Abstract
Introduction
Recent years have seen a dramatic escalation of off-label prescribing for gabapentin and pregabalin (gabapentinoids) owing in part to generic versions of each being released over the past two decades, but also in part as a response to increasing calls for multimodal and non-opioid pain management strategies. In this context, several recent articles have been published alleging widespread misuse, with speculations on the unappreciated addictive potential of the gabapentinoid class of drugs. Reports of a 1% population-level abuse prevalence stem from a single internet survey in the UK, and the vanishingly small adverse event outcomes data do not support such frequency. In this targeted narrative review, we aim to disabuse pain physicians and other clinicians, pharmacists, and policymakers of both the positive and negative myths concerning gabapentinoid medications.
Results
Gabapentinoids inhibit the joint action of voltage-gated calcium channel (VGCC) α2δ subunits in conjunction with the n-methyl-d-aspartate (NMDA) receptor, with subsequent downregulation of VGCC expression and excitatory neurotransmitter release, and possibly synaptogenesis as well, through actions on thrombospondins. These activities reduce the likelihood of central sensitization, which explains in part the efficacy of the gabapentinoids in the management of neuropathic pain. Gabapentinoids also facilitate slow-wave sleep, a relatively rare phenomenon among central nerve system-acting agents, which is also thought to explain some of the therapeutic benefit of the class in conditions such as fibromyalgia. The number needed to treat to see benefit overlaps that of the nonsteroidal anti-inflammatory drugs, but with a considerably improved safety profile. Along these lines, in the context of over 50 million prescriptions per year in the USA alone, the gabapentinoids display remarkably low risk, including risks of misuse, abuse, and dependence. Furthermore, the neurobiology of these agents does not lend plausibility to the allegations, as they have never been shown to elicit dopaminergic activity within the nucleus accumbens, and in addition likely confer a "negative-feedback loop" for habituation and dependence by serving as functional NMDA antagonists, possibly through their actions on thrombospondins. Clinical and epidemiological addictionology studies corroborate the lack of any significant addictive potential of the gabapentinoids, and these drugs are increasingly being used in the treatment of addiction to other substances, with excellent results and no evidence of cross-addiction. However, among individuals with other substance use disorders and, in particular opioid use disorder, there are consistent data showing misuse of gabapentinoids in up to 20% of this population. Although there are allegations of using gabapentinoids to amplify the hedonic effects of opioids, the vast majority of misuse events appear to occur in an attempt to ameliorate opioid withdrawal symptoms. Furthermore, rare but potentially serious respiratory depression may occur, again amplified in the context of opioid or other sedative use. Careful risk:benefit assessment and stratification are warranted when prescription of a gabapentinoid is under consideration, in particular among individuals using opioids.
Conclusions
Gabapentinoids remain a vital tool in the pain physician’s multimodal armamentarium, but these drugs may not be effective in every clinical situation. Individuals with central sensitization and pain associated with slow-wave sleep deficits and potentially persons with comorbid addictions may benefit the most. The gabapentinoids appear to possess no addictive potential on their own, based on laboratory and clinical data, but they may be abused by persons with opioid use disorders; consequently, cautious risk stratification must take place.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gabapentinoids are an important tool in the pain physician’s multimodal armamentarium for the management of many types of pain processes. |
Individuals with central sensitization and pain associated with slow-wave sleep deficits and potentially persons with addictions pain syndromes may benefit the most. |
The gabapentinoids appear to possess no addictive potential themselves, based on laboratory and clinical data. |
Gabapeninoids may be abused by persons with opioid use disorders, and cautious risk stratification must take place. |
Introduction
Chronic neuropathic pain affects a growing segment of the populace, with a prevalence of traditional neuropathic conditions (e.g., diabetic neuropathy, post-herpetic neuralgia, etc.) conservatively estimated at 7–10% [1]. The rubric of central sensitization, a pathologic state wherein nervous system elements display amplified responsiveness and signaling in response to nociceptive or non-nociceptive stimuli [2], has broadened the range (and thus prevalence) of conditions that may be considered neuropathic.
The calcium channel α-2-δ (CCα2δ) subunit ligands gabapentin and pregabalin play an increasingly important role in the post-opioid-centric and multimodal-focused era of pain management, with a primary role in the treatment of neuropathic pain. However, there is a fundamental unanimity among major international clinical practice guidelines in assigning first-line status to the gabapentinoids for the treatment of many of these disorders [3,4,5].
However, these drugs remain highly misunderstood in terms of mechanism of action, indications and, more recently, abuse liability. Few clinicians understand the complex role of CCα2δ in chronic pain states, and many have most likely over-prescribed the drugs [6] under the impression that they will benefit anyone suffering with pain. Conversely, several recent publications written primarily by non-clinicians have called into question both the efficacy and safety of this important drug class, with rather liberal conjecture regarding widespread misuse.
In this review, our aim is to clarify the pharmacology and the highly positive benefit:risk ratio of the gabapentinoids in several difficult clinical scenarios, while acknowledging and supporting the need for appropriate risk stratification and ongoing assessment, as with any therapy.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
The Therapeutic Benefits of Gabapentinoids
The gabapentinoids exhibit a broad spectrum of analgesic benefit, with a wide range of neurobiologic effects and a correspondingly increasing off-label use profile (Table 1), the discussion of which is beyond the scope of this review. Suffice it to say that the literature supports a well-established efficacy of gabapentinoids for traditional neuropathic pain indications, with numerous systematic reviews and meta-analyses demonstrating significant and superior benefit in these states [7, 8]. Gabapentinoids also demonstrate extra-analgesic utility, including anxiolysis [9], amelioration of restless legs syndrome [10], slow-wave sleep enhancement [11], and substance use disorder mitigation [12, 13].
Myth: Universal Utility
Gabapentinoid prescription has tripled in the USA over the past 15 years [6], in part owing to patent expiration of the parent drug, gabapentin. The majority of these prescriptions are written for ‘off-label’ (non-Food and Drug Administration [FDA]-approved) indications, and this phenomenon may be driven by increasing awareness of the need for non-opioid pharmacotherapeutics. Recent criticism of this practice however has highlighted the gabapentinoids’ numbers needed to treat (NNT) to benefit range of 3–8 [7, 8, 14], assuming a cutoff of at least 50% improvement in pain report, which is of course a subjective outcome. It should be noted that this figure overlaps that of (yet with vastly increased safety profile compared to) the nonsteroidal anti-inflammatory drugs (NSAIDs) [15], and is one to two orders of magnitude lower than that needed to see benefit in asthma from treating with long-acting beta-agonists plus inhaled corticosteroids (NNT 73) [16] and in preventing stroke and myocardial infarction by statin treatment (NNT 200–300) [17]. Nonetheless, it is evident that the agents do not benefit everyone, and stratification based upon pathophysiology and symptom profile [14, 18] is logical, as is the prerequisite of an understanding of the mechanism of action of gabapentinoids.
Mechanism: Amelioration of Central Sensitization
Our understanding of neuropathic pain continues to evolve, with various preclinical and clinical investigations elucidating increasingly complex models involving interactions between the peripheral and central nervous systems, neurons and glial cells, and biological and psychosocial dimensions [19,20,21]. The term ‘neuropathic pain’ generally refers to conditions involving direct pathology (e.g., lesion or systemic disease) involving the nervous system, in contradistinction to traditionally conceptualized ‘nociceptive’ or ‘inflammatory’ pain states whereby non-neural tissues, such as bone or viscera, have been implicated as the pain generator. More recently, however, the concept of sensitization (which can occur peripherally or centrally), defined as “increased responsiveness of neurons to their normal input or recruitment of a response to normally subthreshold inputs” [22], has blurred the lines and been invoked in an increasing number of clinical syndromes traditionally thought of as non-neuropathic, such as fibromyalgia, osteoarthritis, and chronic low back pain. Central sensitization (CS) is a maladaptive neural plastic adaptation that essentially uncouples the experience of pain from not only intense noxious peripheral stimuli, but in many cases from any stimulation at all [2].
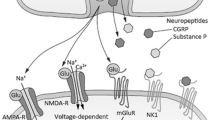
CS arises from a complex series of neuronal, synaptic, and glial changes occurring primarily at the dorsal horn but also at several levels in the brain. Its acute initiation phase appears to be dependent primarily upon the activity of the n-methyl-d-aspartate glutamate receptor (NMDAR) which plays a crucial role in plasticity, potentiation, and learning throughout the central nervous system (CNS). Normally occupied by a magnesium ion, the cationic channel of NMDAR is opened by glutaminergic stimulation to calcium influx (in conjunction with Substance P and calcitonin gene-related peptide, also released by primary afferents), sufficient intracellular accumulation of which leads to a cascade of kinase phosphorylation and translational activities that result in persistence of CS [2].
CCα2δ is primarily an extracellular auxiliary subunit of voltage-gated calcium channels (VGCC) that is expressed throughout the CNS, and this complex among other activities effects excitatory neurotransmitter release (e.g., glutamate and Substance P) via increased intracellular calcium concentration [23]. Following nerve injury CCα2δ is upregulated in afferent fibers in general, and specifically at the terminus/synapse within the dorsal horn [14, 24], where among other activities it participates in increased membrane expression of VGCC. Gabapentinoids have been demonstrated to interfere with the upregulation of CCα2δ and its facilitation of VGCC interaction with presynaptic excitatory neurotransmitter release mechanisms [14, 25].
However, very recent evidence implicates an even more intriguing interaction comprising CCα2δ heteromerization with NMDAR, with resultant regulation of that receptor’s trafficking and expression; gabapentin has also correspondingly been shown to inhibit the activity of this complex [26]. This key finding may explain why gabapentinoids display optimal therapeutic efficacy in CS states [14, 25] given the primacy of the NMDAR in the development of that phenomenon. Gabapentinoids furthermore inhibit descending facilitation while facilitating descending inhibition [14, 25], with both actions serving to decrease sensitization. It has also been suggested that gabapentinoids inhibit excitatory synaptogenesis via inhibition of CCα2δ–thrombospondin activity [27], an astrocyte-driven mechanism that has been proposed to contribute to increased neuronal sensitivity after injury.
Mechanism: Slow-Wave Sleep Enhancement
The development of CS is multifactorial and also multidimensional, with increasing evidence as well as high face value/plausibility of strong behavioral contributors [21]. One such behavioral component that shows important bidirectional relationship with chronic pain is sleep, with disruption of sleep quantity and quality strongly associated with the development of chronic pain in general and in particular CS states such as fibromyalgia [28,29,30].
The mechanisms underlying sleep deprivation-generated or -facilitated pain hypersensitivity are complex and incompletely understood, with multiple dynamic parallel and possibly convergent processes, including glia-mediated neuroinflammation [30], altered hypothalamic–pituitary–adrenal axis (HPA) and cortisol response function [31,32,33], and disrupted reparative mechanisms (including inhibition of growth hormone [hGH] and insulin-like growth factor-1 [IGF-1]) [31]. While most investigations of the detrimental effects of sleep deprivation on physiologic function and pathology (including pain and hypersensitivity, which span both categories) have not incorporated electroencephalographic stratification, emerging evidence supports the primacy of slow-wave sleep (SWS, also known as N3 stage sleep) in physiologic and psychological allostasis, with SWS deprivation specifically reducing hGH and IGF-1 release to a tremendous degree, as these agents are released primarily during these phases of sleep. Furthermore, systemic inflammatory cytokine (e.g., interleukin-1beta and tumor necrosis factor-alfa) expression is increased in SWS deprivation especially, presumably in a homeostatic manner [32], and SWS deprivation also markedly disrupts the normal HPA modulation that occurs during these phases.
Gabapentinoids are fairly unique among pharmacotherapeutic agents in that they specifically facilitate SWS [11], unlike most drugs commonly prescribed for insomnia, and even other anticonvulsant agents, most of which actually reduce SWS quotient. It follows, then, that gabapentinoid-associated improvements in SWS may serve to reduce CS, with considerable speculation that the success of pregabalin in particular in the treatment of fibromyalgia may be related to this phenomenon [34].
The Remarkably Low Risks of Gabapentinoids
The overall safety of the gabapentinoid class of medications, with no known cardiopulmonary nor other end-organ toxicity (outside the context of extreme overdosing), is demonstrated by its considerable therapeutic index and amplified by its lack of hepatic metabolism [35]. The lack of cytochrome P450 inhibition or induction of metabolism of other drugs is a critical and rare feature of the class, in sharp contradistinction to the actions of many other agents including the antidepressants. While these CNS-acting agents certainly confer risks of sedation/somnolence, depression, and suicidality (and in particular via synergistic effect with other CNS-acting agents), they do occupy a strata in line with or less than many commonly used agents, again with antidepressants as a notable comparison. Hard outcomes data from the American Association of Poison Control Centers (AAPCC) from 2000–2014 showed that among psychiatric-related drug overdose deaths, gabapentin resulted in eight deaths (zero from pregabalin) during this 15-year period, compared to 74 deaths from selective serotonin and serotonin/norepinephrine reuptake inhibitors, 115 deaths from benzodiazepines, 115 from atypical antipsychotics, and 261 from tricyclic antidepressants [36].
Myth: Gabapentinoids Carry High Abuse and Addiction Liability
A handful of sensationalized publications however have recently proposed a burgeoning crisis of gabapentinoid abuse and addiction, with support for these allegations primarily comprising data from small polysubstance use populations [37, 38]. At a general population level, attempts to delineate the scope of misuse and abuse have relied primarily upon searches of European national prescription databases for apparent dispensation of drugs in excess of the recommended maximum daily dose, as well as postmortem toxicologic analyses (again, primarily in deceased individuals with numerous other drugs on board) [37]. One article proposes a “potential abuse” rate of between 2 and 8% [39] based upon stratification of prescriptions in a commercially-insured populace by dose, and application of Lorenz curve analysis, which is an economic measure of inequity of distribution of income and goods. It should be noted that congruent skew has been shown for NSAIDs and antibiotics, and obviously may represent heterogeneity of indications among other covariates.
Mechanism: Limited (and Conflicting) Population-Based Epidemiologic Data
Very limited data are currently available on the incidence and prevalence of misuse, abuse, and dependence (which describe very different things) for new psychoactive substances, including the gabapentinoids. Misuse, in its broadest context, is generally defined as any use of the drug outside of the prescribed parameters and includes underuse as well as overuse. Abuse indicates unsanctioned use of the drug for non-intended and usually non-medical purposes, generally recreational. Dependence can be either physical, psychological, or both, and the gabapentinoids may confer physiologic dependence at moderate to high doses and with prolonged application, with a well-known withdrawal profile marked by restlessness/agitation and insomnia and, in some cases, even seizure. When persistent misuse and abuse occur (generally in the context of dependence) in the face of awareness of harms, clinicians generally apply the rubric of addiction.
Incidence of gabapentinoid misuse (defined by the authors as use of higher daily doses than recommended) was recently investigated in the general French population [40], with the authors reporting a 6.6 and 12.8% incidence of gabapentin and pregabalin abuse, respectively, compared to 9.7% for duloxetine which was used as a presumed non-addictive control. It should be noted that the outcome measure of misuse was derived by dividing the total amount of dispensed drug by the duration of treatment interval, which of course cannot account for issues such as loss or hoarding of the drug, or diversion. Within that same population, a disproportionality analysis of the French Pharmacovigilance database found no significant association between exposure to pregabalin and either primary drug abuse or dependence [41].
There are no true population-based prevalence data for misuse or abuse of the gabapentioids. However, the most frequently cited figure among various reports is an approximately 1% rate, which is based solely upon a single 1500-individual online market research survey in the UK [42], with an even greater self-reported abuse rate of baclofen among this sample.
In the USA, the FDA Adverse Events Reporting System (FAERS) showed only 576 cases of gabapentin abuse and 58 cases of pregabalin abuse among reported adverse events over the 5-year period spanning 2012–2016 [43], with the caveat that by the FDA’s admission these reports are unvalidated, possibly duplicated, and are without any mechanism for evaluating association let alone causality. To put these numbers into perspective, over 195 million gabapentin prescriptions and 47 million pregabalin prescriptions were written in the USA during that time period [44].
Dependence data are even more limited, and at this juncture, there are only two published studies among any population sample using a structured face-to-face interview that consider operationalized addiction criteria [45, 46]. The former reported a 12-month prevalence rate of gabapentinoid dependence of 0.25% in a German elderly hospital population, and the latter found point and 24-month prevalence rates of 3 and 7%, respectively, of pregabalin dependence in a German detoxification ward. Of note, all patients identified as abusing or dependent upon gabapentin or pregabalin were dependent on at least one other substance, primarily an opioid.
Mechanism: Inhibition of (CCα2δ-Associated?) Mesolimbic Dopamine Reward
Disproportionate and phasic dopaminergic signal elicitation within the nucleus accumbens (NAc, the terminus of the mesolimbic system) is currently held to comprise the sine qua non of rewarding and addictive drugs or pursuits [47] and forms the cornerstone of modern neurobiology’s mechanistic explanation of how dependence and addiction develops/is maintained. Multiple investigations have corroborated a lack of dopaminergic signal alterations in the NAc with the administration of gabapentin and pregabalin [48,49,50,51,52], or elevation only in experimental groups subjected to chronic painful stimulus via surgical ligation of a spinal nerve. Both drugs have also been shown to decrease or even block opioid-induced NAc dopaminergism [53].
The glutamatergic system is another major neurotransmitter system intricately involved in the multifactorial process of the development of addiction, intertwining with mesolimbic dopaminergism [54]. Not surprisingly, given its pivotal role in neural plasticity, NMDAR activity appears to be important in this process for all drugs of abuse, with a highly complex body of evidence for both NMDAR agonism and antagonism leading to inhibition of self-administration of most abusable substances in animal models, with correlating attenuation of use seen in many clinical studies [55]. In tandem, and again not surprisingly, repetitive use of virtually all drugs of abuse also leads to an upregulation not only of CCα2δ in the mesolimbic system, including the NAc, but also of thrombospondins, which may serve as a (synaptogenic) neurobiologic substrate in the process of reinforcing the pursuit of rewarding substances [56]. As discussed above, the gabapentinoids downregulate expression of all three of these entities (CCα2δ, thrombospondins, and NMDAR), lending further support to the argument that the α2δ-ligands function to prevent the possibility of their own habituation, as well as diminishing reinforcement for the pursuit of other rewarding substances.
Mechanism: (Translational) Addiction Behavior Analysis
In contradistinction to the aforementioned unfounded conjecture regarding addictive potential (and behavior) proliferating primarily among the pharmacy literature, a systematic review of the literature led by one of the authors of this paper (UB) found only four cases of de novo dependence, with only weak evidence of sustained self-administration or other reward pursuit behaviors (“wanting”) and no evidence of social hazards, treatment-seeking, or relapse which also characterize dependence-liable substances [57]. Given that gabapentinoids are meanwhile widely distributed and easily obtainable via the internet or black markets, one would expect many more of these cases if gabapentinoids possessed meaningful addictive power. The limited reward-based (as opposed to co-administered substance withdrawal prophylaxis as described in following text) attractiveness of gabapentinoids among certain populations seems to rest upon their short-term euphoric and sedating effects (“liking”) rather than upon any sustained psychological dependence effects (“wanting”) [57, 58]. At this juncture, there is a lack of clinical studies investigating either the longitudinal course or the severity of gabapentinoid abuse or dependence. Similarly, there is a paucity of reports of people seeking treatment for gabapentinoid abuse or dependence, relapsing behavior, or social hazards involving the use of gabapentinoids. This dearth of evidence corroborates clinical experience suggesting no significant nor sustained addictive power/“wanting”.
Gabapentinoids have been frequently used (off-label) in addiction medicine settings for facilitating detoxification/managing withdrawal symptoms, as well as for chronic conditions, i.e., relapse-prevention efforts, for numerous drugs of abuse, including opioids, cannabis, and alcohol, the latter of which represents the arena of most study [12, 13, 58]. These (and other anticonvulsants) carry substantial advantage over the tradition-rich benzodiazepines in terms of increased safety/no interactions with alcohol, but also in terms of no evident cross-substance dependence and transfer of addiction [35, 57]. One study applied the 49-question version of the Addiction Research Center Inventory (ARCI) in the assessment of abuse potential of gabapentin in alcoholics. The ARCI is the most well-studied and validated instrument used to compare abuse liability of a substance to five standard drug groups (Morphine-Benzadrine, Pentobarbitol-Chlorpromazine-Alcohol, LSD, Benzadrine, and Amphetamine). The investigators found no difference between gabapentin and placebo among all the various axes of subjective effect [58].
Conclusion-Benefit:Risk Stratification, or Patient Selection
Having said that, there does appear to be one patient population with increased risk of gabapentinoid misuse. A variety of data sources and publications suggest that opioid abusers are more likely to misuse and abuse the gabapentinoids [57]. While (disproportionately publicized) self-report from some of these individuals allege concomitant administration to potentiate an altered state of consciousness or euphoria, more intellectually and methodologically rigorous studies generally favor the theory that opioid abusers self-administer α2δ ligands in an attempt to decrease withdrawal symptoms and, conversely, also to ameliorate (likely NMDAR-mediated) tolerance to their drug of choice [13, 52].
The situation is likely much more complex, and possibly even reciprocal. Ample preclinical evidence exists that NMDAR blockade seems to increase opioid pursuit/use under certain circumstances that seem to indicate that this antagonism may elicit a compensatory increase in opioid use to offset reduced reward and reinforcement [54]. As such, it is possible that in the opioid-dependent populace, α2δ ligands, which serve as functional NMDA antagonists (by reducing trafficking and expression of the CCα2δ–NMDAR heteromer) may mitigate undesirable tolerance and withdrawal phenomena while simultaneously driving escalating opioid use. In addition, the rare but potentially serious risk of hypoventilation seen in some animal models and also in some perioperative clinical studies [59] is, as expected, magnified in the context of co-administration of sedatives or opioids.
Regardless of the ultimate mechanism(s) involved, it appears that opioid abusers comprise a high-risk group for gabapentinoid misuse, and as with any prescribed drug or therapy, appropriate risk stratification along with ongoing monitoring is advisable when the treating physician is considering treating this group with gabapentinoids for any reason.
Change history
04 December 2020
The original article can be found online.
References
van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155:654–62.
Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926.
Attal N, Cruccu G, Baron R, et al. European Federation of Neurological Societies. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113–88. https://doi.org/10.1111/j.1468-1331.2010.02999.x.
Centre for Clinical Practice at NICE (UK). Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. London: National Institute for Health and Care Excellence. 2013. https://www.ncbi.nlm.nih.gov/books/NBK266257/.
Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19:328–35.
Goodman CW, Brett AS. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern Med. 2019;179:695–701.
Wiffen PJ, Derry S, Bell RF, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017. https://doi.org/10.1002/14651858.CD007938.pub4.
Derry S, Bell RF, Straube S, et al. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019. https://doi.org/10.1002/14651858.CD007076.pub.
Chen X, Broeyer F, de Kam M, Baas J, Cohen A, van Gerven J. Pharmacodynamic response profiles of anxiolytic and sedative drugs. Br J Clin Pharmacol. 2017;83:1028–38.
Wanner V, Garcia Malo C, Romero S, Cano-Pumarega I, García-Borreguero D. Non-dopaminergic vs. dopaminergic treatment options in restless legs syndrome. Adv Pharmacol. 2019;84:187–205.
Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70:197–245.
Ahmed S, Bachu R, Kotapati P, et al. Use of gabapentin in the treatment of substance use and psychiatric disorders: a systematic review. Front Psychiatry. 2019;10:228. https://doi.org/10.3389/fpsyt.2019.00228.
Freynhagen R, Backonja M, Schug S, et al. Pregabalin for the treatment of drug and alcohol withdrawal symptoms: a comprehensive review. CNS Drugs. 2016;30:1191–200.
Patel R, Dickenson AH. Mechanisms of the gabapentinoids and α 2 δ-1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect. 2016;4(2):e00205. https://doi.org/10.1002/prp2.205.
Ong CK, Lirk P, Tan CH, Seymour RA. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin Med Res. 2007;5:19–34.
Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010. https://doi.org/10.1002/14651858.CD005533.pub2.
Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US preventive services task force. JAMA. 2016;316:2008–244.
Baron R, Maier C, Attal N, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017;158:261–72.
Meacham K, Shepherd A, Mohapatra DP, Haroutounian S. Neuropathic pain: central vs peripheral mechanisms. Curr Pain Headache Rep. 2017;21(6):28. https://doi.org/10.1007/s11916-017-0629-5.Review.
Tozaki-Saitoh H, Tsuda M. Microglia-neuron interactions in the models of neuropathic pain. Biochem Pharmacol. 2019;169:113614. https://doi.org/10.1016/j.bcp.2019.08.016.
Eller-Smith OC, Nicol AL, Christianson JA. Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Front Cell Neurosci. 2018;12:35. https://doi.org/10.3389/fncel.2018.00035.
Loeser JD, Treede RD. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137:473–7.
Hoppa MB, Lana B, Margas W, Dolphin AC, Ryan TA. α2δ expression sets presynaptic calcium channel abundance and release probability. Nature. 2012;486(7401):122–5. https://doi.org/10.1038/nature11033.
Dolphin AC. Voltage-gated calcium channel α (2)δ subunits: an assessment of proposed novel roles. F1000Research. 2018;7:F1000 Faculty Rev-1830. https://doi.org/10.12688/f1000research.16104.1.
Tuchman M, Barrett JA, Donevan S, Hedberg TG, Taylor CP. Central sensitization and Ca(V)α2δ ligands in chronic pain syndromes: pathologic processes and pharmacologic effect. J Pain. 2010;11:1241–9.
Chen J, Li L, Chen SR, et al. The α2δ-1-NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep. 2018;22:2307–21.
Eroglu C, Allen NJ, Susman MW, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–92.
Simpson NS, Scott-Sutherland J, Gautam S, Sethna N, Haack M. Chronic exposure to insufficient sleep alters processes of pain habituation and sensitization. Pain. 2018;159:33–40.
Choy EH. The role of sleep in pain and fibromyalgia. Nat Rev Rheumatol. 2015;11:513–20.
Nijs J, Loggia ML, Polli A, et al. Sleep disturbances and severe stress as glial activators: key targets for treating central sensitization in chronic pain patients? Expert Opin Ther Targets. 2017;21:817–26.
Spaeth M, Rizzi M, Sarzi-Puttini P. Fibromyalgia and sleep. Best Pract Res Clin Rheumatol. 2011;25:227–39.
Goodin BR, Smith MT, Quinn NB, King CD, McGuire L. Poor sleep quality and exaggerated salivary cortisol reactivity to the cold pressor task predict greater acute pain severity in a non-clinical sample. Biol Psychol. 2012;91:36–41.
Irwin MR, Opp MR. Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology. 2017;42:129–55.
Roth T, Lankford DA, Bhadra P, Whalen E, Resnick EM. Effect of pregabalin on sleep in patients with fibromyalgia and sleep maintenance disturbance: a randomized, placebo-controlled, 2-way crossover polysomnography study. Arthritis Care Res (Hoboken). 2012;64:597–606.
Calandre EP, Rico-Villademoros F, Slim M. Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: a review of their clinical pharmacology and therapeutic use. Expert Rev Neurother. 2016;16:1263–77.
Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to US poison control centers. Am J Psychiatry. 2017;174:438–50.
Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77:403–26.
Mersfelder TL, Nichols WH. Gabapentin: abuse, dependence, and withdrawal. Ann Pharmacother. 2016;50:229–33.
Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Investig. 2017;37:763–73.
Driot D, Jouanjus E, Oustric S, Dupouy J, Lapeyre-Mestre M. Patterns of gabapentin and pregabalin use and misuse: results of a population-based cohort study in France. Br J Clin Pharmacol. 2019;85:1260–9.
Bossard JB, Ponté C, Dupouy J, Lapeyre-Mestre M, Jouanjus E. Disproportionality analysis for the assessment of abuse and dependence potential of pregabalin in the french pharmacovigilance database. Clin Drug Investig. 2016;36:735–42.
Kapil V, Green JL, Le Lait MC, et al. Misuse of the γ-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2014;78:190–1.
Evoy KE, Covvey JR, Peckham AM, et al. Reports of gabapentin and pregabalin abuse, misuse, dependence, or overdose: an analysis of the Food And Drug Administration Adverse Events Reporting System (FAERS). Res Social Adm Pharm. 2019;15:953–8.
Agency for Healthcare Research and Quality (AHRQ). Medical Expenditure Panel Survey (MEPS) 2007–2017. Rockville: Agency for Healthcare Research and Quality (AHRQ). 2020. https://meps.ahrq.gov/data_stats/download_data_files.jsp. Accessed 23 Jan 2020.
Cossmann JC, Scherbaum N, Bonnet U. Substance addiction in old age: a cross-sectional study in a German hospital. GeroPsych. 2016;29:17–27.
Snellgrove BJ, Steinert T, Jaeger S. Pregabalin use among users of illicit drugs: a cross-sectional survey in Southern Germany. CNS Drugs. 2017;31:891–8.
Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162:712–25.
Xie JY, Qu C, Patwardhan A, et al. Activation of mesocorticolimbic reward circuits for assessment of relief of ongoing pain: a potential biomarker of efficacy. Pain. 2014;155:1659–66.
Bannister K, Qu C, Navratilova E, et al. Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. Pain. 2017;158:2386–95.
Asaoka Y, Kato T, Ide S, et al. Pregabalin induces conditioned place preference in the rat during the early, but not late, stage of neuropathic pain. Neurosci Lett. 2018;668:133–7.
Coutens B, Mouledous L, Stella M, et al. Lack of correlation between the activity of the mesolimbic dopaminergic system and the rewarding properties of pregabalin in mouse. Psychopharmacology. 2019;236:2069–82.
Vashchinkina E, Piippo O, Vekovischeva O, et al. Addiction-related interactions of pregabalin with morphine in mice and humans: reinforcing and inhibiting effects. Addict Biol. 2018;23:945–58.
Andrews N, Loomis S, Blake R, et al. Effect of gabapentin-like compounds on development and maintenance of morphine-induced conditioned place preference. Psychopharmacology. 2001;157:381–7.
D'Souza MS. Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci. 2015;9:404. https://doi.org/10.3389/fnins.2015.00404.
Hopf FW. Do specific NMDA receptor subunits act as gateways for addictive behaviors? Genes Brain Behav. 2017;16:118–38.
Spencer S, Brown RM, Quintero GC, et al. α2δ-1 signaling in nucleus accumbens is necessary for cocaine-induced relapse. J Neurosci. 2014;34:8605–11.
Bonnet U, Scherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27:1185–215.
Mason BJ, Light JM, Williams LD, Drobes DJ. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addict Biol. 2009;14:73–83.
United States Food and Drug Administration. FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR) when used with CNS depressants or in patients with lung problems. 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-serious-breathing-problems-seizure-and-nerve-pain-medicines-gabapentin-neurontin. Accessed 10 Jul 2020.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Heath McAnally and Udo Bonnet have nothing to disclose. Alan D. Kaye is a member of the journal’s Editorial Board.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12668192.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
McAnally, H., Bonnet, U. & Kaye, A.D. Gabapentinoid Benefit and Risk Stratification: Mechanisms Over Myth. Pain Ther 9, 441–452 (2020). https://doi.org/10.1007/s40122-020-00189-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-020-00189-x