Abstract
Introduction
Understanding the differences between respiratory syncytial virus (RSV) subgroups A and B provides insights for the development of prevention strategies and public health interventions. We aimed to describe the structural differences of RSV subgroups, their epidemiology, and genomic diversity. The associated immune response and differences in clinical severity were also investigated.
Methods
A literature review from PubMed and Google Scholar (1985–2023) was performed and extended using snowballing from references in captured publications.
Results
RSV has two major antigenic subgroups, A and B, defined by the G glycoprotein. The RSV F fusion glycoprotein in the prefusion conformation is a major target of virus neutralizing antibodies and differs in surface exposed regions between RSV A and RSV B. The subgroups co-circulate annually, but there is considerable debate as to whether clinical severity is impacted by the subgroup of the infecting RSV strain. Large variations between the studies reporting RSV subgroup impact on clinical severity were observed. A tendency for higher disease severity may be attributed to RSV A but no consensus could be reached as to whether infection by one of the subgroup caused more severe outcomes. RSV genotype diversity decreased over the last two decades, and ON and BA have become the sole lineages detected for RSV A and RSV B, since 2014. No studies with data obtained after 2014 reported a difference in disease severity between the two subgroups. RSV F is relatively well conserved and highly similar between RSV A and B, but changes in the amino acid sequence have been observed. Some of these changes led to differences in F antigenic sites compared to reference F sequences (e.g., RSV/A Long strain), which are more pronounced in antigenic sites of the prefusion conformation of RSV B. Initial results from the second season after vaccination suggest specific RSV B efficacy wanes more rapidly than RSV A for RSV PreF-based monovalent vaccines.
Conclusions
RSV A and RSV B both contribute substantially to the global RSV burden. Both RSV subgroups cause severe disease and none of the available evidence to date suggests any differences in clinical severity between the subgroups. Therefore, it is important to implement measures effective at preventing disease due to both RSV A and RSV B to ensure impactful public health interventions. Monitoring overtime will be needed to assess the impact of waning antibody levels on subgroup-specific efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Both RSV A and RSV B subgroups substantially contribute to the global RSV burden. |
A tendency for higher disease severity has been attributed to RSV A; however, in more recent years, severity between the RSV A and RSV B has been similar. |
The number of genotypes circulating each year is decreasing but sequence differences in the antigenic RSV F protein between both RSV subgroups potentially requires careful evaluation for vaccine and monoclonal antibody therapy development. |
Initial results from the second season after vaccination suggest specific RSV B efficacy wanes more rapidly than RSV A for RSV PreF-based monovalent vaccines. |
Prevention strategies should ensure that both subgroups are targeted to avoid one subgroup becoming dominant and/or escaping immunity once immunization programs are deployed. |
Introduction
Respiratory syncytial virus (RSV) is a common cause of respiratory tract infections in children and adults [1]. It is one of the main causes of hospitalizations in infants. In 2016, RSV was the second leading cause of death from lower respiratory infections overall, and 54% of deaths from lower respiratory infections attributable to RSV occurred in children under 5 years of age [2]. Mortality due to RSV infection is also high in older adults (≥ 65 years) and adults with underlying medical conditions [3,4,5].
RSV circulates globally. In temperate climates, both in the northern and southern hemispheres, RSV infections generally occur in seasons with peaks lasting between 3 and 5 months during autumn and winter. In tropical regions, the virus circulates all year long [6, 7].
RSV is an RNA virus, split into two major antigenic subgroups, A and B. These subgroups co-circulate annually with relatively equal frequencies [8], but there is considerable debate as to whether clinical severity is impacted by the group of the infecting RSV strain [9, 10]. Differences in severity could be due to differences in structural proteins. RSV subgroups vary primarily in the mucin-like domains of the G protein, which is involved in attachment of the virus to the host cell. The fusion F protein is a primary target for neutralizing antibodies. Though the F protein is highly conserved at the amino acid sequence level between RSV subgroups, substantial heterogeneity exists in the antigenic surface of the prefusion trimer [11].
Although many monoclonal antibodies (mAbs) are cross-reactive against both RSV A and RSV B, some mAbs against RSV A site Ø have shown no or lower neutralization activity against RSV B, suggesting the existence of “subgroup-specific immunity” [12].
The aim of this study is to investigate differences between RSV subgroups A and B on the two key surface proteins, F and G, that might explain differences in the immune response and clinical severity following infection. We will also discuss the potential impact these differences could have on the prevention measures of RSV (mAbs and vaccines).
Methods
We performed a targeted literature review focused on publications evaluating differences between RSV A and RSV B in terms of protein structure, epidemiology, immune response, and clinical severity, from PubMed and Google Scholar. The search was extended using snowballing from references in captured publications. The publication extraction was performed in October and November 2022 with regular updates until April 2023. The review was restricted to publications in English, and conference abstracts were not included. Titles and abstracts were assessed for eligibility by a single reviewer, and relevant full texts were obtained and screened. Any uncertainty on inclusion was resolved by discussion with a second reviewer. Among 176 references identified, 109 were included in this analysis. Publications that did not report data on RSV subgroups were excluded. The search terms used included respiratory syncytial virus (RSV), RSV A and RSV B subgroups or subtypes, in combination with protein structure, epidemiology, immune response, clinical severity, and predominance. Publications extracted ranged in publication date from 1985 to 2023. Data were extracted by one reviewer with key outcomes checked by a second reviewer. Data on country/region, years/seasons, percentage of predominance A/B, population, study design, methods, statistical analysis, disease severity were extracted from eligible publications into a spreadsheet. Where appropriate, data were analyzed by study population, geography, and study design. Formal quality appraisal of the extracted studies was not undertaken, because no quantitative synthesis was planned.
Results
Differences in Protein Structures
RSV is an enveloped virus with an RNA genome encoding a total of 11 proteins (reviewed in more detail in [13]). Four proteins associated with the membrane form the virus envelope: the matrix protein (M), the small hydrophobic protein (SH), the fusion protein (F), and the attachment glycoprotein (G). The virus genome protection and replication are mediated through the nucleoprotein (N), phosphoprotein (P), RNA polymerase (L), and the transcription processivity factor (M2-1). In addition, two nonstructural proteins (NS1 and NS2) are potentially involved in the escape strategy from infected cells.
The virus primarily mediates its viral attachment and entry into host cells via the attachment G protein and the fusion F protein that are on its surface. The interaction between RSV G and the host cell leads to the engagement of RSV F, which then drives fusion between the viral envelope and the host membrane. During fusion, RSV F undergoes a dynamic conformational change from a metastable prefusion trimer to a stable postfusion state. Over the years, as a result of their critical roles in mediating attachment and fusion, and the induction of the majority of neutralizing antibodies in vivo, RSV G and F were the best-studied proteins.
RSV A and RSV B Subgroups are Established by RSV G Antigenic and Sequence Variations
The RSV G is the most variable RSV gene sequence and is used to define RSV genetic variants [14]. RSV is classified into two major subgroups, RSV A and RSV B, initially based on antigenic differences using G protein-specific mAbs [15, 16]. Within each of these two subgroups, several genotypes have been further identified and described. Although there are no criteria for genotype definitions that reach consensus, 15 distinct genotypes have been described for RSV B [17] and nine distinct genotypes for RSV A [18] (Table 1).
RSV keeps evolving worldwide and new genotypes emerged over the years. The genotypic diversity within RSV B is greater than within RSV A (see Table 1). While RSV A strains can be grouped into seven distinct genotypes, at least 37 RSV B genotypes have been described [17], of which only the most dominant ones are listed in Table 1. This is most likely due to RSV B having a higher genome-wide evolutionary rate than RSV A (higher number of substitutions per site per year) [19, 20]. However, strikingly, a recent phylogenetic analysis of the Global Initiative for Sharing All Influenza Data (GISAID) database suggests that the nucleotide diversity in the G protein is three times higher in RSV A than in RSV B [21]. In recent years, several genetic modifications in RSV G have been identified. In the RSV A subgroup, a 72-nucleotide duplication is observed (referred to as the Ontario 1 (ON1) lineage), whereas in the RSV B subgroup, a 60-nucleotide duplication is observed (referred to as the Buenos Aires (BA) genotype) [22, 23]. Although there is still debate on whether they can be considered as new distinct genotypes, both became predominant circulating strains. Genotype diversity decreased over the last two decades, and ON1 and BA9 have become the sole lineages detected for RSV A and RSV B [24,25,26,27,28,29,30,31,32,33]. Whether the G gene duplication plays a role in the predominance of the ON1 and BA9 remains an open question [20]. There is only limited in vitro data suggesting improved infectivity with the duplication and no clinical evidence so far [34].
RSV F, More Conserved Among RSV A and RSV B, is a Major Target for Vaccine and Monoclonal Antibody Development
RSV F is evolutionarily more conserved than RSV G and as such serves as a common therapeutic target for both RSV A and RSV B subgroup viruses. RSV F differs by only 25 amino acids between RSV A and B [35]. The structure of RSV F in prefusion conformation was solved in 2013 and is guiding new vaccine development approaches [36].
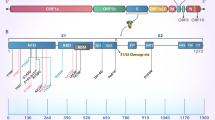
F contains antigenic sites that are both shared by the prefusion and postfusion conformation and sites that are unique to each conformation. Six major antigenic sites have been defined on the RSV F (Ø, I, II, III, IV, V). Some antigenic sites are expressed on both pre-F and post-F, while other sites are presented on only one conformation (see Table 2). The antigenic sites I, II, III, and IV are present in both the pre- and post-F conformation and present different binding affinities; at one end of the spectrum, antibodies targeting site I show a preference for post-F and low neutralizing activity, while at the other end of the spectrum, those targeting site III show a preference for pre-F and have relatively potent neutralizing activities. The antigenic sites Ø and V are only displayed in the pre-F conformation of RSV F and present the highest binding affinity for neutralizing antibodies (see Table 2) [37,38,39].
Almost 50% of the most potent neutralizing antibodies are targeting the pre-F-specific site V in recently studied healthy adults [40]. Importantly, pre-F has been proposed as a superior vaccine candidate since antibodies bind antigenic sites Ø and V with high affinity [41].
Even though the RSV F is relatively well conserved, it evolves continuously. Over time, changes in the amino acid sequence have been observed. Some of these changes led to differences in F antigenic sites compared to reference F sequences (e.g., RSV/A Long strain), which are more pronounced in antigenic sites of the prefusion conformation of RSV B [11, 42]. Data from the INFORM-RSV 2017–2018 pilot season were crucial to establish a molecular baseline of RSV F sequence and antigenic site variation. This can be used to track frequency, geography, and evolutionary trajectory of potential neutralization escape variants, providing an evaluation system for ongoing development of vaccines and mAbs [33]. Despite low variability in the 2017–2018 RSV F sequences, new variants constantly emerge in some regions, which suggests positive selection pressure. Indeed, in a study from China (2014–2016), multiple amino acid differences were found for RSV F, located at multiple antigenic sites, varying between RSV A and RSV B [43]. In a study in the USA (2015–2019), changes at the F antigenic sites of RSV B were more frequent than RSV A, mainly occurring at antigenic sites V (99.6%), Ø (18.6%), and IV (7%) of RSV-B F [44]. In another study in China, the RSV F amino acid sequence homology between RSV A and RSV B was 89–90.6%, with more mutations found in antigenic site II, a target site for mAbs [45].
Subgroups and Immunity
Neutralizing Antibodies: RSV A RSV B Cross-Reactivity
The RSV neutralizing activity in human sera is primarily derived from antibodies targeting the F protein in its prefusion conformation (pre-F specific antibodies), and more specifically antigenic sites Ø and V [38, 40]. Immunological analysis demonstrated that more than 85% of highly potent antibodies were specific for prefusion F. Furthermore, pre-F specific antibodies were more potent than pre- and post-F cross-reactive antibodies and also more potent than specific post-F antibodies; hence, the interest to target prefusion conformation [38]. In contrast, virus neutralization was not observed with G-specific serum [46]. Consequently, most current monoclonal antibody and vaccine design efforts are focused on RSV F.
The majority of neutralizing antibodies induced by natural infection on adults showed activity against both subgroups A and B [38]. They neutralize a diverse panel of clinical isolates and demonstrate in vivo protection. Such studies confirm that the prefusion F has highly conserved epitopes and is therefore a desirable target for RSV vaccines and mAbs [47]. However, some studies have shown that numerous antibodies targeting site Ø had more neutralizing potency toward RSV A than toward the RSV B [41].
Studies evaluating the human antibody response to RSV F in adults following natural infection, who have likely been exposed to RSV A and RSV B, have shown that most anti-RSV F mAbs are subgroup cross-reactive. Very occasionally, mAbs are found that appear to be subgroup-specific or subgroup-preferring [12, 38].
MAbs targeting site Ø showed the most variations in neutralization potencies between the two different RSV subgroups. Some mAbs directed at RSV A site Ø present no or less neutralization activity against RSV B. Indeed, two site Ø-specific mAbs have been well characterized: 5C4 and D25. They recognize antigenic site Ø but at different angles. 5C4 potently neutralizes a panel of RSV A subgroup strains but has limited neutralization activity against subgroup RSV B strains. In contrast, D25 potently neutralizes strains of both RSV subgroups. D25 is a mAb that has been isolated from human B cells derived from an adult most probably infected throughout life with RSV strains of both RSV A and B subgroups [12]. These results show the existence of differences in binding of neutralizing antibodies to key RSV epitopes such as the antigenic site Ø, which can yield neutralization of both RSV subgroups or only a single subgroup.
Finally, clinical proof of concept for prefusion F vaccine design came from immunogenicity data of phase I clinical trial showing a booster effect in neutralizing activity in serum. These findings suggest that developing a successful RSV vaccine is feasible. The boost in neutralizing activity toward subgroup B after immunization with a subgroup A F vaccine shows the high conservation of F between subgroups, and immunity acquired most probably from multiple prior infections by both RSV A and B subgroups [48, 49].
It remains difficult to study the genotype-specific immune response in human cohorts, due to diversity in the hosts, which makes it hard to assign differences to pathogen diversity alone. For instance, when the innate immune response was studied by analyzing the expression level of interferon (IFN)-related genes in infants with RSV bronchiolitis, it was shown that induced interferons (IFN)-stimulated genes (ISGs) differed between patients infected with different genotypes [50]. Furthermore, these ISG expression levels were associated with severity in bronchiolitis clinical manifestations; ISGs expression level was lower in ON1- and BA- than in the NA1-infected patients. The lineage ON1 demonstrated a high replicative capacity but seemed to be clinically less severe than the previously circulating RSV A, NA1. But whether the ISG downregulation in ON1-infected infants is a consequence or a cause of high viral load increase remains to be established [50].
Cellular Immune Response
While inducing potent neutralizing antibodies is the primary objective in most vaccine development programs, both CD4 and CD8 T cells play a key role in the clearance of infected cells and hence the protection against RSV infection [51].
Amino acid variation and the appearance of novel epitopes on RSV proteins of recent circulating RSV strains have been described [43]. Using a computational approach, RSV surface proteins were predicted to have a strong potential to elicit T cell immunity, with the F protein potentially having a high T cell epitope density [52]. Computational results suggest that RSV surface proteins may stimulate T cells that are essential for protective immunity. However, some epitopes were mutated in some strains. The percentage of conserved T cell epitopes in the RSV F protein when comparing the vaccine and corresponding wild type strains is substantially similar in the two subgroups. The proportion of conserved epitopes was higher than 78% for RSV A and higher than 85% for RSV B [52].
Polyfunctionality refers to the ability of T cells to perform a range of functions, including the secretion of cytokines, chemokines, or cytotoxic granules simultaneously at the single-cell level. The presence of polyfunctional T cells could be a sign of immunological disease control; their role in RSV protective immunity is unknown. Blunck et al. [53] found that, in healthy adults under the age of 65, both the acutely and recently RSV-infected groups had lower T cell polyfunctionality, for both CD4+ and CD8+ memory T cells, than the uninfected group at enrollment. The uninfected group was defined as adults who remained uninfected through the season. This suggests that the presence of polyfunctional RSV-specific memory T cells may protect against re-infection. More importantly, while RSV B was the dominant circulating subgroup during the study period, and stronger neutralizing antibody responses were observed against RSV B than against RSV A, polyfunctionality across all T cell subsets was higher after exposure to RSV-A F than RSV-B F protein peptide libraries. It is unclear whether this difference in response is reflective of what these adults were primed with in prior respiratory seasons. The authors conclude that “bivalent vaccines containing both RSV subgroup antigens may be warranted, at least for the older adult population” [53].
Monoclonal Antibodies: Cross-Reactivity Between RSV A and RSV B
Studies on crystal structures have shown that the antigenic site Ø presents various conformations and that even though individual mAbs may have preferential binding affinities to a specific subgroup, the antibody epitopes overlap substantially between RSV A and RSV B [54]. However, site Ø is the most variable surface epitope between RSV A and RSV B.
The anti-RSV fusion protein mAb nirsevimab received marketing authorization from the European Medicines Agency (EMA; October 2022) and US Food and Drug Administration (FDA; July 2023). This mAb binds the prefusion RSV fusion protein at a conserved discontinuous neutralizing epitope in site Ø. It blocks viral entry into host cells and a single dose confers protection for 5 months [55, 56]. However, decreased susceptibility to nirsevimab does occur, as observed in the randomized clinical trial, where two RSV B clinical isolates were identified in the study arm, that had decreased susceptibility to nirsevimab [57]. In a study of 322 samples from three European countries [58] conducted before the nirsevimab approval, isolates collected from 0.8% of the immunoprophylaxis-naïve participants were found to contain a nirsevimab resistance-associated substitution. Similarly, using nearly 6000 RSV-positive nasal samples from 17 countries, another study assessed the frequency of mutations and evaluated the effect of these mutations on the in vitro potency of nirsevimab neutralization [59]. Only one mutation in RSV A, Lys68Glu, resulted in a tenfold or higher change in the inhibitory concentration. Four mutations in RSV B, Lys68Asn, Lys68Gln, Asn201Ser, and Asn201Thr, resulted in a tenfold or higher change in the inhibitory concentration. Each of these binding-site substitutions was rare (< 1%) in circulating RSVs between 2015 and 2021 [59]. Following the introduction of nirsevimab, it is unclear whether its broad use could lead to selection of these resistant viruses over time [33].
Suptavumab, a monoclonal antibody which binds antigenic site V, a conserved epitope on both RSV A and RSV B subgroups, was tested in healthy infants. In a phase III trial, isolated RSV A virus had no suptavumab epitope changes while all isolated RSV B virus had a two-amino acid escape mutation in the suptavumab epitope. This substitution on this newly circulating mutant strain of RSV B led to complete loss of neutralization antibody activity; as a result, suptavumab did not meet its primary efficacy endpoint [60].
Clesrovimab, a monoclonal antibody currently in a phase III trial, binds to RSV F site IV [47, 61]. Site IV is relatively well conserved between RSV A and RSV B [11, 42], and is more conserved than sites Ø and V [47]. Site Ø and V reside at the top half of the RSV prefusion F trimer in contrast to site IV, which resides on the lower half of the prefusion F trimer. This may make site IV mAbs less vulnerable to evolutionary pressure [42]. However further evaluation will be required as other studies have shown variation in site IV of the RSV B subgroup [44].
The current data with these three mAbs shows that close surveillance will be necessary to follow the emergence of mutated RSV strains that could impact the efficacy of mAbs. It also suggests that in the absence of immune pressure, a polyclonal vaccine response is anticipated to be less vulnerable than mAbs to RSV F surface amino acid substitutions, particularly those targeting antigenic site Ø. As mAbs target a single epitope, these may be more prone to drive immune escape compared to a polyclonal vaccine response.
Clinical Course of Infection, and Severity Outcomes
RSV can cause a range of symptoms from a mild cold to a serious respiratory illness. Complications are similar to those caused by influenza and other respiratory viruses, and can include pneumonia, cardiopulmonary complications, intensive care unit (ICU) admission, the need for mechanical ventilation, and might lead to death.
RSV surveillance is essential to improve our understanding of RSV incidence and diversity. Yet, currently, we do not fully understand how this diversity impacts clinical outcomes. A comprehensive collection of papers reporting on the impact of RSV subgroup on clinical severity is shown in Table S1 in the electronic supplementary material.
Two studies, performed in West Virginia, showed that the clinical categorization of the illnesses associated with subgroup A and subgroup B strains appeared to differ between the two subgroups. Bronchiolitis occurred at a lower frequency in children with RSV B (p = 0.025), whereas croup occurred more often (p value not reported) [62, 63]. Similar results were obtained in a study from Finland, where bronchiolitis was observed in 71% of children with group A infection and 59% of children with group B infection (relative risk 1.21; confidence interval 1.02–1.43; p = 0.02) [64]. However, using previously described diagnostic criteria [62, 63], these findings could not be verified in a Japanese study population [65].
Out of 46 studies reporting RSV subgroup impact on clinical severity, 29 studies reported no significant differences in severity between subgroups A and B [9, 65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93], 14 studies reported that subgroup A infection resulted in more severe outcomes [63, 64, 92,93,94,95,96,97,98,99,100,101,102,103], whereas two studies reported that subgroup B infection resulted in more severe outcomes [104, 105], and one was inconclusive [106]. Given this variation, no consensus has been reached on this topic. Several study characteristics can hinder firm conclusions on clinical severity differences between RSV A and RSV B. Shorter studies are more likely to be influenced by a temporarily dominating subgroup than longer study periods. Strain virulence may be variable between epidemics, which impacts shorter studies more than longer ones. Hence, an association of increased severity with longer study periods would argue in favor of a true difference across the entire review period. The studies showing more serious outcomes for infections with subgroup B were short (one or two RSV seasons), whereas other studies differ more widely. A log-rank analysis using the Mann–Whitney U test shows a trend (p = 0.09) toward longer study periods in the group showing no difference in severity than in the group with RSV A leading to more severe outcomes. This argues against a true difference between RSV A and RSV B in clinical severity.
Although anecdotal reports of differences at the genotype level exist, suggesting that GA2 [102], GA3 [100], GA5 [77], or ON1 [80, 84, 91] might lead to more severe outcomes than other genotypes, this is not consistently shown. Moreover, in contrast, others showed lower severity for ON1 [79, 81].
Differences in assay sensitivity may impact the ability to detect true differences in disease severity between subgroups. However, looking at the methodology used for subgrouping, eight of 19 studies using immunofluorescence showed RSV A as more severe, compared to six out of 25 studies using polymerase chain reaction (PCR) or sequencing, resulting in a p value of 0.34.
The ON (RSV A subgroup) and BA (RSV B subgroup) genotype global predominance began around 2014. To assess whether this had an impact on differences in disease severity, we analyzed studies with a study period including 2014 and beyond separately. None of the studies with post-2014 data reported a difference in disease severity between the two subgroups [9, 79, 84,85,86, 88, 89], while 14 of 39 studies performed before 2014 reported that subgroup A led to more severe disease. This may suggest that RSV disease severity for subgroups A and B has become more balanced after the emergence of ON and BA.
Investigating other factors that might impact disease severity, we looked at geography (northern hemisphere vs southern hemisphere vs (sub)tropical zone), study design (prospective vs retrospective studies), and age of the study population (infants vs children vs adults). However, we did not find any association with disease severity.
A major source of heterogeneity between studies was the definition of disease severity. Eight studies did not provide any definition of disease severity, 28 studies defined disease severity, using a wide variety of factors, and 11 studies used a severity index. Studies using a severity index were performed in Canada, Cyprus, Denmark, Greece, Italy, USA (n = 4), United Kingdom, and Vietnam. The earliest study started in 1985 and the latest ended in 2017, with six studies including only data before 1999, and five studies including data from this century. Of note, eight of 11 studies reported a difference in severity (seven times suggesting RSV A as more severe, and once RSV B).
Only a few studies used outpatient data [66], a combination of outpatients and hospitalized patients [95], or subjects presenting to the emergency department [101]. For outpatients, the observation of differences in severity was restricted to the diagnosis of upper versus lower respiratory tract infections, fever, restriction of daily activity, and physician consultation. Most studies included hospitalized patients. These studies looked at a wide range of parameters to assess severity, including the presence of rales, wheezes, fever, stridor, retractions, cyanosis, pallor, the occurrence of apnea, supplemental oxygen use, arterial blood gas values or oximetry, requirement for mechanical ventilation, ribavirin therapy, need for ICU, and length of stay.
Finally, the statistical analysis approach might also impact the likelihood of finding differences between subgroups in disease severity. This is especially important given the wide range of parameters investigated to reflect disease severity. Two studies did not report how their statistical analyses were performed. Thirty-one studies used univariate analysis, of which (only) two used a correction for the frequency of testing (Bonferroni). Fourteen studies used multivariate analysis. Although the statistical comparison approach was not associated with the disease severity outcome (Chi2 [1, N = 45] = 0.4728, p = NS), using multivariate analysis would seem to be more appropriate.
Focusing on those manuscripts that report differences in disease severity, the majority do not explain how structural differences could impact differences in disease severity between subgroups [62, 92,93,94,95,96, 99,100,101,102,103,104]. Generally, it is acknowledged that while the F, M, and N proteins show only minor differences between subgroups, a profound variation is observed in the G protein, with only 53% homology in the amino acid sequences between proteins of the RSV A and RSV B subgroups [64, 98]. However, the mechanism linking this variation to differences in severity is unknown. Nevertheless, it has been suggested that RSV B subgroup viruses may induce less vigorous immune stimulation with its associated immunopathological effects or are less likely to stimulate IgE or inflammatory cytokines than RSV A subgroup, therefore inducing less airway reactivity [98]. Alternatively, the interleukin (IL)-6/tumor necrosis factor (TNF) alpha ratio may be indicative of severity, with high ratios found in relatively benign cases [105]. Again, how structural differences could lead to different ratios remains unclear.
In conclusion, the use of a globally recognized severity index would make studies more easily comparable and would lead to more conclusive data. In this review, we did not analyze nonstructural and regulatory proteins which may be critical in clinical severity.
RSV A and RSV B Global Distribution
As observed in 15 recent publications reporting distribution of RSV A and RSV B, both strains generally co-circulate within a season, with predominance varying over seasons and countries [8, 24,25,26,27,28,29,30,31,32,33, 107,108,109]. Among 138 RSV seasons analyzed in 29 countries (covering both hemispheres) between 1990 and 2021, RSV A was predominant in 76 seasons (55.1%), RSV B was predominant in 54 seasons (39.1%), and eight seasons (5.8%) had no clear predominance (defined as a RSV A/B ratio range between 0.45 and 0.55). For 63 of these seasons, the exact proportions of RSV A and RSV B co-circulating were available (Fig. 1). See Table S2 in the electronic supplementary material for details on RSV A and RSV B predominance with data from 15 studies included.
Proportions of RSV A to RSV B per season (n = 63 seasons) plotted per decreasing frequency of RSV A. Countries included are Algeria, Australia, Brazil, China, Egypt, Finland, Germany, Great Britain, Iran, Iraq, Israel, Japan, Jordan, Kuwait, Lebanon, Netherlands, Pakistan, Saudi Arabia, South Africa, South Korea, Spain, Taiwan, Tunisia, USA, Yemen
For 104 seasons analyzed, data were available on the specific season subgroup predominance which allowed us to compare the circulation of both subgroups before and after 2014 ON and BA genotype predominance. The percentage of predominance of RSV A decreased from 76% (37 out of 49 seasons) before 2014 to 40% after 2014 (22 out of 55 seasons). The predominance of RSV B subgroup increased from 24% (12 out of 49 seasons) to 53% (29 out of 55 seasons), indicating that RSV A and B predominance are at a somewhat similar level. Both subgroups continue to circulate each year, with predominance varying from country to country.
While RSV A infections appear more frequently than RSV B, the persistence of both of these distinct groups may explain how previously infected individuals remain susceptible to RSV infections.
Our results are similar to a recent review by Cantú-Flores et al. [110], covering data from 1961 until 2019 from 83 countries covering all continents. In that review, a total of 60% RSV A and 40% RSV B positive samples were found. The co-circulation of both RSV groups was noted in all years; the only exception was the 1969–1970 season where only one RSV A positive sample was identified. RSV A was the predominant group (> 50% detections) during most seasons/years. In general, season/years with only a single RSV subgroup occur infrequently (3/63 seasons), both subgroups remaining concurrently present with varying frequencies. The most frequently identified RSV A genotype was NA1 (including ON1 viruses) (76.30%), and the most frequently identified RSV B genotype was BA (70.65%) [110].
Discussion
Both RSV A and RSV B subgroups cause severe disease in children and adults, which can lead to hospitalizations or death from lower respiratory infections. Each year, dominance of the circulating subgroup varies, but generally both subgroups co-circulate in given season. Most preventive measures are targeting RSV pre-F which is highly conserved between the subgroups. Despite some degree of cross-reactivity in antibodies elicited against RSV F between both subgroups, vaccination with a monovalent RSV A prefusion F can lead to differences in elicited subgroup-neutralizing activity. Vaccine subgroup-specific host immunity is therefore a theoretical possibility. Vaccination with a bivalent RSV A/RSV B vaccine may provide better protection against both RSV subgroups compared to a monovalent vaccine or mAbs, particularly as antibody levels wane.
Most prevention strategies target the RSV F in its prefusion conformation to confer protection against RSV disease. Though F is highly conserved at the amino acid sequence level between both RSV subgroups, substantial heterogeneity exists in the antigenic surface, mainly on the antigenic site Ø, which also shows the highest neutralizing potency. Neutralizing antibodies present a degree of cross-activity against both subgroups A and B, and antibodies targeting site Ø may have more neutralizing potency toward RSV A than toward RSV B. While there is no established correlate of protection for RSV, there is a clear relationship of higher neutralizing titers with reduced risk of RSV disease [111]. This taken together with the heterogeneity of prefusion F concentrated in the region where antibodies with the most potent neutralizing activity may suggest that a bivalent prefusion F vaccine or mAbs with equivalent neutralization potency against RSV A and RSV B strains are important considerations for optimal RSV prophylactic approaches.
Two recent stabilized prefusion F protein-based vaccines, Arexvy [112, 113] and Abrysvo [114, 115], have been authorized in the USA and Europe to protect for older adults against RSV-associated lower respiratory tract illness. Abrysvo is also indicated to protect infants from RSV via maternal vaccination. While the monovalent vaccine, Arexvy, is based on the RSV PreF3 antigen derived from the F protein of the RSV A2 strain [116], the bivalent RSV vaccine, Abrysvo, is based on contemporary strains, including the F antigen from RSV A subgroup genotype Ontario (ON) and RSV B subgroup genotype Buenos Aires (BA) [117].
Vaccination based on RSV prefusion F generates diverse immune responses with cross-reactivity against RSV A and RSV B subgroups [118]. Despite this degree of cross-reactivity, some studies have shown that vaccinations targeting monovalent RSV A prefusion F can lead to differences in elicited subgroup-neutralizing activity, supporting the theoretical possibility of vaccine subgroup-specific host immunity [119]. The key risk identified by researchers is that if vaccines based on prefusion F are widely used, with greater effectiveness against one of the subgroups over time, the other subgroup may subsequently spread in the community. Both approved monovalent (RSV A) and bivalent (RSV A and RSV B) prefusion F vaccines generate neutralizing antibody responses against the RSV A and RSV B subgroups [49, 120,121,122]. However, the monovalent vaccine Arexvy has recently been reported to have lower vaccine efficacy in the second season for RSV B-related lower respiratory tract disease (LRTD) compared to RSV A LRTD (vaccine effectiveness 44% for RSV B and 76% for RSV A) [123], while it was similar (> 80%) for RSV A and RSV B for the bivalent vaccine [124]. This suggests protection from monovalent vaccines against RSV B may not be as long-lasting as for RSV A. Further, monovalent mRNA vaccine had lower RSV B vaccine efficacy estimates after 8.6 months of follow-up (RSV B vaccine efficacy 20% points lower than A for both two and three-symptom LRTD) [125].
Although RSV F is relatively well conserved between RSV A and RSV B subgroups, recent amino acid variations have been observed, especially at key antigenic sites in both subgroups [11, 35, 42, 126]. Continuous surveillance will be important to ensure vaccines, especially those using monovalent prefusion F from the historical RSV A genotype, remain effective. While analyses such as those by Mas et al. (2018) show that a large variability in amino acid is tolerated in RSV F, continuing sample collection to evaluate RSV F evolution during immunization programs will probably be required [42]. Some researchers suggest establishing a baseline dataset to monitor future changes in RSV F-gene sequence that might be induced by large immunization programs.
In one study, the majority of antibodies generated against RSV natural infection were subgroup cross-reactive in adults, suggesting multiple infections throughout life and likely due to infections with both RSV A and RSV B viruses; a small proportion of characterized RSV prefusion F-specific human monoclonal antibodies have been shown to be subgroup-specific [12]. Based on this, it was postulated that vaccines targeting infants should be designed to protect against both RSV subgroups [12]. Indeed, the immune response in some infants could be elicited only against the RSV subgroup encountered in the first months/years of life, leaving them at risk of reinfections when encountering the other subgroup virus. The literature search for this publication was not performed systematically, which is a limitation in this review. Indeed, some publications containing pertinent information on this subject may therefore not have been collected despite over 100 publications being included.
Conclusion
The key amino acid sequence differences between RSV A and RSV B lie mostly within the G and in surface epitopes in the prefusion F trimer. The RSV G antigenic and sequence variations are used to classify RSV strains into the RSV A or RSV B subgroup. The fusion F is a primary target for neutralizing antibodies as it is most conserved between both subgroups. Although RSV A occurs more frequently than RSV B, both contribute to the global RSV burden. Our data and those from a recent systematic review show that RSV A is reported slightly more frequently than RSV B (60% vs 40%), while both subgroups mostly co-circulate within the same season/year [110]. Additionally, since 2015, worldwide, ON1 and BA9 became the sole genotypes detected for RSV A and RSV B, respectively. Thus, prevention strategies should target both subgroups to avoid one subgroup becoming dominant and/or escaping immunity strategies as prevention programs become more widely deployed.
It remains to be shown whether differences in surface proteins, such as the duplication of the G gene in dominant contemporary strains, are associated with potential differences in clinical severity. In earlier studies, RSV A was suggested to have higher clinical severity than RSV B, but more recent studies since emergence of the ON and BA lineages indicate similar clinical severity for the RSV A and RSV B subgroups. However, it is difficult to draw strong conclusions because of high study heterogenicity regarding study period, population, study design and methodology, and definition of clinical severity. These unclear tendencies also advocate again the need to continue surveillance and to target both subgroups in prevention strategies.
Vaccines and immunization products based on antigens from both subgroups could present various advantages such as limiting the risk of immune escape and potentially limiting the selective pressure on F epitopes. Ongoing RSV prefusion F-based vaccine studies continue to evaluate the duration of vaccine-induced protection in older adults. The differential durability of specific protective immunity against RSV A and B subgroups may become more apparent as neutralizing antibodies wane over time and subgroup dominance naturally varies across seasons.
Data Availability
All data generated or analyzed during this study are included as supplementary information files.
References
Public Health England. Respiratory syncytial virus. The Green Book; 2015.
GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210.
Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–59.
McLaughlin JM, Khan F, Begier E, Swerdlow DL, Jodar L, Falsey AR. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis. 2022;9(7):ofac300.
Li Y, Kulkarni D, Begier E, et al. Adjusting for case under-ascertainment in estimating RSV hospitalisation burden of older adults in high-income countries: a systematic review and modelling study. Infect Dis Ther. 2023;12(4):1137–49.
Janet S, Broad J, Snape MD. Respiratory syncytial virus seasonality and its implications on prevention strategies. Hum Vaccin Immunother. 2018;14(1):234–44.
Chadha M, Hirve S, Bancej C, et al. Human respiratory syncytial virus and influenza seasonality patterns—early findings from the WHO global respiratory syncytial virus surveillance. Influenza Other Respir Viruses. 2020;14(6):638–46.
Staadegaard L, Caini S, Wangchuk S, et al. The global epidemiology of RSV in community and hospitalized care: findings from 15 countries. Open Forum Infect Dis. 2021;8(7):ofab159.
Saravanos GL, Ramos I, Britton PN, Wood NJ. Respiratory syncytial virus subtype circulation and associated disease severity at an Australian paediatric referral hospital, 2014–2018. J Paediatr Child Health. 2021;57(8):1190–5.
Vandini S, Biagi C, Lanari M. Respiratory syncytial virus: the influence of serotype and genotype variability on clinical course of infection. Int J Mol Sci. 2017;18(8):1717.
Hause AM, Henke DM, Avadhanula V, Shaw CA, Tapia LI, Piedra PA. Sequence variability of the respiratory syncytial virus (RSV) fusion gene among contemporary and historical genotypes of RSV/A and RSV/B. PLoS ONE. 2017;12(4):e0175792.
Tian D, Battles MB, Moin SM, et al. Structural basis of respiratory syncytial virus subtype-dependent neutralization by an antibody targeting the fusion glycoprotein. Nat Commun. 2017;8(1):1877.
Pandya MC, Callahan SM, Savchenko KG, Stobart CC. A contemporary view of respiratory syncytial virus (RSV) biology and strain-specific differences. Pathogens. 2019;8(2):67.
Tan L, Lemey P, Houspie L, et al. Genetic variability among complete human respiratory syncytial virus subgroup A genomes: bridging molecular evolutionary dynamics and epidemiology. PLoS ONE. 2012;7(12):e51439.
Anderson LJ, Hierholzer JC, Tsou C, et al. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis. 1985;151(4):626–33.
Mufson MA, Orvell C, Rafnar B, Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol. 1985;66(Pt 10):2111–24.
Muñoz-Escalante JC, Comas-García A, Bernal-Silva S, Noyola DE. Respiratory syncytial virus B sequence analysis reveals a novel early genotype. Sci Rep. 2021;11(1):3452.
Muñoz-Escalante JC, Comas-García A, Bernal-Silva S, Robles-Espinoza CD, Gómez-Leal G, Noyola DE. Respiratory syncytial virus A genotype classification based on systematic intergenotypic and intragenotypic sequence analysis. Sci Rep. 2019;9(1):20097.
Tan L, Coenjaerts FE, Houspie L, et al. The comparative genomics of human respiratory syncytial virus subgroups A and B: genetic variability and molecular evolutionary dynamics. J Virol. 2013;87(14):8213–26.
Schobel SA, Stucker KM, Moore ML, et al. Respiratory syncytial virus whole-genome sequencing identifies convergent evolution of sequence duplication in the C-terminus of the G gene. Sci Rep. 2016;6:26311.
Shishir TA, Saha O, Rajia S, et al. Genome-wide study of globally distributed respiratory syncytial virus (RSV) strains implicates diversification utilizing phylodynamics and mutational analysis. Sci Rep. 2023;13(1):13531.
Eshaghi A, Duvvuri VR, Lai R, et al. Genetic variability of human respiratory syncytial virus A strains circulating in Ontario: a novel genotype with a 72 nucleotide G gene duplication. PLoS ONE. 2012;7(3):e32807.
Trento A, Casas I, Calderón A, et al. Ten years of global evolution of the human respiratory syncytial virus BA genotype with a 60-nucleotide duplication in the G protein gene. J Virol. 2010;84(15):7500–12.
Liang X, Liu DH, Chen D, et al. Gradual replacement of all previously circulating respiratory syncytial virus A strain with the novel ON1 genotype in Lanzhou from 2010 to 2017. Medicine (Baltimore). 2019;98(19):e15542.
Yassine HM, Sohail MU, Younes N, Nasrallah GK. Systematic review of the respiratory syncytial virus (RSV) prevalence, genotype distribution, and seasonality in children from the Middle East and North Africa (MENA) region. Microorganisms. 2020;8(5):713.
Lee CY, Wu TH, Fang YP, et al. Delayed respiratory syncytial virus outbreak in 2020 in Taiwan was correlated with two novel RSV-A genotype ON1 variants. Influenza Other Respir Viruses. 2022;16(3):511–20.
Yun KW, Choi EH, Lee HJ. Molecular epidemiology of respiratory syncytial virus for 28 consecutive seasons (1990–2018) and genetic variability of the duplication region in the G gene of genotypes ON1 and BA in South Korea. Arch Virol. 2020;165(5):1069–77.
Robertson M, Eden JS, Levy A, et al. The spatial-temporal dynamics of respiratory syncytial virus infections across the east-west coasts of Australia during 2016–17. Virus Evol. 2021;7(2):veab068.
Tabatabai J, Ihling CM, Rehbein RM, et al. Molecular epidemiology of respiratory syncytial virus in hospitalised children in Heidelberg, Southern Germany, 2014–2017. Infect Genet Evol. 2022;98:105209.
Luo Q, Li M, Li A, et al. Genetic diversity and epidemiological features of respiratory syncytial virus, Beijing, 2015–2019: a multicenter and all-age groups study. J Infect. 2022;85(1):75–85.
Gimferrer L, Vila J, Piñana M, et al. Virological surveillance of human respiratory syncytial virus A and B at a tertiary hospital in Catalonia (Spain) during five consecutive seasons (2013–2018). Future Microbiol. 2019;14:373–81.
Lee YE, Choi OK, Bang SJ, et al. Molecular epidemiological study of the G protein of human respiratory syncytial virus detected in patients with acute respiratory infections in Gyeonggi Province, South Korea. J Med Virol. 2022;94(2):549–56.
Tabor DE, Fernandes F, Langedijk AC, et al. Global molecular epidemiology of respiratory syncytial virus from the 2017–2018 INFORM-RSV study. J Clin Microbiol. 2020;59(1):e01828-20.
Hotard AL, Laikhter E, Brooks K, Hartert TV, Moore ML. Functional analysis of the 60-nucleotide duplication in the respiratory syncytial virus buenos aires strain attachment glycoprotein. J Virol. 2015;89(16):8258–66.
Graham BS, Modjarrad K, McLellan JS. Novel antigens for RSV vaccines. Curr Opin Immunol. 2015;35:30–8.
McLellan JS, Chen M, Leung S, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340(6136):1113–7.
Ruckwardt TJ, Morabito KM, Graham BS. Immunological lessons from respiratory syncytial virus vaccine development. Immunity. 2019;51(3):429–42.
Gilman MS, Castellanos CA, Chen M, et al. Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci Immunol. 2016;1(6):eaaj1879.
Mousa JJ, Kose N, Matta P, Gilchuk P, Crowe JE Jr. A novel pre-fusion conformation-specific neutralizing epitope on the respiratory syncytial virus fusion protein. Nat Microbiol. 2017;2:16271.
Ngwuta JO, Chen M, Modjarrad K, et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med. 2015;7(309):309ra162.
Xiao X, Tang A, Cox KS, et al. Characterization of potent RSV neutralizing antibodies isolated from human memory B cells and identification of diverse RSV/hMPV cross-neutralizing epitopes. MAbs. 2019;11(8):1415–27.
Mas V, Nair H, Campbell H, Melero JA, Williams TC. Antigenic and sequence variability of the human respiratory syncytial virus F glycoprotein compared to related viruses in a comprehensive dataset. Vaccine. 2018;36(45):6660–73.
Chen X, Xu B, Guo J, et al. Genetic variations in the fusion protein of respiratory syncytial virus isolated from children hospitalized with community-acquired pneumonia in China. Sci Rep. 2018;8(1):4491.
Lu B, Liu H, Tabor DE, et al. Emergence of new antigenic epitopes in the glycoproteins of human respiratory syncytial virus collected from a US surveillance study, 2015–17. Sci Rep. 2019;9(1):3898.
Zhou X, Jiang M, Wang F, et al. Immune escaping of the novel genotypes of human respiratory syncytial virus based on gene sequence variation. Front Immunol. 2022;13:1084139.
Widjaja I, Wicht O, Luytjes W, et al. Characterization of epitope-specific anti-respiratory syncytial virus (anti-RSV) antibody responses after natural infection and after vaccination with formalin-inactivated RSV. J Virol. 2016;90(13):5965–77.
Tang A, Chen Z, Cox KS, et al. A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein. Nat Commun. 2019;10(1):4153.
Crank MC, Ruckwardt TJ, Chen M, et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science. 2019;365(6452):505–9.
Falsey AR, Walsh EE, Scott DA, et al. Phase 1/2 randomized study of the immunogenicity, safety, and tolerability of a respiratory syncytial virus prefusion F vaccine in adults with concomitant inactivated influenza vaccine. J Infect Dis. 2022;225(12):2056–66.
Pierangeli A, Viscido A, Bitossi C, et al. Differential interferon gene expression in bronchiolitis caused by respiratory syncytial virus-A genotype ON1. Med Microbiol Immunol. 2020;209(1):23–8.
Russell CD, Unger SA, Walton M, Schwarze J. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev. 2017;30(2):481–502.
Chen J, Tan S, Avadhanula V, et al. Diversity and evolution of computationally predicted T cell epitopes against human respiratory syncytial virus. PLoS Comput Biol. 2023;19(1):e1010360.
Blunck BN, Angelo LS, Henke D, et al. Adult memory T cell responses to the respiratory syncytial virus fusion protein during a single RSV season (2018–2019). Front Immunol. 2022;13:823652.
Jones HG, Battles MB, Lin CC, Bianchi S, Corti D, McLellan JS. Alternative conformations of a major antigenic site on RSV F. PLoS Pathog. 2019;15(7):e1007944.
Zhu Q, McLellan JS, Kallewaard NL, et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med. 2017;9(388):eaaj1928.
Simões EAF, Madhi SA, Muller WJ, et al. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: a pooled analysis of randomised controlled trials. Lancet Child Adolesc Health. 2023;7(3):180–9.
Griffin MP, Yuan Y, Takas T, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383(5):415–25.
Lin GL, Drysdale SB, Snape MD, et al. Distinct patterns of within-host virus populations between two subgroups of human respiratory syncytial virus. Nat Commun. 2021;12(1):5125.
Wilkins D, Langedijk AC, Lebbink RJ, et al. Nirsevimab binding-site conservation in respiratory syncytial virus fusion glycoprotein worldwide between 1956 and 2021: an analysis of observational study sequencing data. Lancet Infect Dis. 2023;23(7):856–66.
Simões EAF, Forleo-Neto E, Geba GP, et al. Suptavumab for the prevention of medically attended respiratory syncytial virus infection in preterm infants. Clin Infect Dis. 2021;73(11):e4400–8.
Phuah JY, Maas BM, Tang A, et al. Quantification of clesrovimab, an investigational, half-life extended, anti-respiratory syncytial virus protein F human monoclonal antibody in the nasal epithelial lining fluid of healthy adults. Biomed Pharmacother. 2023;169:115851.
Mufson MA, Akerlind-Stopner B, Orvell C, Belshe RB, Norrby E. A single-season epidemic with respiratory syncytial virus subgroup B2 during 10 epidemic years, 1978 to 1988. J Clin Microbiol. 1991;29(1):162–5.
Mufson MA, Belshe RB, Orvell C, Norrby E. Respiratory syncytial virus epidemics: variable dominance of subgroups A and B strains among children, 1981–1986. J Infect Dis. 1988;157(1):143–8.
Heikkinen T, Waris M, Ruuskanen O, Putto-Laurila A, Mertsola J. Incidence of acute otitis media associated with group A and B respiratory syncytial virus infections. Acta Paediatr. 1995;84(4):419–23.
Tsutsumi H, Onuma M, Nagai K, Yamazaki H, Chiba S. Clinical characteristics of respiratory syncytial virus (RSV) subgroup infections in Japan. Scand J Infect Dis. 1991;23(6):671–4.
Monto AS, Ohmit S. Respiratory syncytial virus in a community population: circulation of subgroups A and B since 1965. J Infect Dis. 1990;161(4):781–3.
Hendry RM, Talis AL, Godfrey E, Anderson LJ, Fernie BF, McIntosh K. Concurrent circulation of antigenically distinct strains of respiratory syncytial virus during community outbreaks. J Infect Dis. 1986;153(2):291–7.
Russi JC, Delfraro A, Arbiza JR, et al. Antigenic characterization of respiratory syncytial virus associated with acute respiratory infections in Uruguayan children from 1985 to 1987. J Clin Microbiol. 1989;27(7):1464–6.
Wilson E, Orvell C, Morrison B, Thomas E. Pediatric RSV infection during two winter seasons in British Columbia: a role for subgroup analysis in young children? Can J Infect Dis. 1990;1(4):112–6.
McIntosh ED, De Silva LM, Oates RK. Clinical severity of respiratory syncytial virus group A and B infection in Sydney. Aust Pediatr Infect Dis J. 1993;12(10):815–9.
Fletcher JN, Smyth RL, Thomas HM, Ashby D, Hart CA. Respiratory syncytial virus genotypes and disease severity among children in hospital. Arch Dis Child. 1997;77(6):508–11.
Kneyber MC, Brandenburg AH, Rothbarth PH, de Groot R, Ott A, van Steensel-Moll HA. Relationship between clinical severity of respiratory syncytial virus infection and subtype. Arch Dis Child. 1996;75(2):137–40.
Wang EE, Law BJ, Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr. 1995;126(2):212–9.
Agoti CN, Mwihuri AG, Sande CJ, et al. Genetic relatedness of infecting and reinfecting respiratory syncytial virus strains identified in a birth cohort from rural Kenya. J Infect Dis. 2012;206(10):1532–41.
Kaplan NM, Dove W, Abd-Eldayem SA, Abu-Zeid AF, Shamoon HE, Hart CA. Molecular epidemiology and disease severity of respiratory syncytial virus in relation to other potential pathogens in children hospitalized with acute respiratory infection in Jordan. J Med Virol. 2008;80(1):168–74.
Paulus SC, Hirschfeld AF, Victor RE, Brunstein J, Thomas E, Turvey SE. Common human Toll-like receptor 4 polymorphisms-role in susceptibility to respiratory syncytial virus infection and functional immunological relevance. Clin Immunol. 2007;123(3):252–7.
Rodriguez-Fernandez R, Tapia LI, Yang CF, et al. Respiratory syncytial virus genotypes, host immune profiles, and disease severity in young children hospitalized with bronchiolitis. J Infect Dis. 2017;217(1):24–34.
Fodha I, Vabret A, Ghedira L, et al. Respiratory syncytial virus infections in hospitalized infants: association between viral load, virus subgroup, and disease severity. J Med Virol. 2007;79(12):1951–8.
Midulla F, Nenna R, Scagnolari C, et al. How respiratory syncytial virus genotypes influence the clinical course in infants hospitalized for bronchiolitis. J Infect Dis. 2019;219(4):526–34.
Yoshihara K, Le MN, Okamoto M, et al. Association of RSV-A ON1 genotype with increased pediatric acute lower respiratory tract infection in Vietnam. Sci Rep. 2016;6:27856.
Panayiotou C, Richter J, Koliou M, Kalogirou N, Georgiou E, Christodoulou C. Epidemiology of respiratory syncytial virus in children in Cyprus during three consecutive winter seasons (2010–2013): age distribution, seasonality and association between prevalent genotypes and disease severity. Epidemiol Infect. 2014;142(11):2406–11.
Gamiño-Arroyo AE, Moreno-Espinosa S, Llamosas-Gallardo B, et al. Epidemiology and clinical characteristics of respiratory syncytial virus infections among children and adults in Mexico. Influenza Other Respir Viruses. 2017;11(1):48–56.
Oladokun R, Muloiwa R, Hsiao NY, Valley-Omar Z, Nuttall J, Eley B. Clinical characterisation and phylogeny of respiratory syncytial virus infection in hospitalised children at Red Cross War Memorial Children’s Hospital, Cape Town. BMC Infect Dis. 2016;16:236.
Hibino A, Saito R, Taniguchi K, et al. Molecular epidemiology of human respiratory syncytial virus among children in Japan during three seasons and hospitalization risk of genotype ON1. PLoS ONE. 2018;13(1):e0192085.
Valley-Omar Z, Tempia S, Hellferscee O, et al. Human respiratory syncytial virus diversity and epidemiology among patients hospitalized with severe respiratory illness in South Africa, 2012–2015. Influenza Other Respir Viruses. 2022;16(2):222–35.
Yoon JG, Noh JY, Choi WS, et al. Clinical characteristics and disease burden of respiratory syncytial virus infection among hospitalized adults. Sci Rep. 2020;10(1):12106.
Espinosa Y, San Martín C, Torres AA, et al. Genomic loads and genotypes of respiratory syncytial virus: viral factors during lower respiratory tract infection in chilean hospitalized infants. Int J Mol Sci. 2017;18(3):654.
Utsunomiya T, Hibino A, Taniguchi K, et al. Factors contributing to symptom duration and viral reduction in outpatient children with respiratory syncytial virus infection. Pediatr Infect Dis J. 2020;39(8):678–83.
Ciarlitto C, Vittucci AC, Antilici L, et al. Respiratory syncityal virus A and B: three bronchiolitis seasons in a third level hospital in Italy. Ital J Pediatr. 2019;45(1):115.
Devincenzo JP. Natural infection of infants with respiratory syncytial virus subgroups A and B: a study of frequency, disease severity, and viral load. Pediatr Res. 2004;56(6):914–7.
Moreira FB, Rosario CS, Santos JS, et al. Molecular characterization and clinical epidemiology of human respiratory syncytial virus (HRSV) A and B in hospitalized children, Southern Brazil. J Med Virol. 2017;89(8):1489–93.
Papadopoulos NG, Gourgiotis D, Javadyan A, et al. Does respiratory syncytial virus subtype influences the severity of acute bronchiolitis in hospitalized infants? Respir Med. 2004;98(9):879–82.
Laham FR, Mansbach JM, Piedra PA, et al. Clinical profiles of respiratory syncytial virus subtypes A AND B among children hospitalized with bronchiolitis. Pediatr Infect Dis J. 2017;36(8):808–10.
Taylor CE, Morrow S, Scott M, Young B, Toms GL. Comparative virulence of respiratory syncytial virus subgroups A and B. Lancet. 1989;1(8641):777–8.
Hall CB, Walsh EE, Schnabel KC, et al. Occurrence of groups A and B of respiratory syncytial virus over 15 years: associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis. 1990;162(6):1283–90.
McConnochie KM, Hall CB, Walsh EE, Roghmann KJ. Variation in severity of respiratory syncytial virus infections with subtype. J Pediatr. 1990;117(1 Pt 1):52–62.
Salomón HE, Avila MM, Cerqueiro MC, Orvell C, Weissenbacher M. Clinical and epidemiologic aspects of respiratory syncytial virus antigenic variants in Argentinian children. J Infect Dis. 1991;163(5):1167.
Walsh EE, McConnochie KM, Long CE, Hall CB. Severity of respiratory syncytial virus infection is related to virus strain. J Infect Dis. 1997;175(4):814–20.
Imaz MS, Sequeira MD, Videla C, et al. Clinical and epidemiologic characteristics of respiratory syncytial virus subgroups A and B infections in Santa Fe, Argentina. J Med Virol. 2000;61(1):76–80.
Martinello RA, Chen MD, Weibel C, Kahn JS. Correlation between respiratory syncytial virus genotype and severity of illness. J Infect Dis. 2002;186(6):839–42.
Tabarani CM, Bonville CA, Suryadevara M, et al. Novel inflammatory markers, clinical risk factors and virus type associated with severe respiratory syncytial virus infection. Pediatr Infect Dis J. 2013;32(12):e437–42.
Gilca R, De Serres G, Tremblay M, et al. Distribution and clinical impact of human respiratory syncytial virus genotypes in hospitalized children over 2 winter seasons. J Infect Dis. 2006;193(1):54–8.
Tran DN, Pham TM, Ha MT, et al. Molecular epidemiology and disease severity of human respiratory syncytial virus in Vietnam. PLoS ONE. 2013;8(1):e45436.
Straliotto SM, Roitman B, Lima JB, Fischer GB, Siqueira MM. Respiratory syncytial virus (RSV) bronchiolitis: comparative study of RSV groups A and B infected children. Rev Soc Bras Med Trop. 1994;27(1):1–4.
Hornsleth A, Klug B, Nir M, et al. Severity of respiratory syncytial virus disease related to type and genotype of virus and to cytokine values in nasopharyngeal secretions. Pediatr Infect Dis J. 1998;17(12):1114–21.
Liu W, Chen D, Tan W, et al. Epidemiology and clinical presentations of respiratory syncytial virus subgroups A and B detected with multiplex real-time PCR. PLoS ONE. 2016;11(10):e0165108.
Chen X, Zhu Y, Wang W, et al. A multi-center study on molecular epidemiology of human respiratory syncytial virus from children with acute lower respiratory tract infections in the mainland of China between 2015 and 2019. Virol Sin. 2021;36(6):1475–83.
Adhikari B, Hassan F, Harrison CJ, et al. A multi-center study to determine genetic variations in the fusion gene of respiratory syncytial virus (RSV) from children <2 years of age in the U.S. J Clin Virol. 2022;154:105223.
Tramuto F, Maida CM, Di Naro D, et al. Respiratory syncytial virus: new challenges for molecular epidemiology surveillance and vaccination strategy in patients with ILI/SARI. Vaccines (Basel). 2021;9(11):1334.
Cantú-Flores K, Rivera-Alfaro G, Muñoz-Escalante JC, Noyola DE. Global distribution of respiratory syncytial virus A and B infections: a systematic review. Pathog Glob Health. 2022;116(7):398–409.
Falsey AR, Singh HK, Walsh EE. Serum antibody decay in adults following natural respiratory syncytial virus infection. J Med Virol. 2006;78(11):1493–7.
European Medicines Agency. First vaccine to protect older adults from respiratory syncytial virus (RSV) infection [press release]. 2023. https://www.ema.europa.eu/en/news/first-vaccine-protect-older-adults-respiratory-syncytial-virus-rsv-infection
US FDA. FDA approves first respiratory syncytial virus (RSV) vaccine—arexvy approved for individuals 60 years of age and older [press release]. 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine
European Medicines Agency. First RSV vaccine to protect infants up to 6 months of age and older adults [press release]. 2023. https://www.ema.europa.eu/en/news/first-rsv-vaccine-protect-infants-6-months-age-and-older-adults
US FDA. FDA approves first vaccine for pregnant individuals to prevent RSV in infants [press release]. 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-pregnant-individuals-prevent-rsv-infants
Schwarz TF, Johnson C, Grigat C, et al. Three dose levels of a maternal respiratory syncytial virus vaccine candidate are well tolerated and immunogenic in a randomized trial in nonpregnant women. J Infect Dis. 2022;225(12):2067–76.
Walsh EE, Pérez Marc G, Zareba AM, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465–77.
Mukhamedova M, Wrapp D, Shen CH, et al. Vaccination with prefusion-stabilized respiratory syncytial virus fusion protein induces genetically and antigenically diverse antibody responses. Immunity. 2021;54(4):769–80.e6.
Joyce MG, Bao A, Chen M, et al. Crystal structure and immunogenicity of the DS-Cav1-stabilized fusion glycoprotein from respiratory syncytial virus subtype B. Pathog Immun. 2019;4(2):294–323.
Leroux-Roels I, Davis MG, Steenackers K, et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F (RSVPreF3) candidate vaccine in older adults: phase 1/2 randomized clinical trial. J Infect Dis. 2023;227(6):761–72.
Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med. 2023;388(7):595–608.
Walsh EE, Falsey AR, Scott DA, et al. A randomized phase 1/2 study of a respiratory syncytial virus prefusion F vaccine. J Infect Dis. 2022;225(8):1357–66.
Ison MG, Papi A, Athan E, et al. Efficacy and safety of respiratory syncytial virus prefusion F protein vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons. Clin Infect Dis. 2024;78(6):1732–44.
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two [press release]. 2024. Accessed 29 Feb 2024.
Das R. Overview of Moderna’s investigational RSV vaccine (mRNA-1345) in adults ≥ 60 years of age. CDC Advisory Committee on Immunization Practices (ACIP). 19-Feb-2024. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2024-02-28-29/02-RSV-Adults-Das-508.pdf.
Che Y, Gribenko AV, Song X, et al. Rational design of a highly immunogenic prefusion-stabilized F glycoprotein antigen for a respiratory syncytial virus vaccine. Sci Transl Med. 2023;15:6422.
Medical Writing, Editorial, and Other Assistance
The authors would like to thank Alejandra Gonzalez (P95 Epidemiology & Pharmacovigilance) for editorial review, which was funded by Pfizer, and Kena Swanson (Pfizer) for critical review.
Funding
This work and the journal’s rapid service fee was funded by Pfizer Inc.
Author information
Authors and Affiliations
Contributions
Charles Nuttens, Daniel Curcio, Elizabeth Begier, and Marc Baay contributed to the study conception and design. Material preparation, data collection and analysis were performed by Juliette Moyersoen and Marc Baay. The first draft of the manuscript was written by Juliette Moyersoen and Marc Baay and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Juliette Moyersoen, Marc Baay, Zuleika Aponte-Torres, and Hilde Vroling are employees of P95, which received funding from Pfizer in connection with the development of this manuscript. JM owns shares in the GSK group of companies as part of her previous employee remuneration. Charles Nuttens, Daniel Curcio, Bradford Gessner and Elizabeth Begier are employees of Pfizer and may own Pfizer stock.
Ethical Approval
This study is an integrative review of published data and, therefore, ethical approval was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior Presentation: RSV subgroups A and B epidemiology and differences in clinical severity data have been presented at the 7th ReSVINET conference in Lisbon, Portugal, on 22–24 February 2023 as a poster.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nuttens, C., Moyersoen, J., Curcio, D. et al. Differences Between RSV A and RSV B Subgroups and Implications for Pharmaceutical Preventive Measures. Infect Dis Ther (2024). https://doi.org/10.1007/s40121-024-01012-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40121-024-01012-2