Abstract
Introduction
Recurrent Clostridioides difficile infection (rCDI) often occurs after standard-of-care antibiotics. VOWST oral spores (VOS, previously SER-109), an FDA-approved orally administered microbiome therapeutic, is indicated to prevent rCDI following antibiotics for rCDI.
Objective, Design, and Patients
To evaluate safety and efficacy of VOS from two phase 3 trials, (randomized, placebo-controlled [ECOSPOR III: NCT03183128] and open-label, single arm [ECOSPOR IV: NCT03183141]) of 349 adults with rCDI and prevalent comorbidities.
Methods
VOS or placebo [ECOSPOR III only] (4 capsules once daily for 3 days). Integrated analysis of treatment-emergent adverse events (TEAEs) collected through week 8; serious TEAEs and TEAEs of special interest collected through week 24; and rates of rCDI (toxin-positive diarrhea requiring treatment) evaluated through weeks 8 and 24.
Results
TEAEs were mostly mild or moderate and gastrointestinal. Most common treatment-related TEAEs were flatulence, abdominal pain and distension, fatigue, and diarrhea. There were 11 deaths (3.2%) and 48 patients (13.8%) with serious TEAEs, none treatment-related. The rCDI rate through week 8 was 9.5% (95% CI 6.6–13.0) and remained low through 24 weeks (15.2%; 95% CI 11.6–19.4). Safety and rCDI rates were consistent across subgroups including age, renal impairment/failure, diabetes, and immunocompromise/immunosuppression.
Conclusions
VOS was well tolerated and rates of rCDI remained low through week 24 including in those with comorbidities. These data support the potential benefit of VOS following antibiotics to prevent recurrence in high-risk patients.
Trial Registration
ClinicalTrials.gov identifier, NCT03183128 and NCT03183141.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
VOWST oral spores (VOS, previously SER-109) is an FDA-approved orally administered microbiome therapeutic indicated to prevent Clostridioides difficile infection (CDI) following antibiotics for recurrent CDI. |
In this integrated analysis of two phase 3 studies in adults with recurrent CDI and prevalent comorbidities, VOS was well tolerated. |
What was learned from the study? |
Most treatment-emergent adverse events were mild or moderate and gastrointestinal in nature. |
Rates of recurrent CDI were low through 24 weeks after treatment. |
Introduction
Clostridioides difficile infection (CDI) is associated with severe, debilitating diarrhea, poor quality of life, and significant morbidity and mortality. The main risk factor for CDI is prior broad-spectrum antibiotic exposure [1]. Antibiotics can be life-saving, but also kill beneficial bacteria in the gastrointestinal microbiome that play a key role in host defense against C. difficile [2, 3]. In particular, depletion of Firmicutes facilitates metabolic alterations that support favorable conditions for C. difficile spore germination, vegetative bacterial replication, and toxin production, leading to acute CDI [2, 4]. Approximately 75–80% of patients with primary CDI respond to standard-of-care antibiotics (e.g., vancomycin, fidaxomicin) owing to efficient killing of the toxin-producing bacteria [5]. However, antibiotics do not directly affect the metabolically inactive C. difficile spores that serve as a reservoir for recurrent infection and symptomatic disease [2, 6]. In addition, antibiotics used to treat CDI maintain or exacerbate the disrupted microbiome, supporting the two-phase life cycle of C. difficile, and increasing the risk for future recurrences due to antibiotic impairment of microbiome resilience [2, 7]. In fact, the strongest predictor of rCDI is a prior CDI episode [8]. Paradoxically, the standard-of-care for recurrent CDI (rCDI) is more antibiotics, leading to a vicious cycle of recurrence [9] and placing a heavy burden on patients and greatly diminishing quality of life [10]. Thus, rCDI is a quintessential example of disease caused by microbiome disruption, signaling the need for a two-pronged treatment approach (antibiotics followed by microbiome restoration).
Other risk factors for rCDI include age > 65 years and comorbidities (e.g., immunosuppression, malignancy, renal insufficiency) [11,12,13,14,15,16]. Unfortunately, many clinical trials exclude patients with comorbidities. Fecal microbiota spores, live or VOWST™ (formerly SER-109 and hereafter referred to as VOS for VOWST™ oral spores), is an orally administered microbiome-based live biotherapeutic product (LBP) predominantly comprising Firmicutes bacterial spores. LBPs are a therapeutic class defined as non-vaccine products containing live organisms intended for the treatment or prevention of disease. VOS was developed to restore the composition and function of the gastrointestinal microbiome in patients with a history of rCDI based on their modulatory role in the life cycle of C. difficile and disease pathogenesis and is approved by the US Food and Drug Administration (FDA) to prevent recurrence of CDI in adults following antibacterial treatment for rCDI.
In the ECOSPOR III trial, CDI recurrence at week 8, the primary endpoint, was significantly lower in VOS-treated vs. placebo-treated patients (12% vs. 40%, respectively; relative risk, 0.32; 95% CI 0.18–0.58) and VOS was well tolerated [17]. In the open-label, single-arm trial, ECOSPOR IV, VOS was well tolerated and the rCDI rate was 8.7% (95% CI 5.6%–12.8%) [18]. The CDI recurrence rate through 24 weeks was 21% in ECOSPOR III and 14% in ECOSPOR IV [18, 19]. We report integrated safety and efficacy data for VOS in a broad patient population at increased risk of rCDI through week 24 from both studies.
Methods
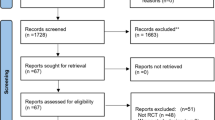
An overview of the studies is presented in Fig. 1. ECOSPOR III was a double-blind, randomized, placebo-controlled trial conducted at 56 US and Canadian sites from July 2017 to September 2020. A total of 182 adults with ≥ 2 CDI recurrences, confirmed via toxin EIA, were randomized 1:1 to VOS or placebo. Patients were stratified by age (< 65; ≥ 65 years) and antibiotic regimen (vancomycin, fidaxomicin).
Phase 3 clinical studies included in the integrated analysis of VOS safety and efficacy. RCT randomized controlled trial. Patients in ECOSPOR III who experienced CDI recurrence prior to 8 weeks post-treatment (indicated by rectangular box) were eligible to rollover into ECOSPOR IV. The same VOS treatment regimen (3 × 107 spore colony forming units [SCFUs] in four capsules once daily for three consecutive days) was administered in both ECOSPOR III and ECOSPOR IV. Blue solid lines indicate data included in the integrated datasets. Placebo data (dashed blue lines) were not included in the integrated datasets. Integrated data was available from 349 unique patients treated with at least one VOS dosing regimen (90 patients from ECOSPOR III [note that 4 of these patients received a second regimen in ECOSPOR IV], 25 placebo rollover patients in ECOSPOR IV, and 234 de novo patients in ECOSPOR IV
ECOSPOR IV was an open-label, single-arm study conducted at 72 US and Canadian sites from October 2017 to April 2022. ECOSPOR IV included 263 patients with rCDI enrolled in two cohorts: (1) rollover patients from ECOSPOR III with on-study recurrence diagnosed by toxin EIA (N = 29); and (2) de novo patients with ≥ 1 CDI recurrence (diagnosed by PCR or toxin EIA), inclusive of the current episode (N = 234).
In both studies, VOS or placebo was administered orally as four capsules over three consecutive days following symptom resolution after standard-of-care antibiotics. Patients were instructed to take magnesium citrate (10 oz) or polyethylene glycol (250 mL) 1 day before treatment initiation. Patients were followed for safety and CDI recurrence through 24 weeks. On-study CDI recurrence was defined as ≥ 3 unformed stools per day for two consecutive days with a positive C. difficile toxin test (EIA or cell cytotoxicity neutralization assay) and a decision by the investigator that antibiotic treatment was needed. Recurrence was imputed for patients who were lost to follow-up, terminated the trial prematurely, or died. Recurrence was also imputed if a patient had one missing criterion (e.g., toxin test result) but the other two criteria were documented (e.g., diarrhea, investigator decision to treat).
Both trials were performed in accordance with Good Clinical Practice and with the Helsinki Declaration of 1964 and its later amendments. The protocols and amendments were reviewed and approved by local or central investigational review boards. Written informed consent was obtained from all patients at screening.
In both trials, all treatment-emergent adverse events (TEAEs), serious AEs (SAEs), and AEs of special interest (AESIs), predefined as any invasive infection (e.g., bacteremia, abscess, meningitis, etc.), were collected weekly via telephone after treatment initiation up to week 8. From weeks 8 to 24, all treatment-emergent SAEs and AESIs, and related data, were collected every 4 weeks via telephone. In ECOSPOR III only, solicited AEs (i.e., diarrhea, gas or flatulence, abdominal distention or bloating, abdominal pain or cramping, nausea, anorexia, vomiting, fatigue, chills or shivering, and constipation) were recorded in a diary card daily for 7 days after treatment completion on day 3.
Additional details are published elsewhere [17,18,19].
Endpoints
Safety endpoints were AEs, including SAEs, and AESIs, clinical laboratory evaluations, and vital signs. In both studies, different types of invasive infection events were designated as AESIs by the investigators. To account for all events, a retrospective AESI analysis was performed using the integrated dataset (see Supplementary Material).
Efficacy endpoints were rCDI (recurrent toxin + diarrhea requiring treatment) through week 8 (i.e., the primary efficacy endpoint in each trial) and through week 24. An analysis of rCDI rates up to 8 weeks after treatment was conducted for the following subgroups: prior CDI episodes (including the qualifying episode (2, ≥ 3); age (< 65, ≥ 65); sex (male, female); qualifying episode definition (PCR alone [diagnostic method permitted in ECOSPOR IV only], toxin with or without PCR); acid-suppressing medication usage (i.e., proton pump inhibitors and/or H2-receptor antagonists); creatinine clearance rate (< 30 ml/min, ≥ 30 to ≤ 50 ml/min to ≤ 80 ml/min, > 80 ml/min); prior antibiotic regimen (vancomycin, fidaxomicin); age-adjusted Charlson Comorbidity Index [20] (CCI) score category (0, 1–2, 3–4, ≥ 5), and immunocompromised/immunosuppressed status (see Supplementary Material Tables 1 and 2).
Statistical Analysis
In the integrated datasets, only data from VOS-treated patients were included from the following populations:
-
1.
VOS-treated patients in ECOSPOR III who did not roll over into ECOSPOR IV.
-
2.
VOS-treated patients in ECOSPOR III who rolled over and received VOS in ECOSPOR IV (cohort 1).
-
3.
Placebo-treated patients in ECOSPOR III who rolled over and received VOS in ECOSPOR IV (cohort 1).
-
4.
De novo patients enrolled and treated with VOS in ECOSPOR IV (cohort 2).
For each study, up to 24 weeks of follow-up was included. Where appropriate, confidence intervals (CIs) were presented at the 5% level of significance.
All continuous variables are summarized using descriptive statistics: N, mean, standard deviation (SD), median, maximum, and minimum. All categorical variables are summarized using frequency counts and percentages.
rCDI rates and non-recurrence rates (proportion of patients with and without recurrence) are presented with 95% CIs (calculated via Clopper-Pearson exact method).
Descriptive summaries are by the overall integrated population.
TEAEs were coded using the Medical Dictionary for Regulatory Activities version 24.1. All statistics were completed using SAS® version 9.4.
Results
Integrated Phase 3 Study Population
In total, 349 patients received at least one dose of VOS in ECOSPOR III or ECOSPOR IV. Four patients received blinded VOS in ECOSPOR III followed by a second regimen of open-label VOS in ECOSPOR IV. The treatment adherence rate for VOS-treated patients was 98.9% in ECOSPOR III and 99.2% in ECOSPOR IV. Retention rates for the VOS arm in ECOSPOR III and for the overall population in ECOSPOR IV were 86.5% and 94.7%, respectively. Overall, more patients were female (68.8%), predominantly White (> 90%), not Hispanic or Latino (> 90%), with a mean age of 64.2 years. Most patients had at least two prior CDI episodes (Table 1), but 77 patients (22.1%) with first recurrence were enrolled in ECOSPOR IV. The majority (72.2%) of patients were treated with vancomycin for the qualifying episode. Concomitant chronic illnesses included cardiac disease (31.2%), immunocompromise/immunosuppression (21.2%), diabetes (18.9%), and renal impairment/failure (13.2%).
Overview of Adverse Events
Overall, 63.3% (221/349) of VOS-treated patients experienced TEAEs through week 24 (Table 2). Most were mild or moderate and gastrointestinal (Table 2 and Supplementary Material Table 3).
Most common TEAEs (> 5% of patients) were flatulence, diarrhea, abdominal pain, fatigue, and abdominal distension (Supplementary Material Table 3). Most common treatment-related TEAEs were flatulence, abdominal pain, abdominal distension, fatigue, and diarrhea (Supplementary Material Table 4).
One patient experienced a TEAE leading to study withdrawal, which was considered unrelated to treatment by the investigator, and was a fatal SAE related to a pre-existing glioblastoma.
Deaths
There were 11 deaths (3.2%; 3 in ECOSPOR III and 8 in ECOSPOR IV) (Supplementary Material Table 5), none of which were deemed treatment-related by the investigators. Patients who died were 64–93 years old and 7 of 11 deaths were associated with pre-existing conditions. There were no trends in the cause or timing of death that would suggest an association with VOS. The interval between treatment initiation and death varied from 5 to 164 days. Three patients died within 30 days due to TEAEs of cardiomyopathy with longstanding cardiomegaly (day 5), subdural hematoma after a fall while taking anticoagulation therapy (day 12), and SARS-CoV-2 infection/C. difficile recurrence and intestinal perforation (day 28). The remaining 8 patients died more than 30 days after the last dose of VOS (range 39–164 days).
Serious Adverse Events
A total of 48 patients (13.8%) (Supplementary Material Table 6) reported SAEs, none of which were deemed treatment-related. The most frequently reported SAEs (≥ 2 patients) included urinary tract infection (UTI), cellulitis, C. difficile colitis, and C. difficile infection. None of the UTIs were related to VOS drug species.
Adverse Events of Special Interest
AESIs were observed in 28 patients (8%) with no consistent pattern and none were considered treatment-related (Supplementary Material Table 7). The organisms isolated from available cultures were not VOS drug species.
Safety in Subgroups
Incidence of TEAEs and SAEs were analyzed by subgroups including age, immunosuppressed/immunocompromised patients, and by concomitant illnesses associated with immune dysfunction, including renal impairment/failure and diabetes (Supplementary Material Tables 8 and 9). There were no distinct or clinically important differences in the AE profile of VOS for any subgroup examined compared with the overall population.
Clinical Laboratory Abnormalities and Vital Signs
Treatment-emergent abnormalities in hematology or biochemistry parameters were rare with 18 patients (5.2%) who had at least one abnormal value (Supplementary Material Table 10). There was no evidence of drug-induced liver injury based on absence of Hy’s law cases [21].
Abnormal post-baseline vital sign measurements were also rare, with 8 patients (2.3%) who had at least one abnormal value (Supplementary Material Table 11).
Efficacy
CDI recurrence through week 8 in patients who received any dose of VOS was 9.5% (33/349); 95% CI [6.6–13.0] (Fig. 2). Most rollover patients in ECOSPOR IV (25 of 29; 86.2%) were from the placebo arm in ECOSPOR III; 13.8% (4/29) had CDI recurrence by week 8. Of the 33 recurrences through week 8, 23 were confirmed and 10 were imputed as recurrence due to loss to follow-up, early termination, or death (n = 5), or missing components for defining rCDI (n = 5) (Supplementary Material Table 12).
The rCDI rate remained low through 24 weeks (15.2% [53/349]; 95% CI [11.6–19.4]) (Fig. 2). Of 53 recurrences documented through 24 weeks, 32 were confirmed and 21 were imputed recurrences due to loss to follow-up, early termination, or death (n = 14) or missing components for defining rCDI (n = 7) (Supplementary Material Table 12).
Non-recurrence rates through weeks 8 and 24 were 90.5% (95% CI [87.0–93.4]) and 84.8% (95% CI [80.6–88.4]), respectively (Fig. 2). Among those patients with non-recurrence through week 8 (316/349; 90.5%), 93.7% (296/316) maintained a durable response through week 24 (Fig. 3).
Durability of response at 24 weeks among patients without recurrence through 8 weeks in patients who received at least one dose of VOS in ECOSPOR III and/or ECOSPOR IV. Among those patients with non-recurrence through week 8 (316/349; 90.5%), 93.7% (296/316) maintained a durable response through week 24
CDI Recurrence Rates by Subgroup
Analyses by select baseline characteristics or concomitant medication use, including age, antibiotic regimen for the qualifying episode, sex, creatine clearance, diagnostic test used for the qualifying episode, acid-suppressing medication use, CCI score category, or immunocompromise/immunosuppression also showed consistent low rCDI rates through week 8 in all subgroups, ranging from 4.2% to 22.2% (Fig. 4). In accordance with data from ECOSPOR IV [18], in the integrated dataset, CDI recurrence rates were lower in younger patients (i.e., < 65 years) vs. those ≤ 65 years (4.2% vs. 14.2%, respectively), whereas recurrence rates by prior antibiotic regimen were similarly low. Also consistent with previously reported data from ECOSPOR IV [18], CDI recurrence rates in patients enrolled by PCR alone were numerically lower (5.8%, 4/69 patients) compared with those enrolled by toxin + EIA (10.5%, 29/276 patients) with overlapping 95% CIs. Recurrence rates range from 4.2% to 6.1% in patients with CCI scores between 1 and 4 while CDI recurrence rates were highest in patients with CCI scores ≥ 5 (19%, 22/116 patients). There were no CDI recurrences among the 26 patients with a CCI score of 0 (Fig. 4). In patients who were immunosuppressed or immunocompromised, CDI recurrence rate was numerically higher (16.2%; 12/74) compared with patients who were not immunosuppressed or immunocompromised (7.6%; 21/275) with overlapping 95% CIs.
CDI recurrence rates in subgroups. CDI Clostridioides difficile infection; PPI proton pump inhibitor; VOS VOWST™ oral spores. Two patients enrolled in ECOSPOR III had missing or negative assay results for the qualifying episode and are not included. Two patients were enrolled in ECOSPOR IV on the basis of a positive loop-mediated isothermal amplification assay result and are also not included. One rollover patient in ECOSPOR IV was diagnosed by PCR alone; however, in the integrated analysis, all baseline information for rollover patients was recorded from ECOSPOR III and this patient is therefore included in the category “qualifying episode defined by toxin with/without PCR”. Four patients had missing CCI scores; 12 patients had missing baseline creatine clearance rate; one patient had missing data for number of prior CDI episodes. [1] Charlson Comorbidity Index (CCI) scores adjusted for age [20]
Consistent with the low overall rCDI rate of 9.5%, CDI recurrence rates through week 8 in patients with first recurrence (i.e., 2 prior CDI episodes) were similarly low (6.5% [5/77]) as those with ≥ 2 recurrences (i.e., ≥ 3 prior CDI episodes; 10.3% [28/271]) (Fig. 4). Non-recurrence rates were also similar in patients with first recurrence (93.5%) vs. those with ≥ 2 recurrences (89.7%) as compared with the overall non-recurrence rate of 90.5% through week 8.
Discussion
Patients with rCDI would benefit from an effective, safe, and convenient treatment option, in addition to antibiotics alone, to restore the microbiome and achieve sustained clinical response. In this integrated analysis of two phase 3 trials, oral administration of VOS was well tolerated in a vulnerable patient population with rCDI and prevalent comorbidities, as reflected by the CCI scores of ≥ 3 in 72% of the study population. This safety profile might be expected since VOS comprised predominantly Firmicutes spores, normally abundant in the healthy microbiome [2]. VOS is manufactured using a series of inactivation, filtration, and purification steps to mitigate risk of transmission of infectious agents, not detected through comprehensive donor screening [22, 23]. Spores are resistant to gastric acid, which enables formulation of these live bacteria into oral capsules suitable for room temperature storage.
The most common AEs were gastrointestinal and most were mild or moderate in severity. There were no notable differences in the safety profile of VOS for any subgroup examined. Specifically, there were no important safety differences observed in patients with renal impairment/failure, diabetes, or cardiac disease. Likewise, the safety of VOS in patients who were immunosuppressed or immunocompromised was similar to the overall population. Additionally, there were no differences in the safety profile of VOS observed in patients ≥ 65 years compared with the overall population. Finally, there were no clinically relevant trends suggestive of a safety signal in laboratory results or vital signs. Although the numbers of patients are limited, these data on the safety profile of VOS in relevant subgroups may be reassuring to physicians in their clinical decisions.
Historically, CDI has been noted to increase all-cause mortality, in association with older age and comorbid conditions [24,25,26]. In this analysis, there were no trends in the timing or patterns of death that would suggest an association with VOS treatment. Moreover, more than half of the population were ≥ 65 years and some of the observed deaths may be explained by enrollment of patients with underlying diseases associated with short-term survival (e.g., glioblastoma, pancreatic cancer), consistent with the broad inclusion/exclusion criteria.
The strongest predictor of CDI recurrence is a prior CDI episode [27, 28]. Patients with a history of rCDI are at high risk of recurrence because antibiotics used to treat the debilitating symptoms of the disease do not address the antibiotic-induced microbiome disruption facilitating the two-phase life cycle of C. difficile. Rates of recurrence after antibiotics alone in trials of patients with a history of ≥ 2 recurrences range from 37% to 52% [29, 30]. Moreover, rates of recurrence in patients with rCDI treated with standard-of-care antibiotics followed by placebo in the ECOSPOR III trial as well as in the integrated phase 2 and phase 3 trials (PUNCH CD2 and PUNCH CD3) of another FDA-approved LBP, REBYOTA RBL™ (formerly RBX2660), were 40% and 42.5%, respectively [17, 31]. In the integrated efficacy analysis of VOS-treated patients, the recurrence rate at week 8 was 9.5%, which is notably lower than rates reported in these populations as well as in other large randomized trials of patients with a history of prior CDI treated with standard-of-care antibiotics [32,33,34]. Furthermore, in a Bayesian analysis of the combined phase 3 PUNCH CD3 and phase 2 PUNCH CD2 trials, the modeled estimated treatment success for RBL in the mITT population, defined as the absence of CDI diarrhea within 8 weeks of study treatment, was 70.6% [35] corresponding to a CDI recurrence rate of 29.4%. It should be noted, however, that differences in study design and study populations complicate direct comparisons of efficacy between these two products.
Although patients in the ECOSPOR IV trial could enroll on the basis of PCR testing alone, the majority of patients (79.1%) in the integrated dataset were enrolled on the basis of a positive toxin test with or without PCR. Given that epidemiologic and observational treatment studies, toxin positivity has been correlated with an increased risk of rCDI compared to PCR in epidemiologic and observational treatment studies [28, 36], the rCDI rate of 15.2% at week 24 is also notably low.
Among patients without CDI recurrence at 8 weeks, the durability of the clinical response at 24 weeks after VOS treatment was high (93.7%). The low recurrence rate and durability support the potential benefit of microbiome restoration with VOS following antibiotics for rCDI to prevent recurrence in high-risk patients. Integrated subgroup analyses at 8 weeks revealed consistently low CDI recurrence rates across all subgroups including age, sex, number of prior CDI episodes, diagnostic method for the qualifying episode, CCI score, usage of acid-suppressing medications, baseline creatinine clearance, and immunocompromised/immunosuppressed status. The CDI recurrence rate was numerically greater in the ≥ 65 years vs. < 65 years age subgroup, consistent with the greater risk of recurrence observed in older populations [37], which may be attributable to immunosenescence of the microbiome [11]. Recurrence rates were similarly low between vancomycin and fidaxomicin subgroups, giving clinicians greater therapeutic options for management. There was also no difference between rCDI rates among patients enrolled by PCR alone compared to those with positive toxin EIA, which may reflect the high likelihood of active disease in these patients enrolled under strict criteria.
To our knowledge, this is the first report of an FDA-approved microbiome therapeutic to demonstrate comparable response rates between patients with first and multiply rCDI. The low recurrence rates suggest that microbiome disruption may be a key contributor to the pathogenesis of recurrent disease, as previously demonstrated (Straub et al., in submission).
Strengths and Limitations
As a result of the small within-subgroup sample sizes, there was insufficient power to conduct formal statistical comparisons of safety or efficacy across subgroups. Strengths include the high enrollment of elderly patients who are at increased risk for recurrence. Additionally, the inclusion criteria of both studies allowed enrollment of a broad study population, including patients on maintenance chemotherapy, dialysis, and those with neutropenia (absolute neutrophil count < 1500 cells/mm3 but > 500 cells/mm3), patient groups often excluded from other trials. Estimates of safety are strengthened by high retention rates of nearly 94% and estimates of efficacy are governed by conservative statistical analysis. Finally, the size of the safety database is estimated to have > 95% detection probability for AEs occurring with a frequency of at least 1%.
Conclusions
rCDI is a debilitating infection with significant morbidity and mortality. In this integrated population, including complex patients with multiple comorbidities, VOS was associated with low recurrence rates and was well tolerated. Absence of CDI recurrence is associated with improved quality of life and may mitigate other untoward outcomes, such as hospitalization and death [10, 38,39,40]. Use of microbiome restoration therapy to manage a first recurrence of CDI may potentially reduce risk of untoward clinical outcomes and is supported by these safety and efficacy data in a vulnerable population.
Data Availability
Data supporting this study are included within the article and in the supplementary material.
References
Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemoth. 2013;68(9):1951–61. https://doi.org/10.1093/jac/dkt129.
Theriot CM, Young VB. Interactions between the gastrointestinal microbiome and Clostridium difficile. Annu Rev Microbiol. 2015;69(1):445–61. https://doi.org/10.1146/annurev-micro-091014-104115.
Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197(3):435–8. https://doi.org/10.1086/525047.
Crobach MJT, Vernon JJ, Loo VG, et al. Understanding Clostridium difficile colonization. Clin Microbiol Rev. 2018;31(2):e00021-e117. https://doi.org/10.1128/cmr.00021-17.
Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile Infection. New Engl J Med. 2011;364(5):422–31. https://doi.org/10.1056/nejmoa0910812.
Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(Suppl 2):S154–61. https://doi.org/10.1093/cid/cis462.
Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2(1):16020. https://doi.org/10.1038/nrdp.2016.20.
Shields K, Araujo-Castillo RV, Theethira TG, Alonso CD, Kelly CP. Recurrent Clostridium difficile infection: from colonization to cure. Anaerobe. 2015;34:59–73. https://doi.org/10.1016/j.anaerobe.2015.04.012.
Feuerstadt P, Theriault N, Tillotson G. The burden of CDI in the United States: a multifactorial challenge. BMC Infect Dis. 2023;23(1):132. https://doi.org/10.1186/s12879-023-08096-0.
Garey KW, Jo J, Gonzales-Luna AJ, et al. Assessment of quality of life among patients with recurrent Clostridioides difficile infection treated with investigational oral microbiome therapeutic SER-109. Jama Netw Open. 2023;6(1): e2253570. https://doi.org/10.1001/jamanetworkopen.2022.53570.
Jump RL. Clostridium difficile infection in older adults. Aging Heal. 2013;9(4):403–14. https://doi.org/10.2217/ahe.13.37.
Negrut N, Bungau S, Behl T, et al. Risk factors associated with recurrent Clostridioides difficile infection. Healthcare. 2020;8(3):352. https://doi.org/10.3390/healthcare8030352.
Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36(4):452–60. https://doi.org/10.1017/ice.2014.88.
Abu-Sbeih H, Choi K, Tran CN, et al. Recurrent Clostridium difficile infection is associated with treatment failure and prolonged illness in cancer patients. Eur J Gastroen Hepat. 2018;31(1):128–34. https://doi.org/10.1097/meg.0000000000001288.
Pant C, Deshpande A, Anderson MP, Sferra TJ. Clostridium difficile infection is associated with poor outcomes in end-stage renal disease. J Invest Med. 2012;60(2):529. https://doi.org/10.2310/jim.0b013e318242b313.
Guh AY, Li R, Korhonen L, et al. Characteristics of patients with initial Clostridioides difficile infection (CDI) that are associated with increased risk of multiple CDI recurrences. Open Forum Infect Dis. 2024;11(4):ofae127. https://doi.org/10.1093/ofid/ofae127.
Feuerstadt P, Louie TJ, Lashner B, et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. New Engl J Med. 2022;386(3):220–9. https://doi.org/10.1056/nejmoa2106516.
Sims MD, Khanna S, Feuerstadt P, et al. Safety and tolerability of SER-109 as an investigational microbiome therapeutic in adults with recurrent Clostridioides difficile infection. Jama Netw Open. 2023;6(2):e2255758. https://doi.org/10.1001/jamanetworkopen.2022.55758.
Cohen SH, Louie TJ, Sims M, et al. Extended follow-up of microbiome therapeutic SER-109 through 24 weeks for recurrent Clostridioides difficile infection in a randomized clinical trial. JAMA. 2022. https://doi.org/10.1001/jama.2022.16476.
Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82. https://doi.org/10.1093/aje/kwq433.
Regev A, Björnsson ES. Drug-induced liver injury: morbidity, mortality, and Hy’s law. Gastroenterology. 2014;147(1):20–4. https://doi.org/10.1053/j.gastro.2014.05.027.
McChalicher CWJ, Lombardo MJ, Khanna S, et al. Manufacturing processes of a purified microbiome therapeutic reduce risk of transmission of potential bacterial pathogens in donor stool. J Infect Dis. 2023. https://doi.org/10.1093/infdis/jiad298.
McChalicher C, Abdulaziz A, Zhou SS, et al. Manufacturing process of SER-109, a purified investigational microbiome therapeutic, reduces risk of coronavirus transmission from donor stool. Open Forum Infect Dis. 2022;9(9):ofac448. https://doi.org/10.1093/ofid/ofac448.
Appaneal HJ, Caffrey AR, Beganovic M, Avramovic S, LaPlante KL. Predictors of mortality among a national cohort of veterans with recurrent Clostridium difficile infection. Open Forum Infect Dis. 2018;5(8):ofy175. https://doi.org/10.1093/ofid/ofy175.
Czepiel J, Krutova M, Mizrahi A, et al. Mortality following Clostridioides difficile infection in Europe: a retrospective multicenter case-control study. Antibiotics. 2021;10(3):299. https://doi.org/10.3390/antibiotics10030299.
Olsen MA, Stwalley D, Demont C, Dubberke ER. Clostridium difficile infection increases acute and chronic morbidity and mortality. Infect Control Hosp Epidemiol. 2019;40(1):65–71. https://doi.org/10.1017/ice.2018.280.
Gerding DN, Kelly CP, Rahav G, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis. 2018;67(5):649–56. https://doi.org/10.1093/cid/ciy171.
Guh AY, Hatfield KM, Winston LG, et al. Toxin enzyme immunoassays detect Clostridioides difficile infection with greater severity and higher recurrence rates. Clin Infect Dis. 2019;69(10):ciz009. https://doi.org/10.1093/cid/ciz009.
Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med. 2016;165(9):609–16. https://doi.org/10.7326/m16-0271.
Hota SS, Sales V, Tomlinson G, et al. Oral vancomycin followed by fecal transplantation versus tapering oral vancomycin treatment for recurrent Clostridium difficile infection: an open-label, randomized controlled trial. Clin Infect Dis. 2017;64(3):265–71. https://doi.org/10.1093/cid/ciw731.
Ferring Pharmaceuticals, Inc. Rebyota [package insert]. US food and drug administration website. Published online 2024. https://www.fda.gov/vaccines-blood-biologics/vaccines/rebyota. Accessed 30 Apr 2024
Cornely OA, Vehreschild MJGT, Adomakoh N, et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection: EXTEND study subgroup analyses. Eur J Clin Microbiol. 2019;38(6):1187–94. https://doi.org/10.1007/s10096-019-03525-y.
Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12(4):281–9. https://doi.org/10.1016/s1473-3099(11)70374-7.
Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. New Engl J Med. 2017;376(4):305–17. https://doi.org/10.1056/nejmoa1602615.
Khanna S, Assi M, Lee C, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs. 2022;82(15):1527–38. https://doi.org/10.1007/s40265-022-01797-x.
Allegretti JR, Marcus J, Storm M, et al. Clinical predictors of recurrence after primary Clostridioides difficile infection: a prospective cohort study. Dig Dis Sci. 2020;65(6):1761–6. https://doi.org/10.1007/s10620-019-05900-3.
van Rossen TM, Ooijevaar RE, Vandenbroucke-Grauls CMJE, et al. Prognostic factors for severe and recurrent Clostridioides difficile infection: a systematic review. Clin Microbiol Infect. 2021;28(3):321–31. https://doi.org/10.1016/j.cmi.2021.09.026.
Armstrong EP, Malone DC, Franic DM, Pham SV, Gratie D, Amin A. Patient experiences with Clostridioides difficile infection and its treatment: a systematic literature review. Infect Dis Ther. 2023;12(7):1775–95. https://doi.org/10.1007/s40121-023-00833-x.
Perez R, Khanna S, Tillotson GS, Lett JE, Prince MA, Lattimer C. Reducing recurrence and complications related to Clostridioides difficile infection. Prof Case Manag. 2022;27(6):277–87. https://doi.org/10.1097/ncm.0000000000000585.
Khanna S, Pardi DS, Aronson SL, Kammer PP, Baddour LM. Outcomes in community-acquired Clostridium difficile infection. Aliment Pharm Therap. 2012;35(5):613–8. https://doi.org/10.1111/j.1365-2036.2011.04984.x.
Acknowledgements
We would like to acknowledge the work of many contributors to this study and manuscript, including all the study investigators who made this trial possible. We would also like to acknowledge Genesis Research, LLC for their work in deriving Charlson Comorbidity Index scores. Finally, we are indebted to all the patients who participated in ECOSPOR III and ECOSPOR IV.
ECOSPOR III and IV Investigators: Anmar Hemaidan, Bharat Misra, Richard Nathan, Hien Nguyen, John Pullman, Jeffrey Williams, Idalia Acosta, Huy Tran, Kent Smith, Leonard Weinstock, Val Hansen, Michael Georgetson, Aasim Sheikh, Julia Garcia-Diaz, Calin Arimie, Gladys Andrade, Steven O’Marro, Tuba Esfandyari, Timothy Ritter, Ian Mcnicol Baird, Ronald Colman, Meenakshi Patel, Lilliam Hernandez, Atoya Adams, Marie Walton, Razvan Arsenescu, Max Shapiro, Marvin Heuer, Tatiana Bogdanovich, Doria Grimard, Theodore Steiner, Debra Butt, Peter Daley, Stephanie Gauthier, Chantal Guimont, Leonard Weinstock, Michael Kreines, Larry Berman, Michael Bennett, Ronald Fogel, Juan Carlos Moises Gutierrez, Peder Pedersen, Adam Bressler, Venkatesh Nadar, Eric Newton, Jorge Diaz, Jalal Abbas, Herbert DuPont, Aamir Jamal, Neetu Talreja, Sabrina Benjamin, Kamran Ayub, Godson Oguchi, Jose Pinero, Gowrappala Ramesh, Paul Sepe, Loren Brook, Frederick Ruthardt, Lindsey Surace, Ayub Hussain, Travis Rutland, Michael Schmalz, Gourisankar Degala, Raymond Phillips, Kent Stock, Jeffrey Bullock, Kenolisa Onwueme, Kenneth Johnson, Suzy Kim, Edward Portnoy, Scott Wofford, John Gancayco, Yoav Golan, Charles Barish, JeanMarie Houghton, Benton Oubre, Zeid Kayali, Magued Beshay, John Curran, Issa Ephtimios, Michael Tan, Angelo Coppola, Syed Naqvi, Richard Caradonna, Subhash Gumber, Sebastian Stanciu, Keith Friedenberg, Satinder Gill, Jaynier Moya, Olayemi Osiyemi, Jerry Stern, Alfred Bacon, Matthew Hall, Gail Hecht, Tariq Mehmood, James Haaksma, Lucky Flores, Brian Behm, Jeffrey Garber, Thomas Welton, James Welker, Alex Sherman, Charles Okolo, Ravish Parekh, Richard Black, Peter Higgins, Patricia Henry, Alexander Dela Llana, Shalini Katikaneni, Sanjeev Kumar, Raymond Mason, Jennifer Vincent, Ghassan Hadi, Mark Kogan, Ifzal Bangash, Robert Orr, Saad Jazrawi, Michael Galambos, Robert Jaeger, Rizwana Thanawala, Magued Beshay, John Curran, Ernest Hendrix, Matthew Parker, Mohammed Mazen Jamal, Ralf Gebhard, Sadia Dar, Bruce Branin, Rodolfo Hanabergh, Syed Nasir Husain, Govinda Lohani, Shatishkumar Patel, Mousab Tabbaa, Teresa Alfonso, Anubha Gupta, Antonio Terrelonge, Satish Rao, Debra Powell, Robert Brennan, Allan G. Coates, Andrew Gentry, Jason Wilson, Shiwali Rai, Kenneth Boren, Chandar Singaram, Todd B. Ellerin, Myung Choi, David Dulitz, Emil Valle, Atsushi Skuraba, John De Beixedon, Diane Carbonneau, Bruce Musgrave, Zahid N. Zafar, Pradeep Kumar Bekal, Eliot Godofsky, Harry Sarles, Yaneicy Gonzalez-Rojas, Miguel E. Trevino, Ahmed A. Arif, Chad M. Gonzales, Maria Cubillas, Agadasah Kuliev, Vivaik Tyagi, George Dickstein, Rukan Daccak, Roberto Fernandez, Ankur A. Doshi, Kofi W. Nauako, Sushma V. Gorrela, Babatunde Adeyafa, Harold G. Preiksaitis, James A. Maher, Eugene F. Yen, Najwa El-Nachef, Larry E. Clark, John Hong, Naval Parikh, Juan Sarol, Syed M. Rehman, John M. Joseph, Markian R. Bochan, Marco Zahedi, Patricial Salvato, Dhaval Patel, Feliz P. Tiongco, Shari E. Rozen.
Funding
The ECOSPOR III and ECOSPOR IV studies were funded by Seres Therapuetics. The Rapid Service Fee was funded by Seres Therapeutics and Nestle Health Science. Contributors from Seres Therapeutics were responsible for the design and conduct of the studies; the collection, management, analysis, and interpretation of the data; and the preparation, review, and approval of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
Colleen Kraft had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Lisa von Moltke, Elaine Wang. Data collection and study support: Clinical Research Organization ICON with oversight from Kelly Brady, Seres Therapeutics, Inc. Data analysis: Asli Memisoglu and David Lombardi, Seres Therapeutics, Inc., conducted and are responsible for the data analysis. Drafting of the manuscript: Barbara McGovern and Brooke Hasson. Critical revision of the manuscript for important intellectual content: All authors.
Corresponding author
Ethics declarations
Conflict of interest
Colleen Kraft reported serving on the scientific advisory board for Seres Therapeutics and serves as a consultant for Rebiotix/Ferring. Matthew Sims reported serving as an advisory board member for Prenosis, consultant for Applied BioCode, CorMedix, and Venatorx, and also reported serving as a principal investigator or co-investigator for the following companies: AstraZeneca, ContraFect, Crestone, Curetis GmBH, Pfizer, DiaSorin Molecular LLC, Epigenomics Inc, EUROIMMUN US, Finch Therapeutics, Adaptive Phage Genetics Biotest AG—PI, Dompe, Pfizer, Genentech USA Inc, Janssen Research and Development, LLC, Kinevant Sciences GmBH, Leonard-Meron Biosciences, Merck, Prenosis, QIAGEN Sciences LC, Regeneron Pharmaceuticals, Roche, Seres Therapeutics, Shire, and Summit Therapeutics. Christine Lee reported receiving grants from Rebiotix/Ferring, Seres, Merck and Summit Therapeutics to conduct clinical trials. Paul Feuerstadt reported serving as a consultant for Merck and Co. and also reported serving on the speakers bureau and on consulting/advisory boards for the following companies: Seres Therapeutics, Ferring/Rebiotix, and Takeda Pharmaceuticals. Sahil Khanna receives research support from Rebioitx/Ferring, Vedanta, Finch, Seres and Pfizer and serves as a consultant for ProbioTech, Takeda, Niche and Immuron. Colleen Kelly reported serving as a site investigator for Seres Therapeutics and Finch Therapeutics; serving as a clinical advisory board member (unpaid) for Openbiome; as well as serving as a consultant for Sebela Pharmaceuticals. Princy Kumar reported serving as an investigator/receiving research funds from ViiV, Gilead, Merck, and Theratechnologies; serving as a consultant for Viiv, Gilead, and Merck; serving on the Advisory Committee/Board for GSK, Gilead, and Merck; and is a shareholder of Pfizer, GSK, Gilead, and Merck. Brooke Hasson and Lisa von Moltke are employees and shareholders of Seres Therapeutics. Lisa von Moltke is an employee and shareholder of Seres Therapeutics and is also a shareholder and serves on the Board of Directors for Cara Therapeutics. Barbara McGovern, Ananya De, Elaine Wang, Asli Memisoglu, and David Lombardi are all former employees of Seres Therapeutics. Darrell Pardi reported receiving research grants from the following companies: Seres Therapeutics, Vedanta, Finch, Takeda, Applied Molecular Transport and also reported serving as a consultant for: Seres Therapeutics, Vedanta, Immunic Therapeutics, Abbvie, Otsuka, Ferring, Rise Therapeutics, Boehringer Ingelheim, and Summit. Paul Cook is a principal investigator for Gilead, Pfizer, Abbvie and the National Institutes of Health. Louis Korman, Charles Berenson, Mayur Ramesh, Bret Lashner, Alberto Odio, Edward Huang, and Stuart Cohen were study investigators. No other disclosures were reported.
Ethical Approval
Both trials were performed in accordance with Good Clinical Practice and with the Helsinki Declaration of 1964 and its later amendments. The protocols and amendments were reviewed and approved by local or central investigational review boards. There was no main center for either study, but the central investigational review board was WIRB Copernicus. A table of all investigational review boards is provided in the Supplementary Material. Written informed consent was obtained at screening. All patients were informed that by signing the informed consent document that they agreed to take part in the study and that they read and understood the information presented within, including that the results of the study may be shared with regulatory agencies, at scientific conferences, or through publications.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior Presentation: This manuscript is based on data that was presented at SHEA Spring 2023; April 11–14, Seattle, WA, USA and at Digestive Disease Week 2023; May 6–9, Chicago, IL, USA.
Sims M, Silverman M, Louie T, et al. Integrated efficacy analysis from phase 3 studies of investigational microbiome therapeutic, SER-109, in recurrent Clostridioides difficile infection. Antimicrobial Stewardship & Healthcare Epidemiology. 2023;3(S2):s5-s5. doi:10.1017/ash.2023.214.
Sims M, Berenson C, Cohen S, et al. Integrated safety analysis of phase 3 studies for investigational microbiome therapeutic, SER-109, in recurrent CDI. Antimicrobial Stewardship & Healthcare Epidemiology. 2023;3(S2):s44-s45. doi:10.1017/ash.2023.281.
Korman L, et al. Durability of the clinical response to SER-109, an investigational oral microbiome therapeutic, in a phase III open-label trial (ECOSPOR IV) in patients with recurrent Clostridioides difficile. Lecture 694, DDW 2023, 6–9 May, Chicago, IL, USA.
The collaborators of “The ECOSPOR III, ECOSPOR IV investigators” are listed in acknowledgement section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kraft, C.S., Sims, M., Silverman, M. et al. Integrated Safety and Efficacy Analyses of Phase 3 Trials of a Microbiome Therapeutic for Recurrent CDI. Infect Dis Ther (2024). https://doi.org/10.1007/s40121-024-01007-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40121-024-01007-z