Abstract
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) infections are a serious threat to public health. Vancomycin (VAN) remains the primary treatment for these infections, and achieving the recommended area under the curve (AUC) target has been linked to improved clinical outcomes. The current VAN therapeutic monitoring guidelines recommend a loading dose (LD) of 20–35 mg/kg to rapidly attain targeted VAN exposures within 24 h of therapy. However, there is a paucity of data describing the impact of VAN LD on day 1 area under the curve (AUC0–24). This study aims to employ pharmacokinetic (PK) equations to calculate and describe the AUC0–24 following a VAN LD of 20 mg/kg.
Methods
This was a retrospective study of adult patients who were loaded with VAN 20 mg/kg, received ≥ 48 h of treatment, and had two consecutive serum VAN levels collected within 24 h. Linear, non-trapezoidal PK equations and two post-infusion VAN levels were used to calculate AUC0–24. Therapeutic AUC0–24 was defined as 400–600 mg/l*h.
Results
Among 123 included patients, the median age was 46 years (IQR 36, 62), 54% (67/123) of the patients had a body mass index (BMI) ≥ 30 kg/m2 and 27% (33/123) were admitted to the intensive care unit (ICU). Following a LD of 20 mg/kg, 50% (61/123) of the patients met the therapeutic AUC0–24, while 22% (27/123) of the patients were subtherapeutic, and 28% (35/123) were supratherapeutic. Compared with patients who achieved therapeutic AUC0–24, patients with subtherapeutic AUC0–24 were more likely to be younger (44 vs. 37 years old) and have a BMI ≥ 30 kg/m2 (67 vs. 52%). In contrast, patients with supratherapeutic AUC0–24 were more likely to be older (64 vs. 44 years old) and to have chronic kidney disease diagnosis (23 vs. 7%) when compared to patients who achieved a therapeutic AUC0–24.
Conclusions
Only 50% of patients achieve the target AUC0-24 following a VAN 20 mg/kg LD, with younger, heavier patients underexposed and older patients with renal impairment overexposed, suggesting that different dosing strategies are needed for these populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Appropriate vancomycin exposure within 24 h of therapy improves clinical outcomes for serious methicillin-resistant Staphylococcus aureus infections. |
The utilization of a vancomycin loading dose facilitates the rapid attainment of therapeutic exposure; however, data describing the true effect of loading dose on day 1 area under the curve (AUC0–24) estimated by non-Bayesian methods is scarce, especially in patients not admitted to the intensive care unit (ICU). |
This study aims to employ linear non-trapezoidal (pharmacokinetic) PK equations to elucidate AUC0–24 values following the administration of a fixed 20 mg/kg vancomycin loading dose in adult hospitalized patients. |
What was learned from the study? |
Following the vancomycin loading dose of 20 mg/kg, 50% (61/123) of the patients attained therapeutic AUC0–24, however, the other half were either subtherapeutic (22%, (27/123)) or supratherapeutic (28%, (35/123)). |
For most non-critically ill adult patients with good renal function, a 20 mg/kg vancomycin loading dose is adequate to achieve target vancomycin AUC0–24; however, patient-specific characteristics, such as weight, age, and renal function can substantially affect vancomycin PK, necessitating further dose adjustment. |
Introduction
Vancomycin (VAN) remains the primary treatment for serious methicillin-resistant Staphylococcus aureus (MRSA) infections [1]; however, the efficacy of VAN can be impacted by interpatient pharmacokinetic (PK) variability [1]. The 2020 therapeutic monitoring of vancomycin guidelines from the American Society of Health-System Pharmacy, Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists (ASHP/IDSA/PIDS/SIDP) highlight the importance of achieving therapeutic VAN levels within the initial 24 h for serious MRSA infections [2] and several studies have reported a correlation between day 1 VAN area under the curve (AUC0–24) and improved clinical outcomes [3, 4]. Of note, Lodise et al. found that the risk of 30-day mortality was twice as high in patients with MRSA bacteremia who failed to achieve an AUC0–24/MIC threshold of 521 or more [3].
VAN follows first-order elimination and is primarily cleared through the kidneys [5]. Depending on patients' renal function, the time needed to reach steady-state conditions can usually take 48–72 h, which can delay treatment optimization [5, 6]. To enhance the probability of target attainment within 24 h of therapy, a one-time loading dose (LD) of 20–35 mg/kg is recommended by the 2020 ASHP/IDSA/PIDS/SIDP guidelines [2, 7]. Studies evaluating the impact of an LD on VAN AUC0–24 have mainly focused on patients with critical illness and utilized Bayesian software for AUC estimation [8, 9]. However, the impact of VAN LD on AUC0–24 in hospitalized patients, particularly with linear non-trapezoidal PK equations estimate of AUC, remains inadequately explored.
Linear non-trapezoidal PK equations, utilizing two VAN levels timed after LD administration, is an alternative approach for calculating AUC0–24 [1, 6, 10,11,12]. This method was first proposed by Sawchuk-Zaske et al. to estimate PK parameters based on the first dose and has been utilized by several studies to calculate VAN patient-specific PK parameters during the first 24 h of therapy [11,12,13,14]. This approach is simple, relies on fewer assumptions, and provides a real-time snapshot of the AUC corresponding to the sampling interval [2, 10, 15]. In a single-center, retrospective cohort study comparing Bayesian two-concentration methods to first-order equations, the two-level PK method demonstrated excellent correlation (r = 0.963) and clinical decision agreement (87%) at steady-state conditions [16]. However, data providing a head-to-head comparison between non-trapezoidal PK equations and Bayesian AUC0–24 estimates are lacking. Moreover, implementing the linear PK-equations is a practical and cost-effective method of calculating AUC [17]. This method can be achieved by integrating an Excel sheet-based formula into electronic medical records, thereby expanding the accessibility of this method to institutions that may have limited resources and lack funding to justify access to Bayesian software.
Given the reported clinical benefits of optimizing VAN AUC within the 24 h of therapy and the limited availability of data that utilize non-Bayesian methods to estimate AUC0–24 following fixed LD, our study aims to use linear non-trapezoidal PK equations to elucidate AUC0–24 values following the administration of a fixed 20 mg/kg VAN LD in adult hospitalized patients.
Methods
Study Design and Setting
This was an IRB-approved, retrospective observational cohort study of adult patients (age 18 years or older) admitted to Loma Linda University Medical Center (LLUMC) from May 1, 2022, to December 31, 2022 who received at least 48 h of intravenous VAN therapy and had two consecutive serum levels collected within 24 h of the VAN LD. We excluded patients with an undetectable VAN serum level of < 4 mcg/ml, serum levels collected before 4 h from loading dose administration, and/or only one serum level collected following the loading dose. Additionally, we excluded those who received non-intravenous routes of VAN and continuous infusion VAN, patients requiring renal replacement, pregnant patients, and patients who had VAN minimum inhibitory concentration (MIC) ≥ 2 mcg/ml.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration. The LLUMC Institutional Review Board approved this study (#5210270) and the waiver of informed consent, given the minimal risk and retrospective nature of the study design.
Institutional Vancomycin Dosing and Monitoring Protocol
At our institution, the VAN per Pharmacy protocol recommends a LD of 20–25 mg/kg, followed by two consecutive serum concentrations (mcg/ml). The first level is drawn at least 4 h after the end of infusion to avoid the distribution phase, while the second level is drawn at least 6 h after the first level. These levels were then used to calculate the patient’s specific volume of distribution (Vd), half-life, elimination rate constant (Ke), and VAN clearance within 24 h of therapy. This protocol allows individualized PK dosing of VAN, which another group validated previously [18]. Linear non-trapezoidal PK equations were used to calculate AUC0–24, as detailed in the Supplementary Material (Table S1).
Data Collection and Definitions
Demographic information, including race/ethnicity, age, body mass index (BMI), and comorbid conditions were recorded. Obesity was defined as having a BMI ≥ 30 kg/m2. The APACHE II scores and the Charlson Comorbidity Index were calculated. Intensive care unit (ICU) admission and renal function were recorded within 24 h of therapy. Acute kidney injury (AKI) was defined as an increase in serum creatinine by either ≥ 50% or 0.5 mg/dl, from baseline for two or more consecutive occurrences [2]. VAN treatment details, including indication, dose, infusion duration, frequency, and serum levels, were recorded. Study data were collected and managed using REDCap electronic data capture tools hosted at Loma Linda University Medical Center [19, 20].
Outcomes Data
The primary objective of this study was to describe AUC0–24 following the administration of a fixed 20 mg/kg VAN LD in adult hospitalized patients, using linear non-trapezoidal PK equations. Based on their calculated AUC0–24, patients were categorized into one of the following groups: subtherapeutic AUC0–24 (< 400 mg/l*h), therapeutic AUC0–24 (400–600 mg/l*h), and supratherapeutic AUC0–24 (> 600 mg/l*h). The secondary objective was to compare the characteristics of patients who achieved a therapeutic AUC0–24 to those with either subtherapeutic or supratherapeutic VAN exposure.
Data Analysis
Data were analyzed using IBM SPSS version 26 (IBM, Armonk, NY, USA). Normality tests were performed using the Shapiro–Wilk test on all continuous variables. Continuous variables were represented by either mean (± standard deviation) or median (interquartile range IQR 25–75%) as appropriate. Categorical variables were represented by counts and percentages.
Results
Patient Populations
A total of 260 patients who received VAN were screened for eligibility, out of which 154 patients received two or more days of VAN therapy and had two or more consecutive VAN levels available for PK calculation. Thirty-one patients who had an initial VAN concentration of < 4 mcg/ml were excluded due to the inability to calculate an accurate AUC. Interestingly, half of these excluded patients required admission to the ICU (52%, 16/31) and required vasopressor support (35%, 11/31) at VAN initiation. The final analysis included a total of 123 patients.
The baseline characteristics are summarized in Table 1. The median (IQR) age was 46 years (36, 62). Fifty-four percent (67/123) of the population were patients with obesity (BMI ≥ 30 kg/m2), and 27% (33/123) required admission to the ICU on the day of VAN initiation. A total of 10% (12/123) of the patients had chronic kidney disease (CKD) documented at baseline. The majority of patients (80%, 100/123) had stable renal function on days 1 and 2 of VAN therapy, allowing for a more accurate calculation of the AUC0–24. The leading indications for VAN treatment were skin and soft tissue (38%, 47/123) and pleuro-pulmonary infections (24%, 30/123) (Table 1). In terms of nephrotoxicity, 14% (17/12) of patients developed AKI during VAN treatment; however, only one case was attributed to VAN. Notably, the incidence of AKI was higher in patients who received vasopressors (36%; 8/22) than those who did not (8%; 9/101).
Pharmacokinetic Data
Table 2 summarizes VAN treatment and pharmacokinetic data. All patients received a median (IQR) LD of 20 mg/kg [19,20,21,22] and had two consecutive serum levels collected. All VAN levels were collected at least 4 h after the end of infusion, accounting for the distribution phase. The median duration between the first level and LD was 7 h [6, 8], and the median duration between the second level and LD was 13 h [12, 14]. Of the 123 patients, only 50% (61/123) achieved therapeutic AUC0–24 target (400–600 mg/l*h). Of the remaining patients, 22% (27/123) were subtherapeutic (AUC0–24 < 400 mg/l*h), and 28% (35/123) were supratherapeutic (AUC0–24 > 600 mg/l*h) (Fig. 1).
Comparison of Patients with Subtherapeutic AUC0–24 (AUC0–24 < 400 mg/l*h) vs. Therapeutic AUC0–24
A total of 27 (22%, 27/123) patients had a calculated subtherapeutic AUC0–24 < 400 mg/l*h. These patients were more likely to be younger (37 vs. 44 years old) and have a BMI ≥ 30 kg/m2 (67 vs. 52%) compared to patients with therapeutic AUC0–24. There were no other notable differences in comorbidities or requirement of an ICU admission between both arms. Patients in the subtherapeutic arm had larger Vd (0.86 vs. 0.75 l/kg) and faster VAN clearance (8 vs. 6 l/h) than patients in the therapeutic arm. The elimination rate constant was 33% higher in the subtherapeutic arm (0.12 vs. 0.09 1/h), resulting in a shorter half-life (6 vs. 8 h) in this population. The data are summarized in Table 3.
Comparison of Patients with Supratherapeutic AUC0–24 (AUC0–24 > 600 mg/l*h) vs. Therapeutic AUC0–24
There were 35 (28%, 35/123) patients with a calculated AUC0–24 > 600 mg/l*h. At baseline, these patients were more likely to be older (64 vs. 44 years old), and have known baseline CKD rates (23 vs. 7%). Otherwise, there were no appreciable differences in weight, ICU admission, and comorbidities between both arms. Patients in the supratherapeutic arm had slower VAN clearance (3 vs. 6 l/h) than patients with therapeutic AUC0–24. As expected, the elimination rate constant was lower in the supratherapeutic arm (0.06 vs. 0.09 1/h), resulting in a longer half-life (13 vs. 8 h) in this population.
Discussion
In this study, we investigated the impact of a fixed VAN LD on the AUC0–24 using a non-trapezoidal linear PK approach. We found that only 50% (61/123) of patients who received 20 mg/kg VAN LD achieved the target AUC0–24 of 400–600 mg/l*h, while the remaining 50% (62/123) were either subtherapeutic or supratherapeutic.
Patients in the subtherapeutic cohort exhibited a faster Ke, increased VAN clearance, and larger Vd. These differences can potentially be attributed to the combined effects of younger age and increased body weight, impacting VAN PK [21,22,23]. Specifically, both youth and increased body weight are known to correlate with enhanced VAN clearance, as supported by existing literature [1, 21, 23, 24]. Our findings align with this observation, as VAN clearance in our cohort was higher than that reported in the general population (8 vs. 5 l/h) and more consistent with VAN clearance reported in younger patients with obesity (8 vs. 6–10 l/h) [5, 21, 25]. Conversely, since increased body weight and youth have opposing effects on VAN Vd, this parameter had less impact on VAN disposition in this young cohort with obesity [21,22,23,24]. It is essential to note that VAN Vd does not scale proportionally with actual body weight, underscoring the importance of limiting the LD to mitigate the risk of toxicity [2, 21, 22, 26]. Given that VAN clearance was the primary determinant of underexposure in this cohort, shortening the dosing interval of the maintenance dose rather than increasing the total LD may be a more prudent approach to optimize exposure (\(Maintenance dose = Clearance \times Concentration \times Dosing interval\)) [5].
In the supratherapeutic cohort, patients exhibited lower Ke and VAN clearance, which can potentially be attributed to the combination of older age and poor renal function at baseline. The median age of these patients was 64 years old (IQR 42, 77), with 23% (8/35) of patients having CKD at baseline. Matzke et al. conducted a study investigating the relationship between changes in renal function and VAN PK across different age groups [27]. They employed linear PK equations to characterize VAN disposition after the first dose, similar to the methodology employed in this study. The authors reported a significant decrease in VAN clearance (2.4 l/h) and Ke (0.05 1/h) in patients aged between 46 and 66 years old and creatinine clearance (CrCl) between 40 and 87 ml/min. Similar to the results of these findings, our cohort’s median (IQR) VAN clearance and Ke were 3 l/h [2, 4] and 0.06 1/h (0.03, 0.07), respectively. The author concluded that reduced renal function was associated with a marked impact of VAN clearance, and dosage adjustment is warranted [27]. Since the Vd in this cohort was similar to values reported in the literature (0.7 l/kg), a change in LD is not required. Instead, extending the dosing interval may be a more effective solution to optimize exposure and minimize nephrotoxicity [5].
In our study, a 20 mg/kg LD resulted in a median AUC0–24 of 525 mg/l*h, and achieved AUC > 400 mg/l*h in 78% (96/123) of the patients. Prior research by Hosiamont et al. and Pongchaidecha et al. proposed a higher LD of 25–30 mg/kg to optimize VAN AUC0–24 [8, 9]. However, both studies focused on patients admitted to the ICU, where a higher LD might be warranted. A LD of 25–30 mg/kg resulted in AUC0–24 > 600 mg/l*h in over 50% of patients in both studies [8, 9]. The majority of the patients included in our study did not require an ICU admission (73%, 90/123). Notably, our study excluded a small cohort of critically ill individuals with a subtherapeutic serum vancomycin concentration after receiving a LD of 20 mg/kg. It is unclear whether a higher dose of 25 mg/kg would have been more beneficial for these patients. Importantly, we observed a lower incidence of AKI compared to the findings reported by Hosiamount et al. (14 vs. 38%) [8]. Although the causation between VAN LD and nephrotoxicity remains uncertain, an AUC0–48 > 650 mg/l*h was associated with an increased risk for AKI [28]. Future research should further explore the appropriate dose for the critically ill versus non-critically ill population. Otherwise, a 20 mg/kg LD appears sufficient to optimize VAN exposure without increasing the risk of nephrotoxicity in patients not admitted to the ICU.
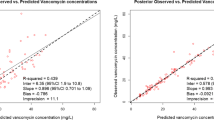
Most studies evaluating the impact of a VAN LD on AUC0–24 utilized Bayesian software to estimate AUC0–24. While Bayesian software provides reliable AUC estimates, cost and clinical experience can limit their implementation [29]. According to a 2019 survey aimed at assessing VAN monitoring practices in academic medical centers in the U.S., 23% (18/78) of surveyed institutions performed AUC-based monitoring. Only 28% (5/18) of institutions have implemented Bayesian software, while 67% (12/18) used linear PK equations for AUC estimation [30]. Bayesian software can potentially underestimate the true AUC by 14% to 23%, depending on the PK model and sampling strategies [31]. In this study, we employed non-trapezoidal linear PK equations to calculate AUC0–24. The main advantages of this method are simplicity, accessibility, and generalizability. Linear PK equations can be incorporated into electronic medical records and applied routinely in clinical practice [17]. By using timed post-distribution peak and trough levels to calculate patient-specific PK parameters (Vd and VAN clearance) and AUC0–24, fewer assumptions were made, resulting in a more accurate estimation of the true AUC [1, 7, 11, 32].
For simplicity, we used VAN total daily dose (TDD) and calculated VAN total body clearance to estimate AUC0–24 (\(AUC=\frac{TDD}{VAN clearance})\)[1, 11, 12, 14]. This approach captures the true AUC0–24 associated with the total VAN dose administered within the initial 24 h of therapy (i.e., LD ± maintenance doses). Given that VAN disposition can be described using one-compartment mono-exponential equations, provided two levels are collected during the elimination phase, VAN clearance was determined using compartmental approaches (\(VAN clearance=K \times V\)) [5, 33]. However, when applying one-compartment equations to drugs with two-compartment dispositions like VAN, drug loss during infusion (α-phase) is not fully accounted for, resulting in a slight underestimation of the true AUC [6]. Yet, intermittent infusion equations were used to account for most drug loss during infusion, which is likely insignificant as the median VAN half-life was five times longer than the infusion time [5, 7, 34]. Furthermore, 78% (96/123) of patients achieved an AUC0–24 > 400 mg/l*h. Even if the true AUC was slightly higher than what was estimated by this approach, this finding does not alter the conclusion that a LD of 20 mg/kg would suffice to optimize VAN exposure within the initial 24 h of therapy for the majority of non-critically ill patients with good renal function.
This study has several strengths, including the use of the specific AUC0–24 values for each patient allowing for minimal assumptions and improved internal validity as well as including patients with varying acuity of illness (27% of the patients were with critical illness) and PK profiles (50% of the patients were with obesity), thereby increasing the study's external validity. Despite the strengths of the study, this study has several limitations that warrant consideration. Firstly, due to the limited study period, our study did not include patients with a BMI below 18.5 kg/m2. Although VAN dosing is weight-based and adjusts for differences in body weight, it has been suggested that VAN’s Vd may not scale proportionally with actual body weight. Further investigation is needed to understand the impact of a 20 mg/kg LD of VAN on this population, as well as on other special populations not well represented in our study.
Secondly, the retrospective design of the study limited our ability to establish causality. Additionally, the small number of subtherapeutic and supratherapeutic patients prevented us from performing robust statistical analyses. Future studies with larger populations should aim to determine whether the variables identified in this study show significant correlations. Finally, the target AUC range of 400–600 mg*h/l is primarily validated in severe MRSA infections. In this study, only 26/123 patients (21%) had confirmed MRSA infection. However, this study focused on describing how fixed VAN LD affects AUC0–24. The clinical implications of achieving the AUC target for non-MRSA infections require further investigations.
Conclusions
Ultimately, our findings offer additional insights into employing simple linear PK equations to estimate VAN AUC0–24 following a fixed LD of 20 mg/kg. Based upon our linear PK calculations, non-critically ill patients with normal renal function are most likely to achieve a sufficient target AUC0–24 with 20 mg/kg VAN LD. However, younger (< 40 years) patients with BMI ≥ 30 kg/m2 are more likely to be subtherapeutic due to increased VAN clearance or/and Vd and may require an adjustment to the dosing frequency. A subset of patients who reside in the ICU may also be subject to subtherapeutic AUC0–24, but further research is required to better characterize this population. Conversely, older patients with impaired renal function require closer monitoring, and a LD may not be necessary. With the results of this study, we aim to contribute to the nuanced understanding of the impact of patient-specific factors in optimizing VAN dosing within 24 h of therapy for the treatment of severe MRSA infections.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bauer LA. In: Applied clinical pharmacokinetics, 3rd Ed. In: Vancomycin. New York: McGraw-Hill Medical; 2015. Accessed on Jan 21, 2024.
Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835–64.
Lodise TP, Drusano GL, Zasowski E, Dihmess A, Lazariu V, Cosler L, et al. Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: how much is enough? Clin Infect Dis. 2014;59(5):666–75.
Casapao AM, Lodise TP, Davis SL, Claeys KC, Kullar R, Levine DP, et al. Association between vancomycin day 1 exposure profile and outcomes among patients with methicillin-resistant Staphylococcus aureus infective endocarditis. Antimicrob Agents Chemother. 2015;59(6):2978–85.
Timothy J. Bensman PMB. Winter’s basic clinical pharmacokinetics / Paul Beringer, PharmD, Associate Professor, University of Southern California, Los Angeles, CA. . In: Vosburgh MHA, editor. Winter's Basic Clinical Pharmacokinetics Sixth edition. Philadelphia Lippincott Williams & Wilkins, a Wolters Kluwer business 2018.
Tozer MRTN. Clinical pharmacokinetics and pharmacodynamics: concepts and applications, fourth edition clinical pharmacokinetics: concepts and applications, 4e. Philadelphia: Lippincott Williams & Wilkins, a Wolters Kluwer business; 2011.
Siwale RC, Sani SN. Multiple-dosage regimens. In: Shargel L, Yu ABC, editors. Applied biopharmaceutics & pharmacokinetics, 7e. New York: McGraw-Hill Education; 2016.
Hodiamont CJ, Juffermans NP, Berends SE, van Vessem DJ, Hakkens N, Mathôt RAA, et al. Impact of a vancomycin loading dose on the achievement of target vancomycin exposure in the first 24 h and on the accompanying risk of nephrotoxicity in critically ill patients. J Antimicrob Chemother. 2021;76(11):2941–9.
Pongchaidecha M, Changpradub D, Bannalung K, Seejuntra K, Thongmee S, Unnual A, et al. Vancomycin area under the curve and pharmacokinetic parameters during the first 24 hours of treatment in critically ill patients using Bayesian forecasting. Infect Chemother. 2020;52(4):573–82.
Zheng H. Intravenous infusion. In: Shargel L, Yu ABC, editors. Applied biopharmaceutics & pharmacokinetics, 7e. New York: McGraw-Hill Education; 2016.
Deryke CA, Alexander DP. Optimizing vancomycin dosing through pharmacodynamic assessment targeting area under the concentration-time curve/minimum inhibitory concentration. Hosp Pharm. 2009;44(9):751–65.
Flannery AH, Delozier NL, Effoe SA, Wallace KL, Cook AM, Burgess DS. First-dose vancomycin pharmacokinetics versus empiric dosing on area-under-the-curve target attainment in critically ill patients. Pharmacotherapy. 2020;40(12):1210–8.
Sawchuk RJ, Zaske DE, Cipolle RJ, Wargin WA, Strate RG. Kinetic model for gentamicin dosing with the use of individual patient parameters. Clin Pharmacol Ther. 1977;21(3):362–9.
Shahrami B, Najmeddin F, Mousavi S, Ahmadi A, Rouini MR, Sadeghi K, et al. Achievement of vancomycin therapeutic goals in critically ill patients: early individualization may be beneficial. Crit Care Res Pract. 2016;2016:1245815.
Taskforce SVT. Vancomycin Area Under the Curve (AUC) Dosing Guideline Template for Institution Adaptation 2021 [Available from: https://sidp.org/Vancomycin-AUC-Implementation-Toolkit-Guide. Accessed on Jan 29, 2024.
Olney KB, Wallace KL, Mynatt RP, Burgess DS, Grieves K, Willett A, et al. Comparison of Bayesian-derived and first-order analytic equations for calculation of vancomycin area under the curve. Pharmacotherapy. 2022;42(4):284–91.
Gregory ER, Burgess DR, Cotner SE, VanHoose JD, Flannery AH, Gardner B, et al. Vancomycin area under the curve dosing and monitoring at an academic medical center: transition strategies and lessons learned. J Pharm Pract. 2020;33(6):774–8.
Truong J, Smith SR, Veillette JJ, Forland SC. Individualized pharmacokinetic dosing of vancomycin reduces time to therapeutic trough concentrations in critically ill patients. J Clin Pharmacol. 2018;58(9):1123–30.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95: 103208.
Blouin RA, Bauer LA, Miller DD, Record KE, Griffen WO Jr. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21(4):575–80.
Ducharme MP, Slaughter RL, Edwards DJ. Vancomycin pharmacokinetics in a patient population: effect of age, gender, and body weight. Ther Drug Monit. 1994;16(5):513–8.
Bearden DT, Rodvold KA. Dosage adjustments for antibacterials in obese patients: applying clinical pharmacokinetics. Clin Pharmacokinet. 2000;38(5):415–26.
Guay DR, Vance-Bryan K, Gilliland S, Rodvold K, Rotschafer J. Comparison of vancomycin pharmacokinetics in hospitalized elderly and young patients using a Bayesian forecaster. J Clin Pharmacol. 1993;33(10):918–22.
Adane ED, Herald M, Koura F. Pharmacokinetics of vancomycin in extremely obese patients with suspected or confirmed Staphylococcus aureus infections. Pharmacotherapy. 2015;35(2):127–39.
Hong J, Krop LC, Johns T, Pai MP. Individualized vancomycin dosing in obese patients: a two-sample measurement approach improves target attainment. Pharmacotherapy. 2015;35(5):455–63.
Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25(4):433–7.
Aljefri DM, Avedissian SN, Rhodes NJ, Postelnick MJ, Nguyen K, Scheetz MH. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019;69(11):1881–7.
Heil EL, Claeys KC, Mynatt RP, Hopkins TL, Brade K, Watt I, et al. Making the change to area under the curve-based vancomycin dosing. Am J Health Syst Pharm. 2018;75(24):1986–95.
Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. Readiness to implement vancomycin monitoring based on area under the concentration–time curve: a cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 2019;76(12):889–94.
Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014;58(1):309–16.
Flannery AH, Wallace KL, Rhudy CN, Olmsted AS, Minrath RC, Pope SM, et al. Efficacy and safety of vancomycin loading doses in critically ill patients with methicillin-resistant Staphylococcus aureus infection. Ther Adv Infect Dis. 2021;8:20499361211005964.
Ducharme MP. Drug elimination, clearance, and renal clearance. In: Shargel L, Yu ABC, editors. Applied biopharmaceutics & pharmacokinetics, 7e. New York: McGraw-Hill Education; 2016.
Murphy JE, Winter ME. Clinical pharmacokinetic pearls: bolus versus infusion equations. Pharmacotherapy. 1996;16(4):698–700.
Acknowledgements
We acknowledge Sasha Rodriguez for her assistance in collecting the data used in the study. Jacinda Abdul-Mutakabbir receives support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) of the National Institutes of Health (NIH) under Award Number K12HD113189. The content is solely the authors' responsibility and does not necessarily represent the official views of the National Institutes of Health. We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Abdulwhab Shremo Msdi: Concept, design, data analysis, and manuscript writing. Karen K. Tan: Concept, design, data analysis, and manuscript writing. Jacinda C. Abdul-Mutakabbir: data analysis, and manuscript writing.
Corresponding author
Ethics declarations
Conflicts of Interest
Jacinda Abdul-Mutakabbir is an Advisory Board member of Infectious Diseases and Therapy. Jacinda Abdul-Mutakabbir was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Jacinda Abdul-Mutakabbir received an honorarium from Shionogi, GSK, NovaVax, CSL Sequiris, Innoviva Specialty Therapeutics, and AbbVie. She has also received research support from CSL Sequiris. All other authors (Abdulwhab Shremo Msdi and Karen K. Tan) have no conflicts to report.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration. The LLUMC Institutional Review Board approved this study (#5210270) and the waiver of informed consent, given the minimal risk and retrospective nature of the study design.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shremo Msdi, A., Abdul-Mutakabbir, J.C. & Tan, K.K. Characterizing Day 1 Area Under the Curve Following Vancomycin Loading Dose Administration in Adult Hospitalized Patients Using Non-Trapezoidal Linear Pharmacokinetic Equations: A Retrospective Observational Study. Infect Dis Ther (2024). https://doi.org/10.1007/s40121-024-01004-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40121-024-01004-2