Abstract
Introduction
International guidelines recommend definitive combination antibiotic therapy for the management of serious infections involving carbapenem-resistant Acinetobacter (CRAB) species. The commonly available combination options include high-dose sulbactam, polymyxins, tetracyclines, and cefiderocol. Scanty prospective data exist to support this approach.
Methods
Patients with CRAB bacteraemia, ventilator-associated pneumonia (VAP), or both were categorized based on whether they received combination therapy or monotherapy. The 30-day mortality was compared between the two groups. Inverse probability treatment weighting (IPTW) was done using propensity score (PS) for a balanced comparison between groups.
Results
Between January 2021 and May 2023, of the 161 patients with CRAB bacteraemia (n = 55, 34.2%), VAP (n = 46, 28.6%), or both (n = 60, 37.3%) who received appropriate intravenous antibiotic therapy, 70% (112/161) received monotherapy, and the rest received combination therapy. The overall 30-day mortality was 62% (99/161) and not different (p = 0.76) between the combination therapy (31/49, 63.3%) and monotherapy (68/112, 60.7%) groups. The propensity score matching using IPTW did not show a statistical difference (p = 0.47) in 30-day mortality for receiving combination therapy with an adjusted odds ratio (OR) P of 1.29 (0.64, 2.58).
Conclusion
Combination therapy for CRAB infections needs further study in a randomised controlled trial, as this observational study showed no difference in 30-day mortality between monotherapy and combination therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The combination therapy approach to managing carbapenem-resistant Acinetobacter (CRAB) infections, as international guidelines recommend, lacks robust prospective data. |
Our study evaluated the efficacy of combination therapy and monotherapy in treating CRAB bacteraemia or pneumonia using inverse probability treatment weighting (IPTW) adjustments. |
What was learned from the study? |
The 30-day overall mortality after IPTW adjustments was not different among those receiving combination therapy vs monotherapy. |
The receipt of combination therapy was not associated with improved adjusted 14-day mortality (odds ratio [OR] 2.01(0.67–5.99), p = 0.207). |
Routine use of combination therapy for serious CRAB infections in areas with high sulbactam, minocycline, and tigecycline resistance rates may not be effective and needs evaluation in a clinical trial. |
Introduction
Carbapenem-resistant Acinetobacter species infections have limited therapeutic options. Recent Infectious Diseases Society of America (IDSA) guidelines highlight the lack of a “standard of care” for the treatment of these infections and emphasise the urgent need for comparative data on commonly used treatments and strategies [1]. Both IDSA and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) recognise sulbactam as a key agent for the treatment of CRAB infections [1, 2]. However, the susceptibility cut-offs for sulbactam are not clearly defined and the minimum inhibitory concentrations (MICs) are often high in many areas of the world [3]. Polymyxins, despite their wide availability and high rates of in vitro susceptibility, are clearly associated with poor outcomes [4, 5]. Tigecycline is seldom considered a standalone therapy for serious CRAB infections [6, 7]. The clinical experience with eravacycline for CRAB infections is still emerging. Cefiderocol and sulbactam durlobactam are often not available in areas where CRAB infections are common.
Given such limitations and pending further data, the IDSA guidelines suggest consideration of combination therapy consisting of two active agents with high-dose sulbactam as a core component [1]. The second agent could be tetracyclines, polymyxins, or cefiderocol, regardless of sulbactam susceptibility. ESCMID also supports the use of combination therapy with any of the above agents in the treatment of such infections [2]. As the guidelines highlight, scanty prospective real-world data exist for these approaches. We report outcomes within a prospective cohort of CRAB bacteraemia and pneumonia patients for polymyxin monotherapy and combination therapy with the above agents.
Methods
Study Design
This study evaluated the efficacy of combination therapy and monotherapy in treating CRAB bacteraemia or pneumonia on a large ongoing prospective cohort of patients admitted to Christian Medical College, Vellore, India. Adult patients (age > 15 years) with laboratory-confirmed CRAB bacteraemia or pneumonia, from January 2021 to May 2023, receiving appropriate antibiotic therapy, were included in the study. The screening for CRAB pneumonia was started after the bacteraemia cohort and included patients from May 2022 to May 2023.
The study designated the date when the first positive culture sample was obtained as the “index date”. Each patient was included only once in the study. All the relevant data of the patients were recorded from the medical charts and electronic platform available at the institution onto a standardised case report form (CRF). CRF included demographic details such as age and gender, information regarding previous antibiotic usage within the 30 days preceding the index date, any previous or concurrent infections, and known comorbidities summarised using the Charlson Comorbidity Index. In addition, clinical details like the presence of septic shock, neutropenia, existing malignancies, renal injury, need for dialysis, duration of hospital stay, type of antibiotic therapy, and any delays in receiving appropriate treatment were also recorded. Mortality risk scores like Pitt bacteraemia score (PBS) and increment score (ICS) were calculated for all the CRAB bacteraemia cases in the study. The primary clinical team was responsible for all management decisions of the patients, including the selection of antibiotics and implementation of source control measures. Though our institutional antibiotic guideline prefers polymyxin B for the management of CRAB pneumonia, prescribers can still tailor the therapy accounting for severity, duration of illness, concurrent bacteraemia, and polymyxin toxicity, allowing them to choose between any monotherapy and combination therapy. The primary treatment of CRAB in the Indian setting is polymyxin-based therapy, as access to sulbactam and susceptibility testing is not available. The dosages of these drugs are based on a uniform hospital antibiotic policy. Our regular dose used in the study is polymyxin B 15 lakh unit loading dose followed by 7.5 lakh units twice daily and colistin-9MU loading dose followed by 3MU IV thrice daily, with colistin renal adjusted whenever indicated. Similarly, 9 to 12 g of sulbactam was used as part of combination therapy. Both tigecycline and minocycline were used with a 200 mg loading dose followed by 100 mg twice daily. The primary study outcome was the assessment of all-cause mortality at day 30. The patients were followed up at day 30, either in person or via telephone to assess the primary outcome.
Definitions
As per the Clinical Laboratory and Standards Institute (CLSI) guidelines, resistance to either meropenem or imipenem was identified as carbapenem resistance. Colistin and sulbactam MIC were done by microbroth dilution, while tigecycline and minocycline were done by either E test or microbroth dilution. Sulbactam breakpoint for non-susceptibility was considered to be > 8 mg/l by CLSI guidance for Acinetobacter baumannii based on ampicillin sulbactam combination [8]. Intermediate susceptibility for colistin, tigecycline, minocycline, and sulbactam was considered susceptible for analysis. VAP was confirmed by a two-step process. The research nurse visited all the ICUs daily to identify patients with potential VAP. An infectious diseases consultant reviewed these patient details based on clinical, radiological, and microbiological findings in a patient intubated for > 48 h. There should be a discrete clinical syndrome of sustained respiratory worsening with new or worsening infiltrate on chest imaging and microbiological culture isolating organisms with CFUs > 105 with validating smear to be included in the study. We intentionally used specific criteria to confirm the diagnosis to reduce the misdiagnosis. Previous serious bacterial infection was defined as a severe bacterial infection, such as pneumonia, bacteraemia, or meningitis, isolated within a span of 30 days prior to the initial presentation. A delay in appropriate antibiotic therapy was identified as a failure to start treatment using antibiotics, to which the pathogen has no documented resistance, within 24 h following the collection of a positive blood sample.
Statistical Analysis
Patients were stratified as combination therapy and monotherapy groups for analysis based on the treatment received from the index date to the 30-day follow-up period. All clinical and microbiology data were compared between treatment groups. Baseline characteristics and clinical events were presented as frequencies with proportions for categorical variables and mean with standard deviation (SD) or median with interquartile range (IQR) for continuous variables. Statistical comparisons for continuous variables were done using Student’s t test and Mann-Whitney U test, while Pearson’s chi-square test or Fisher’s exact test was applied to categorical variables. All estimates were presented with 95% confidence intervals (CI).
To adjust for baseline differences between the treatment groups, we estimated propensity scores (PS) for all patients in the study. The PS for each patient was calculated as a probability from a logistic regression model with treatment groups as the dependent variable and all clinically relevant characteristics that can affect the treatment selection and outcome as independent variables. We used inverse probability of treatment weighting (IPTW) based on PS to balance baseline differences between the two treatment groups after comparing other matching techniques (see Table S2a and S2b). Variables in the model were well balanced after IPTW adjustment with no standardised differences > 0.1. The distribution of standardised differences before and after matching was plotted to visually diagnose balancing after IPTW (see Figure S3a and S3b). Univariate analysis was performed using logistic regression analysis for each variable with 30-day all-cause mortality as the outcome. Odds ratio (OR) with 95% CI was calculated. Estimates comparing treatment groups were adjusted using IPTW. All variables that were significantly different in the univariate analysis were added to the multivariate model. A multivariate logistic regression analysis was done for the primary and secondary outcomes, including the treatment groups, to estimate the adjusted odds ratio. We used STATA 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC) for statistical analysis and visualisations (Fig. 1).
The study was conducted in accordance with the 1964 Helsinki Declaration and its later amendments. The ethical approval for the study was obtained from the Institutional Review Board and ethics committee of Christian Medical College, Vellore, Tamil Nadu, India (IRB minute nos. 14134 and 14427). Informed consent was obtained from each patient or their family members for access to clinical data and publication prior to enrolment.
Results
Demography
Between January 2021 and May 2023, we identified 164 patients admitted to our hospital with CRAB bacteraemia or VAP who received appropriate intravenous antibiotic therapy. Three patients who were discharged before 30 days could not be recontacted to assess primary outcome and hence were excluded from the analysis. Seventy per cent of included patients received monotherapy (112/161), and the rest received combination therapy. Among our patients, 46 (28.57%) had ventilator-associated pneumonia (VAP) and 55 (34.16%) had bacteraemia; 60 (37.27%) had both. Among the 112 patients who received monotherapy, 25 were treated with colistin and 73 received polymyxin B. Both polymyxins were given interchangeably for 14 patients.
Baseline Characteristics
Baseline characteristics were comparable across the two cohorts (Table 1). The mean age of the cohort was 46.14 (SD 16.24) years with male predominance (114/161, 71%). Neutropenia was present in 9% (14/161) of the study participants, and most received monotherapy (11 vs 3). The presence of existing malignancies was noted among 21 patients with no significant difference between the treatment groups. The median Charlson comorbidity index score observed in the study cohort was 2 (IQR 0–3) and was similar in the two treatment groups. The median duration of antibiotic therapy in our cohort was 6 (IQR 3–9) days with a significant difference between the monotherapy and combination therapy group (5 vs 6, p = 0.046). Previous serious bacterial infection was present in 84 (52.17%) patients. A significant proportion had previous carbapenem exposure (127, 81.41%). Ninety-one (56.52) patients had other concomitant bacterial infections. Septic shock was present in 129 (80.12%) patients and 26 (16.15%) required dialysis. There was a delay in initiating antibiotics (> 24 h from index culture) in 30.6% of combination therapy patients and 42.9% in the monotherapy group (p = 0.143).
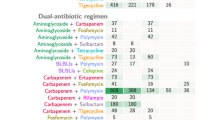
Our cohort consisted of severely ill patients with 97% admitted to an ICU during their hospital stay. All patients (n = 49) in the combination therapy group required ICU stay compared to 95.5% (107/112) in the monotherapy group. PBS and ICS observed were higher with a median PBS of 6 (IQR 4–6) among bacteraemias, similar in the two treatment groups. The mean ICS among the bacteraemias was 11.53 (SD 3.94) and was significantly higher in the combination therapy group (12.77 vs 11.06, p = 0.039). Persistent bacteraemia was noted among 31% (15/49) receiving combination therapy and 12% (13/112) in the monotherapy group. Among the patients who received combination therapy, 38.8% received polymyxin with sulbactam, 26.5% received polymyxin with tigecycline, 22.4% received polymyxin with others, 6.1% received tigecycline with sulbactam, and 4.1% received polymyxin with minocycline. The primary outcome analysed for different combinations did not display any statistical difference between various drug combinations and against monotherapy.
Antimicrobial Susceptibility
The susceptibility for colistin, minocycline, tigecycline, and sulbactam was 156/157 (99.36%), 29/52 (55.76%), 61/94 (64.9%), and 3/50 (6%), respectively. Among patients who received monotherapy, the susceptibility for colistin was 100% while susceptibility for the other drugs using minocycline, tigecycline, and sulbactam was 14/31 (45%), 37/59 (62.7%), and 1/29 (3.4%), respectively. The predominant combination therapies used were polymyxin with sulbactam (19/49, 38.8%) and polymyxin with tigecycline (13/49, 26.5%). Among the patients receiving polymyxin and sulbactam combination, 11/19 had susceptibility reports available for both. Only one isolate was susceptible to both. Among patients who received polymyxin and tigecycline combination, 9/13 had susceptibility reports for both, of which 8/13 patients were susceptible to both.
Forty-three per cent of our patients (21/49) in the combination therapy group received a combination with both drugs susceptible. Among them, 71.4% (15/21) had primary outcome, and 12 succumbed to death within 14 days of the index date. Among people who received a therapy where the combination drug was resistant, 42.8% (6/14) had the primary outcome (p = 0.47). Notably, five patients received sulbactam along with polymyxin, where sulbactam was resistant (MIC > 8), and one patient received tigecycline with colistin, where tigecycline was resistant.
Primary Outcome
The overall 30-day mortality was 62% (99/161), where 63.27% (31/49) and 60.71% (68/112) were recorded in the combination therapy and monotherapy group, respectively. Unadjusted OR for primary outcome among people receiving combination therapy was 1.11 (0.55, 2.22), p = 0.760. The propensity score matching using IPTW did not show any statistical difference in 30-day mortality for receiving combination therapy with an adjusted OR of 1.29 (0.64, 2.58), p = 0.469.
Secondary Outcomes
Among the 99 deaths in our cohort, 87 (87.8%) happened by day 14. The receipt of combination therapy was not associated with improved adjusted 14-day mortality [OR 2.01 (0.67–5.99), p = 0.207].
The other secondary outcomes of interest were the duration of total hospital stay and hospital stay after infection onset. Duration of total hospital stay was significantly higher in the combination therapy group (29 vs 15), and the same was noticed with days of hospital stay after bacteraemia or pneumonia isolation (13 vs 7.5) (Table 2). Increasing age, patients having both bacteraemia and VAP, and septic shock were associated with poorer outcomes, while increased duration of antibiotic therapy was associated with better outcomes in univariate analysis. In multivariate analysis, patients having bacteraemia or both bacteraemia and VAP and septic shock were independent risk factors for mortality (See Table S6).
Discussion
Our study provides real-world outcome data on combination strategies from an area with high rates of sulbactam non-susceptibility. Globally, over half of A. baumannii isolates are carbapenem resistant [3]. Few treatment options exist for these infections, with mortality rates reaching up to 60%. In view of poor clinical outcomes, most guidelines suggest combination therapies for serious CRAB infections. However, multiple RCTs evaluating various combination strategies including both in vitro susceptible and resistant agents have failed [9,10,11,12,13].
In most areas of the developing world, with a significant number of CRAB infections, polymyxins remain the practical first-line treatment option. Non-availability of novel agents like cefiderocol or sulbactam durlobactam and high rates of sulbactam resistance are the important reasons. In this context, our results are particularly important. In this situation, the potential combination options include high-dose sulbactam, minocycline, or tigecycline. Even after adjusting for various clinically relevant factors, combination approaches with any of the above agents were not associated with improved outcomes in our cohort (Table 3).
Conflicting data exist in the literature regarding combination strategies for treating CRAB infections. A large network meta-analysis including 18 studies (7 RCTs and 11 observational studies) and 1835 patients with CRAB infections recently evaluated the efficacy of high-dose sulbactam versus polymyxin-based combinations [14]. No treatment regimen was associated with improved all-cause mortality. Colistin with sulbactam or carbapenem was associated with lesser nephrotoxicity in relation to other regimens. Clinical cure and improvement were better with high-dose sulbactam with carbapenem or levofloxacin compared to colistin alone (RR 2.95, 95% CI 1.03–8.40) or colistin in combination with high-dose sulbactam (RR 2.30, 95% CI 0.79–6.66). However, these findings need a cautious interpretation in view of the limited number of patients in the sulbactam group.
A second network meta-analysis, utilising the Bayesian approach, reported on 2118 adult patients with Acinetobacter spp. pneumonia from 23 studies [15]. The meta-analysis included patients with MDR Acinetobacter spp. in general and did not prespecify carbapenem-resistant isolates alone. The overwhelming majority of patients included in the analysis received colistin alone or in combination with carbapenems. Sulbactam alone was superior to colistin monotherapy but not to high-dose sulbactam therapy. The authors attributed these contradictory findings to probable high rates of sulbactam resistance in the included studies. A third meta-analysis including 29 studies with 2529 patients also reported no difference in mortality rates between the various treatments [16].
Sulbactam susceptibility cannot be assumed in many areas with CRAB infections globally. The current CLSI susceptibility cut-off for sulbactam is 4 mg/l, based on ampicillin-sulbactam at maximum approved doses of 3 g (2 g ampicillin and 1 g sulbactam) every 6 h [8]. Among a global collection of 1722 Acinetobacter isolates, only about half were susceptible [3]. Among carbapenem-resistant isolates, the proportion was close to 90%. Even after increasing the dose (3 g every 8 hourly) and infusion time (4 h), PK/PD targets may not be achieved in more than half the patients with sulbactam MICs ≥ 32 mg/l [17, 18]. This will be 88% of our testing isolates. The clinical experience with sulbactam among patients with such high MICs is also limited. Importantly, the susceptibilities for minocycline and tigecycline are also not high in many areas with high CRAB rates.
Our study has two potential implications. Within the limitations of the study design, in areas with high rates of partner drug resistance, “Combination therapy in CRAB requires further evaluation in randomized controlled trials, as the available trials show no benefit and the current data for potential benefit is from uncontrolled and often unadjusted observational data.” Also, when evaluating novel agents like sulbactam durlobactam, the standard of care antibiotic needs to be decided based on local resistance patterns. Polymyxins probably should be studied as a standard of care in areas with high sulbactam MICs. Novel approaches like the Personalised RAndomised Controlled Trial (PRACTical) design can accommodate such different standards of care within a single trial [19].
Limitations
Our study has several limitations. Observational design, despite the efforts for matching, may be misleading in evaluating interventions. The sample size in our study is relatively small. The timing and use of specific combination agents were not predetermined, which can lead to bias. Our index date for outcome analysis was the date when the positive sample was collected and not the initiation of therapy.
Conclusions
In summary, we suggest caution in the routine use of combination therapy for serious CRAB infections in areas with high rates of sulbactam, minocycline, and tigecycline resistance.
Data Availability
The data sets used during the current study are available from the corresponding author upon reasonable request.
References
Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America 2023 guidance on the treatment of antimicrobial resistant gram-negative infections. Clin Infect Dis Off Publ Infect Dis Soc Am. 2023;ciad428.
Paul M, Carrara E, Retamar P, Tängdén T, Bitterman R, Bonomo RA, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2022;28(4):521–47.
McLeod SM, Moussa SH, Hackel MA, Miller AA. In vitro activity of sulbactam-durlobactam against Acinetobacter baumannii-calcoaceticus complex isolates collected globally in 2016 and 2017. Antimicrob Agents Chemother. 2020;64(4):e02534-e2619.
Oliveira MS, Prado GVB, Costa SF, Grinbaum RS, Levin AS. Ampicillin/sulbactam compared with polymyxins for the treatment of infections caused by carbapenem-resistant Acinetobacter spp. J Antimicrob Chemother. 2008;61(6):1369–75.
Piperaki ET, Tzouvelekis LS, Miriagou V, Daikos GL. Carbapenem-resistant Acinetobacter baumannii: in pursuit of an effective treatment. Clin Microbiol Infect. 2019;25(8):951–7.
Chuang YC, Cheng CY, Sheng WH, Sun HY, Wang JT, Chen YC, et al. Effectiveness of tigecycline-based versus colistin- based therapy for treatment of pneumonia caused by multidrug-resistant Acinetobacter baumanniiin a critical setting: a matched cohort analysis. BMC Infect Dis. 2014;14(1):102.
Ku K, Pogue JM, Moshos J, Bheemreddy S, Wang Y, Bhargava A, et al. Retrospective evaluation of colistin versus tigecycline for the treatment of Acinetobacter baumannii and/or carbapenem-resistant Enterobacteriaceae infections. Am J Infect Control. 2012;40(10):983–7.
Clinical & Laboratory Standards Institute [Internet]. [cited 2023 Dec 11]. M100Ed33|performance standards for antimicrobial susceptibility testing, 33rd Edition. https://clsi.org/standards/products/microbiology/documents/m100/. Accessed 11 Dec 2023.
Durante-Mangoni E, Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013;57(3):349–58.
Park HJ, Cho JH, Kim HJ, Han SH, Jeong SH, Byun MK. Colistin monotherapy versus colistin/rifampicin combination therapy in pneumonia caused by colistin-resistant Acinetobacter baumannii: a randomised controlled trial. J Glob Antimicrob Resist. 2019;17:66–71.
Aydemir H, Akduman D, Piskin N, Comert F, Horuz E, Terzi A, et al. Colistin vs. the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. Epidemiol Infect. 2013;141(6):1214–22.
Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial—PubMed [Internet]. [cited 2023 Dec 11]. https://pubmed.ncbi.nlm.nih.gov/29456043/.
Colistin monotherapy versus combination therapy for carbapenem-resistant organisms|NEJM evidence [Internet]. [cited 2023 Dec 11]. https://doi.org/10.1056/EVIDoa2200131.
Liu J, Shu Y, Zhu F, Feng B, Zhang Z, Liu L, et al. Comparative efficacy and safety of combination therapy with high-dose sulbactam or colistin with additional antibacterial agents for multiple drug-resistant and extensively drug-resistant Acinetobacter baumannii infections: a systematic review and network meta-analysis. J Glob Antimicrob Resist. 2021;1(24):136–47.
Jung SY, Lee SH, Lee SY, Yang S, Noh H, Chung EK, et al. Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: a systemic review and Bayesian network meta-analysis. Crit Care Lond Engl. 2017;21(1):319.
Kengkla K, Kongpakwattana K, Saokaew S, Apisarnthanarak A, Chaiyakunapruk N. Comparative efficacy and safety of treatment options for MDR and XDR Acinetobacter baumannii infections: a systematic review and network meta-analysis. J Antimicrob Chemother. 2018;73(1):22–32.
Abouelhassan Y, Kuti JL, Nicolau DP, Abdelraouf K. 1652. Sulbactam against Acinetobacter baumannii pneumonia: pharmacokinetic/pharmacodynamic appraisal of current dosing recommendations. Open Forum Infect Dis. 2022;9(Supplement_2):ofac492.118.
Jaruratanasirikul S, Nitchot W, Wongpoowarak W, Samaeng M, Nawakitrangsan M. Population pharmacokinetics and Monte Carlo simulations of sulbactam to optimize dosage regimens in patients with ventilator-associated pneumonia caused by Acinetobacter baumannii. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2019;1(136): 104940.
Walker AS, White IR, Turner RM, Hsu LY, Yeo TW, White NJ, et al. Personalised randomised controlled trial designs—a new paradigm to define optimal treatments for carbapenem-resistant infections. Lancet Infect Dis. 2021;21(6):e175–81.
Funding
This work was supported by a grant (IA/CPHE/21/1/505972) from the DBT–Wellcome Trust India Alliance (India Alliance) and a research grant from the International Society for Infectious Diseases (ISID). No funding or sponsorship was received for the publication of this article.
Author information
Authors and Affiliations
Contributions
Abi Manesh conceptualised the study, Abi Manesh, Mithun Mohan George, Kundakarla Bhanuprasad and Prasannakumar Palanikumar developed the methodology, Ramya Krishna and Nivetha G collected the data, Mithun Mohan George and V Nagaraj cleaned and analysed the data, Abi Manesh, Prasannakumar Palanikumar and Mithun Mohan George drafted the manuscript, V Nagaraj, Ramya Krishna, Nivetha G., Binesh Lal, Rajitha Triveni K, Priyanka Gautam, Biju George, Vikram Mathews, K.Subramani, Shoma Rao, Binila Chacko, Anand Zachariah, Sowmya Sathyendra, Samuel George Hansdak, Ooriapadickal Cherian Abraham, Ramya Iyadurai, Rajiv Karthik, Yin Mo, Balaji Veeraraghavan, George M Varghese and David Leslie Paterson critically reviewed the manuscript and all authors provided approval for submission.
Corresponding author
Ethics declarations
Conflicts of Interest
Abi Manesh, Mithun Mohan George, Prasannakumar Palanikumar, V Nagaraj, Kundakarla Bhanuprasad, Ramya Krishnan, Nivetha G, Binesh Lal, Rajitha Triveni K, Priyanka Gautam, Biju George, Uday Kulkarni, Vikram Mathews, K Subramani, Shoma Rao, Binila Chacko, Anand Zachariah, Sowmya Sathyendra, Samuel George Hansdak, Ooriapadickal Cherian Abraham, Ramya Iyadurai, Rajiv Karthik, John Victor Peter, Yin Mo, George M Varghese, and David Leslie Paterson declare no conflict of interest. Balaji Veeraraghavan is an Editorial Board member of Infectious Diseases and Therapy. Balaji Veeraraghavan was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
The study was conducted in accordance with the 1964 Helsinki Declaration and its later amendments. The ethical approval for the study was obtained from the Institutional Review Board and ethics committee of Christian Medical College, Vellore, Tamil Nadu, India (IRB minute nos. 14134 and 14427). Informed consent was obtained from each patient or their family members for access to clinical data and publication prior to enrolment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Manesh, A., George, M.M., Palanikumar, P. et al. Combination Versus Monotherapy for Carbapenem-Resistant Acinetobacter Species Serious Infections: A Prospective IPTW Adjusted Cohort Study. Infect Dis Ther (2024). https://doi.org/10.1007/s40121-024-01042-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40121-024-01042-w