Abstract
Introduction
Many immunization programs in Europe recommend quadrivalent meningococcal vaccinations, which are often administered concomitantly with other vaccines. We compared the immune response of a tetanus toxoid conjugated quadrivalent meningococcal vaccine (MenACYW-TT, MenQuadfi®) with another quadrivalent meningococcal conjugate vaccine (MCV4-TT; Nimenrix®) when administered alone or concomitantly with Tdap-IPV and 9vHPV vaccines in adolescents.
Methods
In this phase IIIb trial, healthy adolescents (MenC-naïve or MenC-primed before 2 years of age) from Spain, Italy, Hungary, and Singapore were randomized in a 3:3:2 ratio to receive either MenACYW-TT or MCV4-TT alone, or MenACYW-TT concomitantly with 9vHPV and Tdap-IPV. The primary objective was to demonstrate the non-inferiority of the seroprotection rate (human serum bactericidal assay [hSBA] titer ≥ 1:8) to serogroups A, C, W, and Y 30 days post-vaccination with a single dose of MenACYW-TT or MCV4-TT. Secondary objectives included describing hSBA titers for the four serogroups before and 1 month following vaccination and according to MenC priming status.
Results
A total of 463 participants were enrolled (MenACYW-TT, n = 173; MCV4-TT, n = 173; MenACYW-TT/9vHPV/Tdap-IPV n = 117). Non-inferiority based on seroprotection was demonstrated for MenACYW-TT versus MCV4-TT for all serogroups. Immune responses were comparable whether MenACYW-TT was administered alone or concomitantly with Tdap-IPV and 9vHPV. Post-vaccination hSBA GMTs were higher in MenACYW-TT vs. MCV4-TT for serogroups C, Y, and W and comparable for serogroup A. The percentages of participants with an hSBA vaccine seroresponse were higher in MenACYW-TT vs. MCV4-TT for all serogroups. For serogroup C, higher GMTs were observed in both MenC-naïve or -primed participants vaccinated with MenACYW-TT vs. MCV4-TT. Seroprotection and seroresponse were higher in MenC-naïve participants vaccinated with MenACYW-TT vs. MCV4-TT and comparable in MenC-primed. The safety profiles were comparable between groups and no new safety concerns were identified.
Conclusions
These data support the concomitant administration of MenACYW-TT with 9vHPV and Tdap-IPV vaccines in adolescents.
Trial Registrations
Clinicaltrials.gov, NCT04490018; EudraCT: 2020-001665-37; WHO: U1111-1249-2973.
Plain Language Summary
MenACYW conjugate vaccine has been made to protect against meningococcal disease caused by four common types of bacteria (germs) called Neisseria meningitidis (or meningococcus), A, C, W, and Y. Many people, particularly adolescents, have the germs of this disease in their nose or throat, and therefore may develop the disease or transmit the bacteria to other people. Hence, adolescent meningococcal vaccination against serogroups ACWY is increasingly recommended in several countries. This study assessed the immune response to these serogroups in healthy adolescents after one dose of MenACYW conjugate vaccine or Nimenrix®, a meningococcal licensed vaccine. Moreover, the immune response and safety were assessed when the vaccines were given alone or when given concomitantly with other adolescent vaccines, including the human papillomavirus (9vHPV) and tetanus, diphtheria, pertussis, and poliomyelitis (Tdap-IPV) vaccines. A total of 463 adolescents (aged 10–17 years) participated in this study and received either MenACYW or Nimenrix® alone, or MenACYW concomitantly with 9vHPV and Tdap-IPV vaccine. The immune response induced by MenACYW was as good as the immune response induced by Nimenrix®, and when given alone or concomitantly with 9vHPV and Tdap IPV vaccines. None of the participants experienced any serious side effects of any vaccine. The most common non-serious side effects were injection site pain, muscle pain, and headache. These data support the use of MenACYW in adolescents, with or without concomitant administration with 9vHPV and Tdap-IPV, which may help to increase the number of adolescents vaccinated.
AbstractSection Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
As carriage rates of Neisseria meningitidis bacteria, which causes invasive meningococcal disease, are highest in adolescents, vaccination against serogroups A, C, W, and Y is increasingly recommended in adolescents in several countries. |
Concomitant administration with other recommended adolescent vaccines may improve vaccine coverage rates. |
We conducted a phase IIIb trial to compare the immunogenicity and safety of MenACYW-TT with MCV4-TT when administered alone or concomitantly with Tdap-IPV and 9vHPV vaccines in adolescents. |
Non-inferiority of seroprotection was demonstrated for MenACYW-TT versus MCV4-TT 30 days after administration of a single dose and immune responses were comparable whether MenACYW-TT was administered alone or concomitantly with Tdap-IPV and 9vHPV. |
The data demonstrate the immunogenicity and safety of MenACYW-TT and support its use in adolescents, alone or concomitantly with 9vHPV and Tdap-IPV. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.26029237.
Introduction
Neisseria meningitidis infection, leading to invasive meningococcal disease (IMD), was notified in the European Economic Area at a rate of 0.57 per 100,000 in 2019 [1]. While the incidence of IMD is greatest in infants, it also peaks in adolescents, who have the highest carriage rates of N. meningitidis compared with other age groups [1, 2].
As a result of meningococcal serogroup C (MenC) outbreaks in 1999 to 2001, MenC conjugate vaccine immunization programs were launched for infants in a number of European countries [3]. Therefore, a substantial proportion of adolescents in Europe are MenC vaccine-primed. Despite a decrease in the incidence of IMD in Europe in recent years, there has been an increase in infections with serogroups W and Y [1, 4], leading to recommendations for adolescent vaccination with quadrivalent meningococcal ACWY vaccine in several countries. Meningococcal ACWY vaccination during adolescence not only directly protects against IMD but also diminishes carriage, thereby sustaining herd protection across all age groups [5,6,7].
The tetanus toxoid conjugated quadrivalent meningococcal vaccine, MenACYW-TT (MenQuadfi®), is approved for use in individuals aged 12 months and older in the European Union (EU) and in those aged 2 years and older in the USA [8, 9]. In the adolescent population, the clinical studies for MenACYW-TT were conducted in the USA using other licensed quadrivalent meningococcal conjugate vaccines as comparators, including MenACWY-CRM (Menveo®) and MenACYW-DT (Menactra®) [10, 11]. MenACYW-TT was shown to have non-inferior immune responses and comparable safety versus MenACWY-CRM in meningococcal vaccine-naïve adolescents [10], and meningococcal vaccine-primed adolescents and adults compared with MenACWY-DT [11]. The meningococcal conjugate vaccine-quadrivalent (MCV4-TT; Nimenrix®) is licensed for use in adolescents in Europe, but not in the USA. Previous studies comparing MenACYW-TT with MCV4-TT in the EU have been conducted in toddlers [12,13,14]. Therefore, it was of interest to compare the immune response of MenACYW-TT with that of MCV4-TT in adolescents, for which there are no current studies in the EU. In the European context, we conducted a study including adolescents who were either meningococcal vaccine-naïve or MenC-primed before 2 years of age to reflect the implementation of MenC immunization programs targeting infants/toddlers in some EU countries since 2000.
Both MenACYW-TT and MCV4-TT are tetanus toxoid conjugate vaccines. Each dose of MenACYW-TT contains 10 μg of capsular polysaccharide from each of serogroups A, C, Y, and W conjugated to 55 μg of tetanus toxoid (TT) carrier protein [15]. MCV4-TT contains 5 μg each of oligosaccharides from serogroups A, C, W, and Y conjugated to 44 μg of tetanus toxoid carrier protein [16]. In contrast to quadrivalent meningococcal conjugate vaccines previously approved for use in the EU (MCV4-TT and MenACWY-CRM), MenACYW-TT is available in a liquid formulation, whereas Nimenrix presentation is powder and solvent for solution for injection (two vials per dose) and it requires reconstitution prior to administration [15, 16]. Therefore, it is of interest to compare the immunogenicity and safety of MenACYW-TT and MCV4-TT.
Of note, Bordetella pertussis and oncogenic human papillomavirus (HPV) serotypes are also significant disease-causing pathogens of which adolescents and young adults are key reservoirs for transmission, and therefore vaccination against these pathogens is recommended in this population [17, 18]. Three or four tetanus and diphtheria vaccine doses before 2 years of age are recommended in all European countries, with up to three booster doses between age 2 to 17 years, as well as vaccination against pertussis in infants (utilizing the diphtheria, tetanus, and acellular pertussis vaccine [DTaP]), with the majority also recommending vaccination in adolescents (utilizing tetanus with reduced diphtheria acellular pertussis doses [Tdap]) [19, 20]. Many national immunization programs now include vaccination against polio with Tdap vaccination (Tdap-IPV), as opposed to Tdap vaccination alone. Since 2006, a number of European countries have implemented vaccination against HPV into their national immunization programs [21]. These programs targeted adolescent girls initially and then extended to boys in some countries, usually starting between 10 and 12 years of age and up to 18 years of age with either a two- or three-dose series according to the national recommendations and age of the subject at the initiation of HPV vaccination [21]. Current guidance published by the European Centre for Disease Prevention and Control now recommends vaccination with the 9-valent HPV (9vHPV) vaccine to provide protection against five further HPV types, thereby gradually phasing out the use of 4vHPV [22]. Similarly, 4vHPV is no longer administered in the USA, with HPV vaccination consisting solely of 9vHPV [23].
Meningococcal vaccines are often administered concomitantly with other adolescent vaccines, including with Tdap, Tdap-IPV, and HPV vaccines. Previous data show that the immunogenicity and safety profile of the MenACYW-TT is comparable when administered with or without Tdap and 4vHPV vaccines [10]. However, concomitant administration of MenACYW-TT with Tdap-IPV and 9vHPV has not been investigated. Here, we present the results of a phase IIIb, partially observer-blind, randomized, active-controlled, parallel-group, multi-center study comparing the immunogenicity and safety of MenACYW-TT versus MCV4-TT, administered alone or concomitantly with 9vHPV and Tdap-IPV vaccines in healthy adolescents, who are either meningococcal vaccine-naïve or MenC-primed before 2 years of age.
Methods
Participants and Study Design
This study was conducted in accordance with the protocol and consensus ethical principles derived from international guidelines including the Declaration of Helsinki and the International Council for Harmonization (ICH) guidelines for Good Clinical Practice (GCP), and all applicable laws, rules, and regulations. Participants turning 18 years old during the study or parents/legal representatives provided informed consent. Ethical approval was obtained from the following committees:
Singapore: NHG Domain Specific Review Boards (DSRB).
Spain: Comité de Ética de la Investigación Con Medicamentos del Hospital General Universitario Gregorio Marañón.
Hungary: Egészségügyi Tudományos Tanács Klinikai Farmakológiai Etikai Bizottság/Medical Research Council Ethics Committee for Clinical Pharmacology.
Italy: Comitato Etico Regionale Liguria Largo Rosanna Benzi; Comitato Etico Milano Area 1—ASST Sacco e Fatebenefratelli; Comitato Etico dell’Area Vasta Emilia Nord; Segreteria Locale di Parma c/o Azienda Ospedaliero; Universitaria di Parma Via Gramsci 14; Comitato Etico Palermo 1; Comitato Etico “Ospedali Riuniti” Di Foggia; Comitato Etico Regione Calabria—Sezione Area Centro.
Participants were recruited from 20 sites in Europe (Spain, Italy, and Hungary) and one site in Asia (Singapore). Healthy adolescents aged 10–17 years who were either MenC vaccine-primed before 2 years of age or MenC-naïve and were covered by health insurance, if required by local regulations, were included. Exclusion criteria included pregnancy, lactation, or not using effective contraception if the individual was of child-bearing age, as well as previous vaccination against meningococcal disease with either the study vaccine or another vaccine, excluding MenC vaccination during infancy. Similarly, participants with any previous history of HPV vaccination or any tetanus, diphtheria, pertussis, or inactivated polio virus vaccine within the last 3 years were excluded. Participants turning 18 years old during the study or parents/legal representatives provided informed consent. Assent forms were completed as per local regulations.
Participants were enrolled and randomized between March 16, 2021 and December 22, 2021 in a 3:3:2 ratio to receive either MenACYW-TT on D01 and 9vHPV + Tdap-IPV on D31 (group 1), MCV4-TT on D01 and 9vHPV + Tdap-IPV on D31 (group 2), or MenACYW-TT and 9vHPV + Tdap-IPV on D01 (group 3) (Fig. 1). Groups 1 and 2 were observer-blind and group 3 was open-label.
Interventions
MenACYW-TT (MenQuadfi, Sanofi Inc., Swiftwater, PA, USA) was presented as a ready-to-use solution in single-dose vials. Each dose contained 10 μg meningococcal capsular polysaccharides from the four serogroups conjugated to approximately 55 μg tetanus toxoid protein carrier. MCV4-TT (Nimenrix, Pfizer Limited, Sandwich, UK) was presented as a lyophilized powder and solvent for resuspension, containing 5 μg meningococcal capsular polysaccharides from the four serogroups conjugated to approximately 44 μg tetanus toxoid protein carrier. 9vHPV (Gardasil® 9, Merck, Sharp & Dohme Limited, Kenilworth, NJ, USA) was presented a ready to use suspension containing: 30 μg HPV type 6 L1 protein; 40 μg HPV type 11 L1 protein; 60 μg HPV type 16 L1 protein; 40 μg HPV type 18 L1 protein; 20 μg HPV type 31 L1 protein; 20 μg HPV type 33 L1 protein; 20 μg HPV type 45 L1 protein; 20 μg HPV type 52 L1 protein; and 20 μg HPV type 58 L1 protein. Tdap-IPV (Repevax®/Triaxis® Polio/Adacel® Polio, Sanofi Limited, Toronto, Canada) was presented as a ready-to-use suspension containing: 5 Lf of tetanus toxoid, 2 Lf of diphtheria toxoid and acellular pertussis antigens [2.5 µg pertussis toxin (PT), 5 µg filamentous hemagglutinin (FHA), 3 µg pertactin, and 5 µg fimbriae], and IPV type 1 (Mahoney strain) 40 D-antigen unit, type 2 (MEF-1 strain) 8 D-antigen unit, and type 3 (Saukett strain) 32 D-antigen unit. All vaccines were given at 0.5 ml per dose intramuscularly (deltoid).
Endpoints
hSBA and baby rabbit complement assay (rSBA) (in a subgroup of participants in each group) were used to measure antibodies against the four meningococcal vaccine serogroups at D0 and D31. Seroprotection against each meningococcal serogroup was defined as hSBA titer ≥ 1:8. [11,12,13, 24, 25]. The proportion of participants with rSBA titers ≥ 1:8 for each meningococcal serogroups was also assessed. The seroprotection thresholds of hSBA and rSBA titers ≥ 1:8 were defined based on previous research on the MenACYW-TT and MCV4-TT vaccines [11,12,13, 24,25,26,27]. Seroresponse to meningococcal vaccination was defined as a post-vaccination hSBA titer ≥ 1:16 in participants with pre-vaccination hSBA titer < 1:8, or a post-vaccination titer at least fourfold greater than the pre-vaccination titer in those with a pre-vaccination titer ≥ 1:8. For rSBA titers, this was defined as a post-vaccination titer ≥ 1:32 for participants with pre-vaccination rSBA titer < 1:8, or a post-vaccination titer ≥ fourfold increase from baseline for participants with pre-vaccination rSBA titer ≥ 1:8. Antibody titers/concentrations against antigens contained in 9vHPV and Tdap-IPV vaccines were measured 30 days after vaccination with 9vHPV and Tdap-IPV. Vaccine seroconversion for each of the HPV types (6, 11, 16, 18, 31, 33, 45, 52, and 58) by D31 (+ 14 days) after vaccination, with seroconversion was defined as change in serostatus from seronegative at D01 to seropositive by D31. A participant with a titer at or above the serostatus cut-off for a given HPV type was considered seropositive for that type. The serostatus cut-offs were 9, 6, 5, 5, 3, 4, 3, 5, and 5 milli-Merck units (mMU)/ml for HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58, respectively. The rSBA testing was performed at the Vaccine Evaluation Unit, Public Health Laboratory, Manchester, UK. All other antibody assessments were performed at Global Clinical Immunology (GCI) (Swiftwater, PA, USA) or at qualified contract laboratories for GCI. Participants were kept under observation for 30 min after vaccination to ensure their safety and to record any immediate adverse events (AEs) and adverse reactions (ARs). Solicited injection-site reactions were collected from D01 to D08 after each vaccination. AEs were collected from D01 until D31 after each vaccination and serious AEs (SAEs) and AEs of special interest (AESIs) were collected throughout the study. Participants, or their parents or legally acceptable representatives, recorded AEs and SAEs in a diary card specifically designed for this study, and were provided rulers for measuring the size of injection site reactions and standard digital thermometers for measuring daily temperatures prior to the study. Participants or their parents/legally acceptable representatives were interviewed at specified intervals to collect the information recorded in the diary card and to clarify anything that was incomplete or unclear. All clinical study information gathered was reported electronically by the investigators using a web-based case report form. Safety outcomes were classified using "MedDRA", the Medical Dictionary for Regulatory Activities (version 25.1) [28].
Objectives
The primary objective was to demonstrate non-inferiority of the seroprotection rate achieved with MenACYW-TT after 30 days (day 31 [D31]) post-vaccination (percentage of participants achieving seroprotection, defined as hSBA titers ≥ 1:8 for meningococcal serogroups A, C, W, and Y) to that achieved with MCV4-TT.
Key secondary objectives included describing the immune response of meningococcal serogroups A, C, W, and Y measured by hSBA before and at D31 post-vaccination with MenACYW-TT or MCV4-TT, and additionally, describing the antibody response to serogroup C measured by hSBA and by serum bactericidal assay using rSBA before and at D31 post-vaccination with MenACYW-TT, and according to MenC priming status (MenC-naïve or MenC-primed during infancy). Other key secondary objectives were to describe the immune response of 9vHPV and Tdap-IPV vaccine antigens before and at D31 post-vaccination when administered alone or concomitantly with MenACYW-TT, and to describe the safety profile in each group after each and any vaccination. Observational objectives included describing the kinetics of antibody titers against the four meningococcal serogroups in the first 60 participants in group 3, measured by hSBA before and at D07 and D31 post-vaccination, and describing the antibody response against the four meningococcal serogroups as measured by rSBA, before and at D31 post-meningococcal vaccination, in the first 50 participants in each group.
Statistical Analyses
A total of 464 participants were planned to be enrolled including 174 subjects in groups 1 and 2 and 116 in group 3. For the primary objective, 378 participants were planned to be enrolled in group 1 and group 2 (174 participants per group), to ensure the study had > 90% power (Farrington and Manning formula [29])) to declare the non-inferiority of MenACYW-TT (group 1) versus MCV4-TT (group 2) based on A, C, W, and Y hSBA seroprotection rates, assuming a 10% drop-out rate from the per-protocol analysis set (PPAS), a one-sided alpha level of 2.5% and a non-inferiority margin of 10%. The sample size was arbitrarily set to 116 participants in group 3, as these data were not intended to be used for hypothesis testing. For the secondary and observational objectives, no formal sample size calculations were performed.
For the primary objective, a non-inferiority approach was used to compare the D31 post-vaccination hSBA seroprotection rates in the MenACYW-TT group (group 1) to that in the MCV4-TT group (group 2). Non-inferiority was demonstrated if the lower limit of the 95% confidence interval (CI) of the difference was greater than – 10% for all four serogroups. The two-sided 95% CI was calculated based on the Wilson score method without continuity correction, as described by Newcombe 1998 [30]. For secondary and observational objectives, no hypotheses were tested and analyses were descriptive. In general, categorical variables were summarized and presented by frequency counts, percentages, and CIs. The 95% CIs of percentages were calculated using the exact binomial distribution (Clopper–Pearson method). For antibody geometric mean titers and geometric mean concentrations, 95% CIs of the point estimates were calculated using a normal approximation assuming these were log-normally distributed.
Analysis Sets
The per-protocol analysis set for meningococcal vaccines using hSBA (hSBA PPASM) and the per-protocol analysis set for meningococcal vaccines using rSBA (rSBA PPASM) were defined as the subset of participants who received a dose of a meningococcal vaccine, excluding those with at least one of the relevant protocol deviations, with a valid hSBA or rSBA result, respectively, on D31. The per-protocol analysis set for concomitant vaccines (PPASC) was defined as the subset of participants who received meningococcal vaccination with concomitant administration of 9vHPV and Tdap-IPV, excluding those with at least one of the relevant protocol deviations. The safety analysis set (SafAS) was defined as participants who received a dose of any vaccine who had safety data available. Safety endpoints are reported for the SafAS overall and at visit 1 (SafAS1) and at visit 2 (SafAS2).
Results
Study Participants
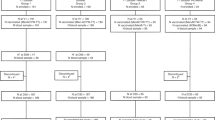
A total of 463 participants were enrolled and randomized between March 2021 and December 2021 into the study (group 1: MenACYW-TT alone, n = 173; group 2: MCV4-TT alone, n = 173; group 3: MenACYW-TT concomitantly with /9vHPV/Tdap-IPV, n = 117). For each country, the distribution of the participants was consistent with the randomization ratio (3:3:2). A total of 167 participants (96.5%) in group 1, 165 participants (95.4%) in group 2, and 116 participants (99.1%) in group 3 completed the study. A summary of the flow of participants through the study is presented in Fig. 2. Of those randomized, there were 312 (67.4%) males and 151 (32.6%) females (Table 1). Overall, the mean age was 12.6 ± 2.38 years and was similar across vaccination group. Of the 462 randomized participants with available history of meningococcal C vaccination, 326 (70.4%) participants were previously vaccinated before 2 years of age with a meningococcal C vaccine (MenC-primed) and 136 (29.4%) participants were not (MenC-naive). There were 120 (69.4%) MenC primed participants in group 1, 119 (68.8%) in group 2, and 87 (74.4%) in group 3. There were 53 (30.6%) MenC-naive participants each in groups 1 and 2, and 30 (25.6%) in group 3.
On D01, 458 participants (98.9%) provided a pre-vaccination blood sample (Fig. 2). In group 1, 170 participants (98.3%) received MenACYW-TT alone; in group 2, 171 participants (99.4%) received MCV4-TT alone and in group 3, 116 participants (99.1%) received MenACYW-TT concomitantly with Tdap-IPV and 9vHPV vaccines (PPASM; Fig. 2). The duration of the study (first participant first visit through to the last participant last visit) was 422 days, and the mean participation duration was 63 (± 19.4) days.
Primary Immunogenicity Objective
Non-inferiority of MenACYW-TT vs MCV4-TT When Administered Alone (Group 1 vs. Group 2)
Non-inferiority based on seroprotection rate against all four serogroups at D31 as determined by hSBA was demonstrated for MenACYW-TT alone versus MCV4-TT alone (Table 2).
Secondary Immunogenicity Objectives
Antibody Response to Meningococcal Vaccination When Administered Alone (Group 1 vs. Group 2)
The antibody responses as measured by hSBA at D31 (percentage achieving hSBA titers ≥ 1:8, GMTs, and percentage with seroresponse) are summarized by serogroup in Table 3 and Fig. 3. Seroprotection rates across the serogroups were similar in the MenACYW-TT alone and MCV4-TT groups. However, 30 days post-vaccination (D31), the hSBA GMTs were higher in the MenACYW-TT group versus the MCV4-TT group for serogroups C, Y, and W and comparable for serogroup A. The percentage of participants with an hSBA vaccine seroresponse were higher for MenACYW-TT compared with MCV4-TT for all serogroups. The same trends were observed when measured with rSBA (Supplementary Table 1).
Comparison of hSBA immune response to serogroups A, C, W and Y 30 days after vaccination on D01 with MenACYW-TT alone (group 1), MCV4-TT alone (group 2) and MenACYW-TT concomitantly with 9vHPV/Tdap-IPV (group 3): A proportion of participants with seroprotection (titers ≥ 1:8, B geometric mean titers, and C proportion of participants achieving seroresponse
Antibody Response to Meningococcal Serogroup C as Measured by hSBA According to MenC Primed Status
For serogroup C, higher GMTs were observed in both MenC-naïve or primed participants vaccinated with MenACYW-TT vs. MCV4-TT. Seroprotection and seroresponse for serogroup C were higher in MenC-naïve participants vaccinated with MenACYW-TT vs. MCV4-TT and comparable in MenC-primed participants (Table 4). Similar trends were observed when measured by rSBA (Supplementary Table 2).
Antibody Response to Meningococcal Vaccination as Measured with hSBA When Administered Alone or Concomitantly with Tdap-IPV and 9vHPV Vaccines (Group 1 vs. Group 3)
The antibody responses in terms of percentage of subjects achieving hSBA titers ≥ 1:8, GMTs, and percentage of subjects achieving seroresponse to the four meningococcal serogroups induced by MenACYW-TT alone or concomitantly with 9vHPV and Tdap-IPV vaccines were generally similar across the groups (Table 3; Fig. 3), except for GMTs serogroup A and W and seroresponses to serogroup A, which tended to be lower in the group that received MenACYW-TT concomitantly with 9vHPV and Tdap-IPV.
Antibody Responses Against 9vHPV and Tdap-IPV Vaccine Antigen When Administered 30 days After MenACYW-TT (Group 1) or Concomitantly with MenACYW-TT (Group 3)
Immunogenicity profiles after 9vHPV vaccination in terms of seroconversion rates (Table 5) and after Tdap-IPV vaccination in terms of response rates (Table 6) were comparable between both groups. No clinically relevant differences were observed in terms of antibody concentrations/titers, which tended to be lower in the group that received MenACYW-TT concomitantly with 9vHPV and Tdap-IPV vaccines compared to the group that received the 9vHPV and Tdap-IPV vaccines sequentially after MenACYW-TT for anti-HPV type-6 and type-58 antigens, and polio type 1 and type 3, and pertussis toxoid. Interestingly, geometric means for antibodies against diphtheria antigens were higher in the group that received the vaccines concomitantly.
Kinetics of Antibody Titers Against the Four Meningococcal Serogroups
The kinetics of the hSBA antibody titers against the four meningococcal serogroups observed in the first 60 participants in the group that received MenACWY-TT concomitantly with 9vHPV/Tdap-IPV vaccines are summarized in Supplementary Table 3. Overall, a rapid and robust immune response was observed with hSBA titers increasing mainly between D01 and D07, and to a lesser extent between D07 and D31.
Safety
Overall, the safety profiles were comparable between groups and no new safety concerns were identified (Table 7). There were no unsolicited AEs/adverse reactions (ARs) within 30 min of vaccination in any group. Unsolicited AEs/ARs within 30 days after vaccination were similar across vaccination groups. One SAE was reported in the group that received MCV4-TT alone (type 1 diabetes mellitus), but assessed as not related to the study vaccine. There were no AESIs, AEs leading to study discontinuation or deaths.
Discussion
During the extensive MenACYW-TT global clinical development program that led to its initial licensure, the vaccine has consistently demonstrated non-inferior immunogenicity against all four meningococcal serogroups compared to other available MenACWY vaccines, across all age groups (from age 12 months) and in both vaccine-naïve and primed participants [10, 11, 13, 14, 24]. Moreover, the immunogenicity of MenACYW-TT often tended to be higher than that for the comparators. This study confirms these previous observations and additionally supports the concomitant administration of MenACYW-TT with 9vHPV and Tdap-IPV vaccines.
Before this study, the studies in the adolescent population were conducted in the USA with locally licensed vaccines as comparators, MenACWY-DT (Menactra®), which is not licensed in Europe, and MenACWY-CRM (Menveo®). The non-inferiority of the immune response of MenACYW-TT was demonstrated versus these vaccines [10, 11]. The present study was conducted using MCV4-TT (Nimenrix), which is licensed in Europe but not the USA. In this study, the non-inferiority of MenACYW-TT compared with MCV4-TT based on hSBA seroprotection rate (≥ 1:8) against the four serogroups was demonstrated, which is in line with previous research [10]. There was a higher proportion of participants achieving hSBA seroresponse rates for all serogroups in those who received MenACYW-TT compared with those who received MCV4-TT. All findings measured by hSBA were similar when measured using rSBA, although titers using rSBA tend to be higher [31]. The safety outcomes for each vaccine were also comparable with previous research [10, 13, 32,33,34,35].
Prior to this study, there was a need for clinical data on immune response to meningococcal vaccination among adolescents primed with the MenC vaccine during infancy (below age 2 years), despite the fact that in Europe, MenC vaccination of infants has been recommended since the 2000s and therefore many adolescents may have been vaccinated with at least one dose of monovalent C vaccine. In the current study, as expected, adolescent participants who were MenC primed had greater hSBA GMTs for serogroup C compared with MenC-naïve participants, since the quadrivalent vaccines acted as a booster for this serogroup in those already primed. Participants who were MenC-naïve showed a greater seroresponse to serogroup C when they received MenACYW-TT compared with MCV4-TT. These findings were similar when immune response was measured using rSBA.
The rapidity of the immune response induced by MenACYW-TT in adolescents after just 6 days post-vaccination may be particularly advantageous in managing outbreaks in closed/semi-closed communities, such as universities, where immediate protection is crucial.
Interestingly, the baseline seroprotection rates for MenA in the current study were high. Despite the lack of circulation of serogroup A in western countries for decades, high pre-vaccination serogroup A titers have been witnessed in clinical trials [36]. This may be explained by protection against serogroup A conferred by other bacteria with cross-reacting polysaccharides, including Neisseria lactamica, Escherichia coli, Bacillus pumilus, and Streptococcus faecium [37, 38].
The World Health Organization (WHO) also recommends vaccination with 9vHPV (in both sexes, as 2- or 3-dose schedules according to age) and Tdap/Tdap-IPV (as a booster dose) during adolescence [39]. However, compliance with existing recommended vaccination schedules among adolescents and young adults is challenging [40], particularly regarding multi-dose schedules such as those needed for protection against HPV [41]. Concomitant administration of recommended vaccines would reduce the number of clinic visits required and maximize each vaccination opportunity, and could improve coverage among adolescents [42].
Overall, the immune response induced by MenACYW-TT was similar regardless of whether administered alone or concomitantly with 9vHPV and Tdap-IPV. When Tdap-IPV and 9vHPV were administered concomitantly with MenACYW-TT, the antibody concentrations/titers tended to be lower as compared to when Tdap-IPV and 9vHPV were administered alone, for anti-HPV type-6 and type-58 antigens, polio type 1 and type 3, and pertussis toxoid. No conclusions about clinical relevance of these findings can be drawn. It should be noted that the immunogenicity profiles after Tdap-IPV vaccination in terms of seroresponse rates and after 9vHPV vaccination in terms of seroconversion rates were comparable between groups. Moreover, for 9vHPV vaccine, it should be noted that it was the first dose of what is ultimately a two or three-dose vaccination regimen. Additional booster doses have been shown to induce high levels of anti-HPV type protection.
Overall, the reactogenicity and safety profiles were favorable and comparable between the study groups. No safety concerns related to MenACYW-TT or the other vaccines utilized in this study were identified; the most common reactions were non‑serious, self-limited. There were no safety issues identified with concomitant administration of MenACYW-TT with 9vHPV and Tdap-IPV vaccines.
One limitation of the current study was that there were substantially more males included compared with females. However, this may be explained by a large number of females meeting the exclusion criteria due to high uptake of vaccination against HPV in this population as a result of immunization recommendations which were implemented first in females [21, 22]. Additionally, as most MenACYW-TT studies have been conducted in the US and EU, it was of interest to conduct investigations in non-US/EU countries. The countries for the current study were selected prior to the COVID-19 pandemic. The impact of the pandemic on study conduct was more pronounced in Singapore than in the EU, which ultimately led to a lack of data from this country. As the study used competitive enrolment, almost all participants were enrolled in EU countries.
Enhancing vaccination coverage is particularly important in adolescents given that this demographic group have lower vaccination rates in comparison to younger age groups. These rates may be lower because adolescents are generally healthy, have infrequent visits to healthcare providers and less opportunity to be vaccinated, and would be transitioning away from pediatric-related care [43]. Concomitant administration of MenACYW-TT with other vaccines could help to increase coverage rates among adolescents for recommended vaccines.
Conclusions
In conclusion, this study provides key data regarding the immunogenicity and safety of MenACYW-TT compared with MCV4-TT in adolescents, when administered alone or concomitantly with 9vHPV and Tdap-IPV vaccines. These results were consistent in both meningococcal vaccine-naïve adolescents and those who have received at least one dose of a monovalent C vaccine before age 2 years. Concomitant administration of MenACYW-TT with other vaccines could help achieve higher vaccination coverage among adolescents for recommended vaccines.
Data Availability
Qualified researchers may request access to patient-level data and related documents, including, for example, the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications). Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://vivli.org/.
References
European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases. 2023. http://atlas.ecdc.europa.eu/public/index.aspx.
Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–61. https://doi.org/10.1016/S1473-3099(10)70251-6.
Knol MJ, Hahné SJM, Lucidarme J, Campbell H, de Melker HE, Gray SJ, et al. Temporal associations between national outbreaks of meningococcal serogroup W and C disease in the Netherlands and England: an observational cohort study. Lancet Public Health. 2017;2(10):e473–82. https://doi.org/10.1016/S2468-2667(17)30157-3.
Nuttens C, Findlow J, Balmer P, Swerdlow DL, Tin Tin Htar M. Evolution of invasive meningococcal disease epidemiology in Europe, 2008 to 2017. Euro Surveill. 2022. https://doi.org/10.2807/1560-7917.Es.2022.27.3.2002075.
Carr JP, MacLennan JM, Plested E, Bratcher HB, Harrison OB, Aley PK, et al. Impact of meningococcal ACWY conjugate vaccines on pharyngeal carriage in adolescents: evidence for herd protection from the UK MenACWY programme. Clin Microbiol Infect. 2022;28(12):1649e1–8. https://doi.org/10.1016/j.cmi.2022.07.004.
Maiden MC, Ibarz-Pavón AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197(5):737–43. https://doi.org/10.1086/527401.
Kristiansen PA, Diomandé F, Ba AK, Sanou I, Ouédraogo AS, Ouédraogo R, et al. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin Infect Dis. 2012;56(3):354–63. https://doi.org/10.1093/cid/cis892.
European Medicines Agency. MenQuadfi. 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/menquadfi.
US Food and Drug Administration. MenQuadfi | FDA. 2023. https://www.fda.gov/vaccines-blood-biologics/menquadfi.
Chang L-J, Hedrick J, Christensen S, Pan J, Jordanov E, Dhingra MS. A Phase II, randomized, immunogenicity and safety study of a quadrivalent meningococcal conjugate vaccine, MenACYW-TT, in healthy adolescents in the United States. Vaccine. 2020;38(19):3560–9. https://doi.org/10.1016/j.vaccine.2020.03.017.
Áñez G, Hedrick J, Simon MW, Christensen S, Jeanfreau R, Yau E, et al. Immunogenicity and safety of a booster dose of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adolescents and adults: a Phase III randomized study. Hum Vaccin Immunother. 2020;16(6):1292–8. https://doi.org/10.1080/21645515.2020.1733867.
Vesikari T, Borrow R, Forsten A, Findlow H, Dhingra MS, Jordanov E. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in healthy toddlers: a Phase II randomized study. Hum Vaccin Immunother. 2020;16(6):1306–12. https://doi.org/10.1080/21645515.2020.1733869.
Knuf M, Rämet M, Breinholt Stærke N, Bertrand-Gerentes I, Thollot Y, B’Chir S, et al. Comparing the meningococcal serogroup C immune response elicited by a tetanus toxoid conjugate quadrivalent meningococcal vaccine (MenACYW-TT) versus a quadrivalent or monovalent C tetanus toxoid conjugate meningococcal vaccine in healthy meningococcal vaccine-naïve toddlers: a randomised, controlled trial. Hum Vaccin Immunother. 2022;18(5):2052657. https://doi.org/10.1080/21645515.2022.2052657.
Martinón-Torres F, Bertrand-Gerentes I, Oster P. A novel vaccine to prevent meningococcal disease beyond the first year of life: an early review of MenACYW-TT. Expert Rev Vaccines. 2021;20(9):1123–46. https://doi.org/10.1080/14760584.2021.1964962.
Electronic Medicines Compendium. MenQuadfi solution for injection—summary of product characteristics (SmPC)-(emc). 2020. https://www.medicines.org.uk/emc/product/12818/smpc#gref.
European Medicines Agency. Nimenrix | Summary of Product Characteristics. 2024.
Macina D, Evans KE. Bordetella pertussis in school-age children, adolescents and adults: a systematic review of epidemiology and mortality in Europe. Infect Dis Ther. 2021;10(4):2071–118. https://doi.org/10.1007/s40121-021-00520-9.
Ribeiro AA, Saddi VA, Carneiro MA, Figueiredo-Alves RR, da Silva Barros NK, de Almeida Carvalho KP, et al. Human papillomavirus and Chlamydia trachomatis infections in adolescents and young women: prevalence and risk factors. Diagn Cytopathol. 2020;48(8):736–44. https://doi.org/10.1002/dc.24460.
Weinberger B. Adult vaccination against tetanus and diphtheria: the European perspective. Clin Exp Immunol. 2017;187(1):93–9. https://doi.org/10.1111/cei.12822.
European Centre for Disease Prevention and Control. Vaccine Scheduler | ECDC. 2023. https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=3&SelectedCountryIdByDisease=-1.
Colzani E, Johansen K, Johnson H, Pastore Celentano L. Human papillomavirus vaccination in the European Union/European Economic Area and globally: a moral dilemma. Euro Surveill. 2021. https://doi.org/10.2807/1560-7917.Es.2021.26.50.2001659.
European Centre for Disease Prevention and Control. Guidance on HPV vaccination in EU countries: focus on boys, people living with HIV and 9-valent HPV vaccine introduction. 2020. https://www.ecdc.europa.eu/en/publications-data/guidance-hpv-vaccination-eu-focus-boys-people-living-hiv-9vHPV-vaccine.
Centers for Disease C, Prevention. HPV Vaccination: What Everyone Should Know | CDC. 2021. https://www.cdc.gov/vaccines/vpd/hpv/public/index.html.
Díez-Domingo J, Simkó R, Icardi G, Chong CP, Zocchetti C, Syrkina O, et al. 1139. Immunogenicity and Safety Study of a Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) Compared to a Meningococcal Reference Vaccine (MCV4-TT) in Healthy Adolescents. Open Forum Infect Dis. 2023. https://doi.org/10.1093/ofid/ofad500.980.
Neveu D, Mallett Moore T, Zambrano B, Chen A, Kürzinger M-L, Marcelon L, et al. Structured benefit-risk assessment of a new quadrivalent meningococcal conjugate vaccine (MenACYW-TT) in individuals ages 12 months and older. Infect Dis Ther. 2023;12(10):2367–86. https://doi.org/10.1007/s40121-023-00864-4.
Zambrano B, Peterson J, Deseda C, Julien K, Spiegel CA, Seyler C, et al. Quadrivalent meningococcal tetanus toxoid-conjugate booster vaccination in adolescents and adults: phase III randomized study. Pediatr Res. 2023;94(3):1035–43. https://doi.org/10.1038/s41390-023-02478-5.
Burman C, Knuf M, Sáfadi MAP, Findlow J. Antibody persistence and revaccination recommendations of MenACWY-TT: a review of clinical studies assessing antibody persistence up to 10 years after vaccination. Expert Rev Vaccines. 2024. https://doi.org/10.1080/14760584.2024.2348609.
Medical Dictionary for Regulatory Activities. MedDRA. 2024. https://www.meddra.org/.
Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med. 1990;9(12):1447–54. https://doi.org/10.1002/sim.4780091208.
Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857–72. https://doi.org/10.1002/(sici)1097-0258(19980430)17:8%3c857::aid-sim777%3e3.0.co;2-e.
Findlow J, Balmer P, Borrow R. A review of complement sources used in serum bactericidal assays for evaluating immune responses to meningococcal ACWY conjugate vaccines. Hum Vaccin Immunother. 2019;15(10):2491–500. https://doi.org/10.1080/21645515.2019.1593082.
Matsuoka O, Ujiie M, Kikuchi H, Otake S, Chansinghakul D, Inoue T, et al. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in meningococcal vaccine-naïve participants across a broad age range (2–55 Years) in Japan: a phase III randomized study. Jpn J Infect Dis. 2023;76(3):174–82. https://doi.org/10.7883/yoken.JJID.2022.272.
Conti A, Broglia G, Sacchi C, Risi F, Barone-Adesi F, Panella M. Efficacy and safety of quadrivalent conjugate meningococcal vaccines: a systematic review and meta-analysis. Vaccines. 2023;11(1):178.
Shimabukuro TT, Su JR, Marquez PL, Mba-Jonas A, Arana JE, Cano MV. Safety of the 9-valent human papillomavirus vaccine. Pediatrics. 2019. https://doi.org/10.1542/peds.2019-1791.
Laurichesse H, Zimmermann U, Galtier F, Launay O, Duval X, Richard P, et al. Immunogenicity and safety results from a randomized multicenter trial comparing a Tdap-IPV vaccine (REPEVAX®) and a tetanus monovalent vaccine in healthy adults: new considerations for the management of patients with tetanus-prone injuries. Hum Vaccin Immunother. 2012;8(12):1875–81. https://doi.org/10.4161/hv.22083.
Dhingra MS, Peterson J, Hedrick J, Pan J, Neveu D, Jordanov E. Immunogenicity, safety and inter-lot consistency of a meningococcal conjugate vaccine (MenACYW-TT) in adolescents and adults: a phase III randomized study. Vaccine. 2020;38(33):5194–201. https://doi.org/10.1016/j.vaccine.2020.06.013.
Filice GA, Hayes PS, Counts GW, Griffiss JM, Fraser DW. Risk of group A meningococcal disease: bacterial interference and cross-reactive bacteria among mucosal flora. J Clin Microbiol. 1985;22(2):152–6. https://doi.org/10.1128/jcm.22.2.152-156.1985.
Tzeng Y-L, Stephens DS. Epidemiology and pathogenesis of Neisseria meningitidis. Microbes Infect. 2000;2(6):687–700. https://doi.org/10.1016/S1286-4579(00)00356-7.
World Health O. Defeating meningitis by 2030: baseline situation analysis. 2010. https://cdn.who.int/media/docs/default-source/documents/health-topics/meningitis/bsa_20feb2019473fd679-4af3-4406-9eb9-6f95540a1c14.pdf?sfvrsn=4812bd88_1&download=true).
Bernstein HH, Bocchini JA, Committee On Infectious D. The need to optimize adolescent immunization. Pediatrics. 2017;139(3):e20164186. https://doi.org/10.1542/peds.2016-4186.
Gallagher KE, Kadokura E, Eckert LO, Miyake S, Mounier-Jack S, Aldea M, et al. Factors influencing completion of multi-dose vaccine schedules in adolescents: a systematic review. BMC Public Health. 2016;16(1):172. https://doi.org/10.1186/s12889-016-2845-z.
Moss JL, Reiter PL, Brewer NT. Concomitant adolescent vaccination in the US, 2007–2012. Am J Prev Med. 2016;51(5):693–705. https://doi.org/10.1016/j.amepre.2016.05.013.
Alderfer J, Srivastava A, Isturiz R, Burman C, Absalon J, Beeslaar J, et al. Concomitant administration of meningococcal vaccines with other vaccines in adolescents and adults: a review of available evidence. Hum Vaccin Immunother. 2019;15(9):2205–16. https://doi.org/10.1080/21645515.2019.1581542.
Acknowledgements
The authors would like to thank all participants who volunteered to take part in the study, and all study investigators. The authors also wish to acknowledge and thank the Sanofi study team for their support during the conduct of this study.
Medical Writing, Editorial, and Other Assistance
Editorial assistance with the preparation of the manuscript was provided by Katie Williams and Vicky Hinstridge of inScience Communications, Springer Healthcare Ltd, UK, and was funded by Sanofi. The authors also thank Isabel Gregoire for editorial assistance and manuscript coordination on behalf of Sanofi.
Funding
The study and the journal’s Rapid Service Fee was funded by Sanofi.
Author information
Authors and Affiliations
Contributions
Concept/design: Javier Díez-Domingo, Róbert Simkó, Giancarlo Icardi, Chan Poh Chong, Isabelle Bertrand-Gerentes, Céline Zocchetti, Siham Bchir. Data acquisition: Javier Díez-Domingo, Róbert Simkó, Giancarlo Icardi, Chan Poh Chong. Data analysis or interpretation: Javier Díez-Domingo, Róbert Simkó, Giancarlo Icardi, Chan Poh Chong, Isabelle Bertrand-Gerentes, Céline Zocchetti, Olga Syrkina, Siham Bchir. Drafting of the publication: Javier Díez-Domingo, Róbert Simkó, Giancarlo Icardi, Chan Poh Chong, Isabelle Bertrand-Gerentes, Céline Zocchetti, Olga Syrkina, Siham Bchir. Accountable for accuracy and integrity: Javier Díez-Domingo, Róbert Simkó, Giancarlo Icardi, Chan Poh Chong, Isabelle Bertrand-Gerentes. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Céline Zocchetti, Olga Syrkina, Siham Bchir and Isabelle Bertrand-Gerentes are employees of Sanofi and may hold shares and/or stock options in the company. Javier Díez-Domingo received sponsorship from Sanofi for the conduct of clinical trial; received grants from GSK, MSD, and Sanofi paid to institution; received consulting fees from GSK, MSD, and Sanofi; received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from GSK, MSD, and Moderna; received support for attending meetings from Sanofi and MSD. Róbert Simkó, Chan Poh Chong and Giancarlo Icardi received sponsorship from Sanofi for the conduct of clinical trial and received payment or honoraria for scientific lectures from Sanofi.
Ethical Approval
This study was conducted in accordance with the protocol and consensus ethical principles derived from international guidelines including the Declaration of Helsinki and the International Council for Harmonization (ICH) guidelines for Good Clinical Practice (GCP), and all applicable laws, rules, and regulations. Participants turning 18 years old during the study or parents/legal representatives provided informed consent. Ethical approval was obtained from the following committees: Singapore: NHG Domain Specific Review Boards (DSRB). Spain: Comité de Ética de la Investigación Con Medicamentos del Hospital General Universitario Gregorio Marañón. Hungary: Egészségügyi Tudományos Tanács Klinikai Farmakológiai Etikai Bizottság/ Medical Research Council Ethics Committee for Clinical Pharmacology. Italy: Comitato Etico Regionale Liguria Largo Rosanna Benzi; Comitato Etico Milano Area 1 – ASST Sacco e Fatebenefratelli; Comitato Etico dell’Area Vasta Emilia Nord; Segreteria Locale di Parma c/o Azienda Ospedaliero; Universitaria di Parma Via Gramsci 14; Comitato Etico Palermo 1; Comitato Etico “Ospedali Riuniti” Di Foggia; Comitato Etico Regione Calabria—Sezione Area Centro.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Díez-Domingo, J., Simkó, R., Icardi, G. et al. Immunogenicity and Safety of a Quadrivalent Meningococcal Conjugate Vaccine Versus Nimenrix in Healthy Adolescents: A Randomized Phase IIIb Multicenter Study. Infect Dis Ther (2024). https://doi.org/10.1007/s40121-024-01009-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40121-024-01009-x