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):553–7. https://doi.org/10.1007/s00192-007-0466-x.
Kessler TM, Buchser E, Meyer S, et al. Sacral neuromodulation for refractory lower urinary tract dysfunction: results of a nationwide registry in Switzerland. Eur Urol. 2007;51(5):1357–63. https://doi.org/10.1016/j.eururo.2006.11.011.
Aboseif S, Tamaddon K, Chalfin S, Freedman S, Kaptein J. Sacral neuromodulation as an effective treatment for refractory pelvic floor dysfunction. Urology. 2002;60(1):52–6. https://doi.org/10.1016/s0090-4295(02)01630-8.
Siegel S, Paszkiewicz E, Kirkpatrick C, Hinkel B, Oleson K. Sacral nerve stimulation in patients with chronic intractable pelvic pain. J Urol. 2001;166(5):1742–5.
Everaert K, Devulder J, De Muynck M, et al. The pain cycle: implications for the diagnosis and treatment of pelvic pain syndromes. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(1):9–14. https://doi.org/10.1007/s001920170087.
Yang KS, Kim YH, Park HJ, Lee MH, Kim DH, Moon DE. Sacral nerve stimulation for treatment of chronic intractable anorectal pain—a case report. Korean J Pain. 2010;23(1):60. https://doi.org/10.3344/kjp.2010.23.1.60.
Uludağ Ö, Melenhorst J, Koch SMP, van Gemert WG, Dejong CHC, Baeten CGMI. Sacral neuromodulation: long-term outcome and quality of life in patients with faecal incontinence: SNM: long-term outcome and QOL in patients with FI. Colorect Dis. 2011. https://doi.org/10.1111/j.1463-1318.2010.02447.x.
Vancaillie T, Kite L, Howard E, Chow J. Sacral neuromodulation for pelvic pain and pelvic organ dysfunction: a case series. Aust N Z J Obstet Gynaecol. 2018;58(1):102–7. https://doi.org/10.1111/ajo.12752.
Falletto E, Masin A, Lolli P, et al. Is sacral nerve stimulation an effective treatment for chronic idiopathic anal pain? Dis Colon Rectum. 2009;52(3):456–62. https://doi.org/10.1007/DCR.0b013e31819d1319.
Sokal P, Zieliński P, Harat M. Sacral roots stimulation in chronic pelvic pain. Neurol Neurochir Pol. 2015. https://doi.org/10.1016/j.pjnns.2015.07.003.
Alonso Guardo L, Cano Gala C, Sánchez Poveda D, et al. Caudal neuromodulation with the transforaminal sacral electrode (InterStim®): experience in a pain center regarding 12 implants. Korean J Pain. 2016;29(1):23–8. https://doi.org/10.3344/kjp.2016.29.1.23.
Lavonius M, Suvitie P, Varpe P, Huhtinen H. Sacral neuromodulation: foray into chronic pelvic pain in end stage endometriosis. Case Rep Neurol Med. 2017;2017:1–4. https://doi.org/10.1155/2017/2197831.
Yakovlev AE, Timchenko AA, Parmentier AM. Spinal cord stimulation and sacral nerve stimulation for postlaminectomy syndrome with significant low back pain. Neuromodulation. 2014;17(8):763–5. https://doi.org/10.1111/ner.12144.
Kim YH, Moon DE. Sacral nerve stimulation for the treatment of sacroiliac joint dysfunction: a case report. Neuromodulation. 2010;13(4):306–10. https://doi.org/10.1111/j.1525-1403.2009.00270.x.
Marcelissen T, Van Kerrebroeck P, de Wachter S. Sacral neuromodulation as a treatment for neuropathic clitoral pain after abdominal hysterectomy. Int Urogynecol J. 2010;21(10):1305–7. https://doi.org/10.1007/s00192-010-1145-x.
Lavano A, Volpentesta G, Piragine G, et al. Sacral nerve stimulation with percutaneous dorsal transforamenal approach in treatment of isolated pelvic pain syndromes: transforamenal SNS in pelvic pain syndromes. Neuromodulation. 2006;9(3):229–33. https://doi.org/10.1111/j.1525-1403.2006.00064.x.
Hunter CW, Stovall B, Chen G, Carlson J, Levy R. Anatomy, pathophysiology and interventional therapies for chronic pelvic pain: a review. Pain Physician. 2018;21(2):147–67.
Spinelli M, Sievert K-D. Latest technologic and surgical developments in using InterStim™ therapy for sacral neuromodulation: impact on treatment success and safety. Eur Urol. 2008;54(6):1287–96. https://doi.org/10.1016/j.eururo.2008.01.076.
Elhilali MM, Khaled SM, Kashiwabara T, Elzayat E, Corcos J. Sacral neuromodulation: long-term experience of one center. Urology. 2005;65(6):1114–7. https://doi.org/10.1016/j.urology.2004.12.016.
Nashold BS, Grimes J, Friedman H, Semans J, Avery R. Electrical stimulation of the conus medullaris in the paraplegic. A 5-year review. Appl Neurophysiol. 1977. https://doi.org/10.1159/000102443.
Buffenoir K, Rioult B, Hamel O, Labat J-J, Riant T, Robert R. Spinal cord stimulation of the conus medullaris for refractory pudendal neuralgia: a prospective study of 27 consecutive cases. Neurourol Urodyn. 2015;34(2):177–82. https://doi.org/10.1002/nau.22525.
Hunter CW, Sayed D, Lubenow T, et al. DRG FOCUS: a multicenter study evaluating dorsal root ganglion stimulation and predictors for trial success. Neuromodulation. 2019. https://doi.org/10.1111/ner.12796.
Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158(4):669–81. https://doi.org/10.1097/j.pain.0000000000000814.
Rowland DCL, Wright D, Moir L, FitzGerald JJ, Green AL. Successful treatment of pelvic girdle pain with dorsal root ganglion stimulation. Br J Neurosurg. 2016;30(6):685–6. https://doi.org/10.1080/02688697.2016.1208810.
Schu S, Gulve A, ElDabe S, et al. Spinal cord stimulation of the dorsal root ganglion for groin pain—a retrospective review. Pain Pract. 2015. https://doi.org/10.1111/papr.12194.
Palecek J, Willis WD. The dorsal column pathway facilitates visceromotor responses to colorectal distention after colon inflammation in rats. Pain. 2003;104(3):501–7. https://doi.org/10.1016/s0304-3959(03)00075-7.
Janicki TI. Chronic pelvic pain as a form of complex regional pain syndrome. Clin Obstet Gynecol. 2003;46(4):797–803. https://doi.org/10.1097/00003081-200312000-00009.
Visnjevac O, Costandi S, Patel BA, et al. A comprehensive outcome-specific review of the use of spinal cord stimulation for complex regional pain syndrome. Pain Pract. 2017;17(4):533–45. https://doi.org/10.1111/papr.12513.
Simopoulos T, Yong RJ, Gill JS. Treatment of chronic refractory neuropathic pelvic pain with high-frequency 10-kilohertz spinal cord stimulation. Pain Pract. 2018. https://doi.org/10.1111/papr.12656.
Krames E, Mousad DG. Spinal cord stimulation reverses pain and diarrheal episodes of irritable bowel syndrome: a case report. Neuromodulation. 2004;7(2):82–8. https://doi.org/10.1111/j.1094-7159.2004.04011.x.
Yakovlev AE, Resch BE. Treatment of intractable abdominal pain patient with Bannayan–Riley–Ruvalcaba syndrome using spinal cord stimulation. WMJ. 2009;108(6):323–6.
Kapural L, Narouze SN, Janicki TI, Mekhail N. Spinal cord stimulation is an effective treatment for the chronic intractable visceral pelvic pain. Pain Med. 2006;7(5):440–3. https://doi.org/10.1111/j.1526-4637.2006.00165.x.
Khan YN, Raza SS, Khan EA. Application of spinal cord stimulation for the treatment of abdominal visceral pain syndromes: case reports. Neuromodulation. 2005;8(1):14–27. https://doi.org/10.1111/j.1094-7159.2005.05216.x.
Tiede JM, Ghazi SM, Lamer TJ, Obray JB. The use of spinal cord stimulation in refractory abdominal visceral pain: case reports and literature review. Pain Pract. 2006;6(3):197–202. https://doi.org/10.1111/j.1533-2500.2006.00085.x.
Elias M. Spinal cord stimulation for post-herniorrhaphy pain: SCS for post-herniorrhaphy pain. Neuromodulation. 2000. https://doi.org/10.1046/j.1525-1403.2000.00155.x.
Labat J-J, Riant T, Robert R, Amarenco G, Lefaucheur J-P, Rigaud J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurourol Urodyn. 2008;27(4):306–10. https://doi.org/10.1002/nau.20505.
Peters KM, Killinger KA, Jaeger C, Chen C. Pilot study exploring chronic pudendal neuromodulation as a treatment option for pain associated with pudendal neuralgia: neuromodulation and pudendal neuralgia. Lower Urinary Tract Sympt. 2015. https://doi.org/10.1111/luts.12066.
Carmel M, Lebel M, Tu LM. Pudendal nerve neuromodulation with neurophysiology guidance: a potential treatment option for refractory chronic pelvi-perineal pain. Int Urogynecol J. 2010;21(5):613–6. https://doi.org/10.1007/s00192-009-1054-z.
Armstrong GL, Vancaillie TG. Combined site-specific sacral neuromodulation and pudendal nerve release surgery in a patient with interstitial cystitis and persistent arousal. BMJ Case Rep. 2016. https://doi.org/10.1136/bcr-2015-213513.
Peters KM, Killinger KA, Boguslawski BM, Boura JA. Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms: chronic pudendal neuromodulation. Neurourol Urodyn. 2010;29(7):1267–71. https://doi.org/10.1002/nau.20823.
Govaert B, Melenhorst J, van Kleef M, van Gemert WG, Baeten CG. Sacral neuromodulation for the treatment of chronic functional anorectal pain: a single center experience. Pain Pract. 2010;10(1):49–53. https://doi.org/10.1111/j.1533-2500.2009.00318.x.
Kim JH, Hong JC, Kim MS, Kim SH. Sacral nerve stimulation for treatment of intractable pain associated with cauda equina syndrome. J Korean Neurosurg Soc. 2010;47(6):473–6. https://doi.org/10.3340/jkns.2010.47.6.473.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
Concept and design: DH, AY, RC, MSO, HO, JH, KP, RDS, SM, ADK, VO; statistical analysis: DH, AY, RC, MSO, HO, JH, KP, RDS, SM, ADK, VO; drafting the manuscript: DH, AY, RC, MSO, HO, JH, KP, RDS, SM, ADK, VO; final edits and proofreading: DH, AY, RC, MSO, HO, JH, KP, RDS, SM, ADK, VO.
Disclosures
David Hao nothing to disclose, Alp Yurter nothing to disclose, Robert Chu nothing to disclose, Mariam Salisu-Orhurhu nothing to disclose, Henry Onyeaka nothing to disclose, Jon Hagedorn nothing to disclose, Kiran Patel nothing to disclose, Ryan D'Souza nothing to disclose, Susan Moeschler nothing to disclose, Alan David Kaye nothing to disclose, Vwaire Orhurhu nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hao, D., Yurter, A., Chu, R. et al. Neuromodulation for Management of Chronic Pelvic Pain: A Comprehensive Review. Pain Ther 11, 1137–1177 (2022). https://doi.org/10.1007/s40122-022-00430-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00430-9