Abstract
Introduction
This study was performed to explore the efficacy and safety of pregabalin and gabapentin in patients with spinal cord injury (SCI)-induced neuropathic pain to determine which treatment is most suitable for such patients.
Methods
We searched the PubMed, MEDLINE, Embase, and Cochrane Library databases from database inception to August 31, 2020. The quality of the included studies was assessed. We selected the average pain intensity after treatment and the proportion of patients who discontinued treatment because of adverse effects as the outcome indicators for efficacy and safety, respectively. Statistical analyses were performed using Stata, v16.0, and RevMan, v5.3, software.
Results
We included eight randomized controlled trials that examined four interventions (pregabalin, gabapentin, carbamazepine, and amitriptyline). Based on the average pain intensity after treatment, the efficacy order from highest to lowest was pregabalin, gabapentin, amitriptyline, carbamazepine, and placebo. Based on the proportion of patients who discontinued treatment because of adverse effects, the order from highest to lowest was pregabalin, amitriptyline, carbamazepine, gabapentin, and placebo. In addition, five studies reported the overall incidence of treatment-related adverse effects for two interventions (pregabalin and gabapentin). According to the pooled analysis of these studies, the order for the overall incidence of treatment-related adverse effects from highest to lowest was pregabalin, gabapentin, and placebo.
Conclusions
This study revealed that for patients with SCI-related neuropathic pain, pregabalin was the most effective for relieving pain, whereas gabapentin performed better in aspects associated with drug therapy-related safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain is a common complication after spinal cord injury (SCI). Approximately 70% of patients with SCI are estimated to experience persistent pain, which can be nociceptive, neuropathic, or both [1, 2]. Neuropathic pain remains one of the most complex and challenging pain syndromes to diagnose and treat [3]. Neuropathic pain not only causes significant impacts on physical and emotional functions and quality of life but also weakens or slows the effectiveness of rehabilitation treatments in SCI patients [4, 5].
Currently, drugs remain the primary treatment strategy for patients with SCI-related neuropathic pain. However, the mechanism underlying this type of pain is not yet fully understood, and no effective theoretical bases have been developed to explain existing data regarding the effects of drug treatments on specific pain characteristics. Often patients are not satisfied with the level of pain control achieved by drug treatments, requiring the further exploration of effective treatment options for pain relief [6,7,8].
Anticonvulsants, antidepressants, and antiepileptics are the most commonly used drugs to treat SCI-related neuropathic pain, and existing neuropathic pain guidelines recommend these three categories as first-line treatments [9,10,11,12]. Among these drugs, gabapentin and pregabalin are the most promising [13, 14], which have been shown to be effective for the treatment of neuropathic pain caused by post-herpetic neuralgia and diabetic peripheral neuropathy [15, 16]. In recent years, continuous research and exploration have confirmed that compared with placebo, gabapentin and pregabalin are effective and safe for the treatment of SCI-related neuropathic pain [17,18,19]. However, which of these two drugs is safer and more effective for pain management and the treatment of neuropathic pain remains unclear. Two randomized crossover trials [20, 21] were conducted in 2014 to explore the effectiveness and safety of gabapentin and pregabalin in patients with SCI-related neuropathic pain, which revealed that both drugs were effective and safe for the treatment of pain in such patients, with no significant differences reported between these two drugs. Subsequently, a traditional, head-to-head, meta-analysis and systematic review comparing these two drugs reported the same results and proposed the use of a network meta-analysis as a future research approach [22].
We applied the principle of indirect comparison using a network meta-analysis to pool and analyze all direct and indirect comparative treatments using gabapentin and pregabalin in patients with SCI-related neuropathic pain. The aim of this study was to compare the efficacy and safety of pregabalin and gabapentin in patients with SCI-associated neuropathic pain to determine which of these two treatment options is the most suitable.
Methods
Inclusion and Exclusion Criteria
All included studies met the following criteria: examined patients with SCI-related neuropathic pain, patients were aged ≥ 18 years, pain scores ≥ 3 by using the numerical rating scale (NRS) or visual analog scale (VAS), and the interventions used gabapentin and pregabalin. After a preliminary search of the primary databases, we identified an insufficient number of high-quality, large-scale, randomized controlled trials. Therefore, both randomized controlled trials and observational studies were included in this study. The included studies aimed to explore the pain relief and safety outcomes of the interventions using gabapentin and pregabalin. Only those studies that included a sample size of participants ≥ 10 were included in the present analysis.
The exclusion criteria were as follows: studies that included patients with neuropathic pain caused by reasons other than SCI (such as stroke); studies that included patients with a history of severe allergy, severe complications (e.g., heart, liver, and kidney disease), or pregnant or lactating women; studies that did not include a control group; and case reports.
Outcome Measures
The primary outcome measure was the evaluation of average pain intensity after treatment, as assessed using the VAS, or the pain intensity relief rate after treatment. The secondary outcome measure was the incidence of moderate to severe adverse effects after treatment or the proportion of patients who discontinued treatment because of adverse effects.
Search Strategy
The following keywords and full-text and medical subject heading terms were used to search PubMed, Cochrane Library, Embase, and MEDLINE databases for studies published on or before August 31, 2020: “spinal cord injury,” “anticonvulsant,” “gabapentin,” and “pregabalin.” We searched the references of all identified studies, magazines, journals, and meeting abstracts, and we searched the World Health Organization International Clinical Trials Registry Platform to identify any ongoing or completed trials that had not yet been published. Finally, we searched for any trials that have been included in relevant systematic reviews or meta-analyses published in the past 2–3 years.
Quality Assessment
Inclusion and exclusion criteria were applied to all identified studies by two systematic reviewers, who independently screened the retrieved literature results and read the full articles. The reviewers used the Cochrane Quality Evaluation Method to assess all identified randomized controlled trials for inclusion. Non-randomized controlled trials were evaluated using the Newcastle-Ottawa scale. Differences in the opinion regarding the inclusion or exclusion of any study were discussed by the two reviewers, and a third reviewer was consulted if consensus could not be achieved.
Data Extraction
The following data were extracted from all included studies: author, year of publication, country, study type, ages of patients, intervention types, numbers of intervention groups, detailed information regarding the drug treatment, and the average pain intensity or the pain intensity relief rate after the end of treatment. Continuous variables were obtained as the mean and mean difference (MD), whereas risk ratios (RRs) were obtained for dichotomous variables. In cases of missing data, we contacted the original authors wherever possible.
Statistical Analysis
The χ2 and I2 statistics were used to analyze the heterogeneity of the data collected in our study. All results with values of P > 0.1 and I2 < 20% were considered to have no heterogeneity, and a fixed-effects model was applied; otherwise, a random-effects model was applied. Consistency checks were performed using RevMan software, version 5.3 (The Cochrane Collaboration, Copenhagen, Denmark), and Stata, version 16.0 (StataCorp, College Station, TX, USA).
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Literature Search Process and Results
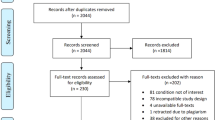
In total, 2919 articles were retrieved from all databases. First, 2251 duplicate articles were removed. Next, 584 articles were removed after the initial screening of the titles and abstracts, which were evaluated according to the inclusion and exclusion criteria. An additional 76 articles were excluded after reading the full texts. Finally, eight randomized controlled trials were included in the network meta-analysis. The screening process is shown in Fig. 1. Among the included studies, three articles were randomized, double-blind, crossover trials [13, 19, 21], three articles were randomized, double-blind, parallel trials [18, 23, 24], one article was a randomized, single-blind, crossover trial [20], and one article was a randomized, double-blind, triple-crossover trial [17]. The study type, intervention protocols, detailed information for each intervention, and the number of intervention groups included in each study are shown in Table 1. The total sample size was 628 individuals and included four interventions: gabapentin, pregabalin, amitriptyline, and carbamazepine.
Quality Assessment
A Cochrane bias risk assessment was performed to evaluate the quality of the eight randomized controlled trials with respect to randomization (allocation concealment), blinding, selective bias, incomplete data, and other biases. For example, if randomization was performed using an appropriate method, the study was classified as having a low risk of bias, whereas if no randomization was performed, the study was classified as having a high risk of bias; if insufficient information regarding the implementation of randomization was provided, the study could not be classified as having either a high or low risk of bias, and these studies were defined as having unclear risk. All other bias-related variables were assessed according to this standard (Fig. 2).
Heterogeneity
We evaluated the average pain intensity after various interventions, as assessed using the VAS (Supplementary Fig. 1a), and identified significant heterogeneity between subgroups (P < 0.1). A random-effect model revealed that compared with pregabalin and placebo, gabapentin treatment was not associated with any significant decrease in average pain intensity after treatment (all P > 0.05). Compared with carbamazepine and placebo, pregabalin significantly reduced the average pain intensity after treatment (all P < 0.05). Amitriptyline was superior to gabapentin in terms of average pain intensity after treatment, and the difference is statistically significant (P = 0.02).
A subgroup analysis was performed to evaluate the incidence of treatment discontinuation due to adverse effects after treatment (Supplementary Fig. 1b), and no heterogeneity was detected (P > 0.1). A fixed-effect model revealed that compared with pregabalin and placebo, gabapentin did not significantly increase the proportion of patients who discontinued treatment because of adverse effects (all P > 0.05). Compared with carbamazepine and placebo, pregabalin did not significantly increase the proportion of patients who discontinued treatment because of adverse effects (all P > 0.05). Compared with amitriptyline, pregabalin did not significantly increase the proportion of patients who discontinued treatment because of adverse effects (P = 0.61).
Network Meta-analysis
Network Chart of Different Interventions
In Fig. 3, a direct network connection between any two intervention groups indicates a direct comparison, whereas no connection indicates a lack of direct comparison. The sizes of the dots in the figure represent the sample sizes. The thickness of each line represents the number of studies. This study included four intervention types: pregabalin, gabapentin, carbamazepine, and amitriptyline.
Analysis of Inconsistency
Both direct and indirect comparative interventions were included in this study. A consistency analysis was performed before merging the interventions, which revealed no significant differences (all P > 0.05), which indicated that the network model had no inconsistencies (Supplementary Fig. 2).
Network Meta-analysis Ranking
To examine the average pain intensity after treatment, we conducted a network meta-analysis of the four different interventions that were included in the analysis (Fig. 4a), which revealed that in patients with SCI-related neuropathic pain, the average reported pain intensities after treatment with pregabalin, gabapentin, carbamazepine, and amitriptyline were lower than that reported for placebo, and the order of pain relief efficacy from best to worst was pregabalin, gabapentin, amitriptyline, carbamazepine, and placebo.
To examine the incidence of treatment discontinuation due to adverse effects after treatment, we conducted a network meta-analysis of the four different interventions (Fig. 4b). The treatments of SCI-related neuropathic pain with pregabalin, gabapentin, amitriptyline, and carbamazepine were all associated with some proportion of patients who discontinued treatment because of drug-related adverse effects, and the proportion order, from highest to lowest, was pregabalin, carbamazepine, amitriptyline, gabapentin, and placebo.
In addition, five studies reported the overall incidence of treatment-related adverse effects for the two treatment options of interest (pregabalin and gabapentin). We performed a pooled analysis of these study results (Supplementary Fig. 3), which revealed that compared with placebo, pregabalin and gabapentin were both associated with an overall incidence of adverse effects, and the order of adverse incidents from highest to lowest was pregabalin, gabapentin, and placebo.
Occurrence of Adverse Effects
We conducted subgroup analyses based on common adverse effects associated with the various interventions. In the groups treated with pregabalin and placebo, the common adverse effects included dry mouth, somnolence, dizziness, edema, and peripheral edema. Compared with placebo, pregabalin significantly increased the incidences of these adverse effects (Table 2, all P < 0.05). In the groups treated with gabapentin and placebo, the common adverse effects included nausea, dizziness, vomiting, edema, and itching. Compared with placebo, gabapentin did not significantly increase the incidences of these adverse effects (Table 3, all P > 0.05). In the groups treated with pregabalin and gabapentin, the common adverse effects included sedation, dizziness, somnolence, and edema, and no significant differences were observed in the incidences of these adverse effects between the two interventions (Table 4).
Discussion
Both gabapentin and pregabalin are derivatives of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) [25, 26]. These drugs bind with presynaptic voltage-gated calcium channels in the posterior horn to reduce the release of excitatory neurotransmitters, such as glutamate and substance P [27, 28]. Current clinical practice guidelines recommend these drugs as first-line treatment options for patients with SCI-related neuropathic pain. However, which of these two interventions is safer and more effective for such patients remains unclear.
To clarify this issue, we applied the principle of indirect comparison using a network meta-analysis to conduct a comprehensive screening of the existing clinical trials that have examined the use of gabapentin and pregabalin for the treatment of SCI-related neuropathic pain. After study retrieval, eight randomized controlled trials were included in the network meta-analysis. The total sample size was 628 and included four interventions. The analysis revealed that in patients with SCI-related neuropathic pain, the average pain intensity after treatment with pregabalin, gabapentin, carbamazepine, or amitriptyline was lower than that following placebo, which demonstrated that compared with placebo, all four interventions were able to effectively alleviate neuropathic pain. The order of pain relief efficacy, from best to the worst, was pregabalin, gabapentin, amitriptyline, carbamazepine, and placebo.
However, based on the analysis of patients who discontinued treatment because of drug-related adverse effects, all four interventions were associated with an increase in the proportion of patients who discontinued treatment because of drug-related adverse effects. The order, from the highest to the lowest proportion of treatment discontinuation, was pregabalin, carbamazepine, amitriptyline, gabapentin, and placebo. In addition, five studies reported the overall incidence of treatment-related adverse effects, and the pooled analysis revealed that compared with placebo, both pregabalin and gabapentin increased the overall incidence of adverse effects, and the order from the highest to the lowest incidence of adverse effects was pregabalin, gabapentin, and placebo.
A subgroup analysis was performed to explore the common adverse effects associated with each intervention, and the common adverse effects caused by pregabalin treatment were dry mouth, somnolence, dizziness, edema, and peripheral edema. Compared with placebo, pregabalin treatment significantly increased the incidences of these adverse effects. Between gabapentin and placebo, the common adverse effects included nausea, dizziness, vomiting, edema, and itching. Compared with placebo, gabapentin did not significantly increase the incidences of these adverse effects. However, compared with the gabapentin, pregabalin had a tendency to increase the rates of adverse effects, such as dizziness, somnolence, and edema, but the differences were not significant, which is similar to the findings reported in previous studies [13, 17, 19, 20, 23]. Moreover, a review [29] showed that gabapentin has risks of abuse and misuse, but previous studies did not consider this potential, and this article also did not consider these. Therefore, we recommend that further studies are necessary to identify risk factors for gabapentin misuse and abuse.
The results of this analysis suggested that although pregabalin was the most effective for the relief of neuropathic pain among SCI patients, the increased incidence of adverse effects associated with pregabalin may reduce patient compliance, reducing the effectiveness of treatment. Although the effectiveness of gabapentin for the relief of SCI-related neuropathic pain was slightly reduced compared with that for pregabalin, the adverse effects associated with gabapentin were significantly reduced compared with those associated with pregabalin. Therefore, considering both aspects of their efficacy and safety, it is still unclear which treatment is most suitable for such patients, and there is a need for comprehensive analyses considering the efficacy and safety of the two interventions. In addition, carbamazepine and amitriptyline were involved in one article respectively in this article. Considering the number of patients was relatively small and both were not our main research aim, we did not carry out further analysis on their efficacy and safety.
Our research also has some limitations. First, the sample sizes of most of the included randomized controlled trials in this study were small, and some studies did not provide sufficient information to determine whether their blinding protocols were performed correctly or whether selective reporting occurred, which may reduce the reliability of the evidence to some extent. Second, the facts of fake studies and the only partial publication of data are not discussed in this article, which may lead to publication bias. Third, the cutoff times for the evaluation of efficacy and adverse events for each intervention differed across studies. For standardization, we only selected a 2-week treatment period as the cutoff time. Whether extending the treatment time might be more effective for relieving pain remains unclear. Therefore, studies that improve upon these limitations are necessary, and further explorations should be performed to obtain more accurate and convincing conclusions that can be used to guide clinical treatment.
Conclusion
This study revealed that in patients with SCI-related neuropathic pain, pregabalin was the most effective treatment for relieving pain, but gabapentin had better drug therapy-related safety characteristics.
References
Cardenas DD, Felix ER. Pain after spinal cord injury: a review of classification, treatment approaches, and treatment assessment. PM R. 2009;1:1077–90.
Felix ER. Chronic neuropathic pain in SCI: evaluation and treatment. Phys Med Rehabil Clin N Am. 2014;25:545–71.
D’Angelo R, Morreale A, Donadio V, Boriani S, Liguori R. Neuropathic pain following spinal cord injury: what we know about mechanisms, assessment and management. Eur Rev Med Pharmacol Sci. 2013;17:3257–61.
Budh CN, Osteraker AL. Life satisfaction in individuals with a spinal cord injury and pain. Clin Rehabil. 2007;21:89–96.
Donnelly C, Eng JJ. Pain following spinal cord injury: the impact on community reintegration. Spinal Cord. 2005;43:278–82.
Murphy D, Reid DB. Pain treatment satisfaction in spinal cord injury. Spinal Cord. 2001;39:44–6.
Siddall PJ, Middleton JW. A proposed algorithm for the management of pain following spinal cord injury. Spinal Cord. 2006;44:67–77.
Vadalouca A, Siafaka I, Argyra E, Vrachnou E, Moka E. Therapeutic management of chronic neuropathic pain: an examination of pharmacologic treatment. Ann N Y Acad Sci. 2006;1088:164–86.
Agarwal N, Joshi M. Effectiveness of amitriptyline and lamotrigine in traumatic spinal cord injury-induced neuropathic pain: a randomized longitudinal comparative study. Spinal Cord. 2017;55:126–30.
Finnerup NB, Sindrup SH, Bach FW, Johannesen IL, Jensen TS. Lamotrigine in spinal cord injury pain: a randomized controlled trial. Pain. 2002;96:375–83.
Norrbrink C, Lundeberg T. Tramadol in neuropathic pain after spinal cord injury: a randomized, double-blind, placebo-controlled trial. Clin J Pain. 2009;25:177–84.
O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122:S22-32.
Levendoglu F, Ogün CO, Ozerbil O, Ogün TC, Ugurlu H. Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine. 2004;29:743–51.
Rekand T, Hagen EM, Gronning M. Chronic pain following spinal cord injury. Tidsskr Nor Laegeforen. 2012;132:974–9.
Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113-e1188.
Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–65.
Rintala DH, Holmes SA, Courtade D, Fiess RN, Tastard LV, Loubser PG. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Arch Phys Med Rehabil. 2007;88:1547–60.
Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006;67:1792–800.
Vranken JH, Dijkgraaf MG, Kruis MR, van der Vegt MH, Hollmann MW, Heesen M. Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain. 2008;136:150–7.
Kaydok E, Levendoglu F, Ozerbil MO, Karahan AY. Comparison of the efficacy of gabapentin and pregabalin for neuropathic pain in patients with spinal cord injury: a crossover study. Acta Med Mediterr. 2014;2014:1343–8.
Yilmaz B, Yasar E, Omac OK, et al. Gabapentin vs pregabalin for the treatment of neuropathic pain in patients with spinal cord injury: a cross-over study. Turk J Phys Med Rehab. 2014;5:239.
Davari M, Amani B, Amani B, Khanijahani A, Akbarzadeh A, Shabestan R. Pregabalin and gabapentin in neuropathic pain management after spinal cord injury: a systematic review and meta-analysis. Korean J Pain. 2020;33:3–12.
Cardenas DD, Nieshoff EC, Suda K, Goto SI, Knapp LE. A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology. 2013;80:533–9.
Yang X, Zhang Y, Yu H, Sun J, He Y. Efficacy of pregabalin in neuropathic pain after spinal cord injury. J Coll Physicians Surg Pak. 2020;30:106–7.
Hagen EM, Rekand T. Management of neuropathic pain associated with spinal cord injury. Pain Ther. 2015;4:51–65.
Tai Q, Kirshblum S, Chen B, Millis S, Johnston M, DeLisa JA. Gabapentin in the treatment of neuropathic pain after spinal cord injury: a prospective, randomized, double-blind, crossover trial. J Spinal Cord Med. 2002;25:100–5.
Taylor CP. The biology and pharmacology of calcium channel α2-δ proteins pfizer satellite symposium to the 2003 Society for Neuroscience Meeting Sheraton New Orleans Hotel New Orleans, LA November 10, 2003. CNS Drug Rev. 2004;10:183.
To TP, Lim TC, Hill ST, et al. Gabapentin for neuropathic pain following spinal cord injury. Spinal Cord. 2002;40:282–5.
Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111:1160–74.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Chen Tong performed the study subject and design, data extraction, statistical analysis, interpretation of data, and manuscript drafting. Zuo Zhengyao contributed to the study design, data extraction, statistical analysis, and manuscript revising. Li Mei contributed to the study design, data extraction, statistical analysis, and interpretation of data. Su Dongpo performed study design, statistical analysis, and critical revision of manuscript. Han Qian extracted the data. Mu Fengqun was involved in critical revision of manuscript.
Disclosures
Chen Tong, Zuo Zhengyao, Li Mei, Su Dongpo, Han Qian, Mu Fengqun have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tong, C., Zhengyao, Z., Mei, L. et al. Pregabalin and Gabapentin in Patients with Spinal Cord Injury-Related Neuropathic Pain: A Network Meta-Analysis. Pain Ther 10, 1497–1509 (2021). https://doi.org/10.1007/s40122-021-00302-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-021-00302-8