Abstract
Introduction
Ceftazidime-avibactam (CAZ-AVI) is a combination of the third-generation cephalosporin ceftazidime and the novel, non-β-lactam β-lactamase inhibitor avibactam that is approved for the treatment of pediatric (≥ 3 months) and adult patients with complicated infections including hospital-acquired and ventilator-associated pneumonia (HAP/VAP), and bacteremia. This systematic literature review and meta-analysis (PROSPERO registration: CRD42022362856) aimed to provide a quantitative and qualitative synthesis to evaluate the effectiveness of CAZ-AVI in treating adult patients with bacteremia or nosocomial pneumonia caused by carbapenem-resistant Enterobacterales (non metallo-β-lactamase-producing strains) and multi-drug resistant (MDR) Pseudomonas aeruginosa infections.
Methods
The databases included in the search, until November 7, 2022, were Embase and PubMed. A total of 24 studies (retrospective: 22, prospective: 2) with separate outcomes for patients with bacteremia or pneumonia were included.
Results
The outcomes assessed were all-cause mortality, clinical cure, and microbiological cure. Qualitative (24 studies) and quantitative (8/24 studies) syntheses were performed. The quality of the studies was assessed using the MINORS checklist and the overall risk of bias was moderate to high.
Conclusions
In studies included in the meta-analysis, lower all-cause mortality for patients with bacteremia (OR = 0.30, 95% CI 0.19–0.46) and improved rates of clinical cure for patients with bacteremia (OR = 4.90, 95% CI 2.60–9.23) and nosocomial pneumonia (OR = 3.20, 95% CI 1.55–6.60) was observed in the CAZ-AVI group compared with the comparator group. Data provided here may be considered while using CAZ-AVI for the treatment of patients with difficult-to-treat infections.
Systematic Review Registration
PROSPERO CRD42022362856.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ceftazidime-avibactam (CAZ-AVI) in bacteremia and nosocomial pneumonia (NP); pathogens carbapenem-resistant Enterobacterales (CRE), multidrug resistant (MDR) P. aeruginosa. |
Bacteremia treated with CAZ-AVI was associated with lower mortality and improved clinical cure. |
Patients with NP who were treated with CAZ-AVI demonstrated improved clinical cure. |
CAZ-AVI is a treatment option for patients with bacteremia and NP with MDR-gram-negative bacteria (GNB). |
Introduction
Gram-negative bacilli, Enterobacterales (such as Klebsiella pneumoniae, Escherichia coli, and Enterobacter spp.) and Pseudomonas aeruginosa are common human pathogens with increasing antibacterial resistance [1]. Carbapenems have been an effective treatment for most infections caused by Gram-negative bacteria; however, increased use clinically has resulted in carbapenem-resistant Enterobacterales (CRE) and P. aeruginosa (CRPA) [2]. Based on World Health Organization (WHO) classification, these are critical-priority pathogens [3]. CRE is one of the three most urgent antimicrobial-resistance threats identified by the Centers for Disease Control and Prevention (CDC) [4]. Both CRE and CRPA cause severe and often fatal infections including bacteremia, hospital-acquired pneumonia (HAP), and ventilator-associated pneumonia (VAP), and may further develop resistance to several antimicrobials [5, 6]. Progressive accumulation of antibiotic resistance mechanisms manifests in multidrug-resistance (MDR; defined as non-susceptible to ≥ 1 agent in ≥ 3 categories of antimicrobials). Invasive infections (e.g., bacteremia, nosocomial pneumonia) caused by MDR Enterobacterales and P. aeruginosa, are associated with mortality rates of ~ 34–56% [4, 7,8,9,10,11].
Ceftazidime-avibactam (CAZ-AVI) is a combination of the third-generation cephalosporin ceftazidime and the novel, non-β-lactam β-lactamase inhibitor avibactam. The combination exhibits in vitro activity against a broad range of Gram-negative bacteria, including against isolates harboring extended spectrum β-lactamase (ESBL)-, AmpC-, OXA-48, and serine carbapenemases (K. pneumoniae carbapenemase [KPC]) as well as MDR P. aeruginosa isolates; but not against metallo-β-lactamase (MBL)-producing strains [12]. CAZ-AVI is a frontline agent for the treatment of CRE infections and was approved by the U.S. Food and Drug Administration (FDA) in 2015 and the European Medicines Agency (EMA) in 2016 for the treatment of adult patients, and later pediatric patients (≥ 3 months), with complicated intraabdominal infections, complicated UTIs, hospital-acquired pneumonia (HAP) including VAP (and in EU for bacteremia in adults associated with any of these infections) [13,14,15]. In the EU specifically, CAZ-AVI is also approved for the treatment of patients with infections caused by Gram-negative organisms with limited treatment options.
A growing body of real-world evidence (RWE) has evaluated the effectiveness of CAZ-AVI in patients with bacteremia or nosocomial pneumonia. In the context of increasing antimicrobial resistance, it is important to assess the usefulness of newer-generation antimicrobials such as CAZ-AVI in treating these severe infections and use them judiciously to ensure continued effectiveness. Previous systematic literature reviews (SLRs) and meta-analyses evaluating the real-world use of CAZ-AVI have generally focused on any infection caused by Gram-negative organisms [16,17,18]. Such reviews have not elucidated outcomes for specific infection types due to the high variability in reported outcomes as well as the potential for bias in outcome assessments as a result of combining infection types with disparate outcomes. Bacteremia and nosocomial pneumonia cause disproportionately worse outcomes than other infection types [1, 5]. This SLR and meta-analysis is a comprehensive, quantitative, and qualitative synthesis of the clinical and microbiological outcomes of treating adult patients with CAZ-AVI, including those with bacteremia or nosocomial pneumonia caused by CRE (non-MBL producing) and MDR P. aeruginosa infections.
Methods
This SLR followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement and guidance [19]. This SLR protocol was registered on the international prospective register of systematic reviews (PROSPERO: CRD42022362856) on October 6, 2022 [20] and was updated to include a meta-analysis due to the availability of quantitative data on bacteremia and nosocomial pneumonia. This article is based on published literature and does not contain any previously unreported studies with human participants or animals.
Search Strategy
Systematic Embase and PubMed searches were conducted to include articles from 2015 onwards to November 7, 2022. Search terms used were “ceftazidime avibactam”, “hospital-acquired pneumonia”, “ventilator-associated pneumonia”, “bacteremia”, “pneumonia”, “bloodstream infection”, P. aeruginosa, Enterobacterales, Klebsiella pneumoniae, E. coli, Enterobacter cloacae and other synonyms (Supplemental Table 1). Additionally, similar reviews were also scanned manually for relevant articles that may not have been captured in the database search.
Study Selection
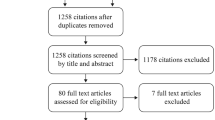
We included real-world studies (both prospective and retrospective), published in the English language. After search results were extracted from the selected databases, duplicates were removed using citation management software (Endnote V20.2 Clarivate Analytics, PA, USA), and a list of all eligible studies was created. The process of screening and inclusion and exclusion criteria are presented in Fig. 1 (PRISMA flow diagram).
PRISMA flow diagram of the search process and study selection. *Studies included patients with multiple infection types and outcomes could not be differentiated by indications and such studies were excluded. AMR antimicrobial resistance, AMS antimicrobial stewardship, PK pharmacokinetics, PD pharmacodynamics, RCTs randomized controlled trials
Screening, Data Extraction, and Quality Assessment
A two-step process involving two reviewers and a third independent reviewer was followed for screening and data extraction. Relevant studies were identified based on a title-abstract screening, followed by a full-text review of the shortlisted studies. Any disagreements on inclusion were resolved by discussion with a third reviewer. Data from eligible studies were extracted in an Excel spreadsheet (Microsoft Corp., Redmond, WA, USA). The quality of relevant studies was assessed using the methodological index for non-randomized studies (MINORS) checklist [21] with cross-review.
Inclusion and Exclusion Criteria
The following article types were excluded, with reasons for exclusion documented in each case: clinical trial data for any of the primary indications for CAZ-AVI, pediatric data, in vitro/animal/modeling simulation studies, studies including microbiological surveillance or population PK and PK/PD modeling, conference materials, review articles, guidelines, commentaries/opinion pieces, and editorials not reporting original outcomes data for patients treated with CAZ-AVI. We also excluded case reports and case series, studies without extractable data for the indication of interest (bacteremia and nosocomial pneumonia) and articles primarily reporting outcomes for bacteria with exclusive intrinsic resistant (MBL, New Delhi metallo-β-lactamases [NDM], Verona integron encoded metallo-β-lactamase [VIM], and imipenemase metallo-β-lactamase [IMP] producing organisms) to CAZ-AVI. Meta-analyses of published literature were also excluded from the analysis to avoid duplication of data; however, the source data/references within such articles were reviewed to ensure all relevant primary data were included.
Statistical Analysis
Meta-analysis was performed for synthesizing information and pooled estimates using package 'meta' on R version 1.1.453. τ2 and I2 (< 50%: no heterogeneity, ≥ 50%: significant heterogeneity) statistics were used to quantify heterogeneity among included studies. A random effects model was applied in case of significant heterogeneity; otherwise, a common/fixed-effect model was applied. Meta-analytical methods used were the Mantel–Haenszel method, restricted maximum-likelihood estimator for τ2, and Q-profile method for the confidence interval (CI) of τ2 and τ.
Separate analysis was conducted for each outcome and plots with ≥ 2 studies were included. CAZ-AVI therapy given as either monotherapy, combination therapy, or a mix of monotherapy and combination therapy was considered as one treatment group. Similarly, data from different comparators such as best available therapy (BAT) were grouped and presented in a combined manner as part of a single comparator group.
Results
Literature Search
A total of 1006 publications were identified. After further screening and exclusion, 24 relevant full-length articles were included in the qualitative synthesis and 8/24 articles in the quantitative synthesis (meta-analysis) (Fig. 1). Included publications reported data for a total of 1114 patients (bacteremia: 946, nosocomial pneumonia: 168) treated with CAZ-AVI either as a part of monotherapy, combination therapy, or both, and 640 patients (bacteremia: 539, nosocomial pneumonia: 101) treated with alternative or comparator antibiotics. The majority of patients included had bacteremia or pneumonia due to Enterobacterales except for two studies where patients with P. aeruginosa (bacteremia: CAZ-AVI—24, other antibiotic—37) [22, 23] infections were included.
For completeness, data from five relevant case series with aggregate data reported for bacteremia and nosocomial pneumonia is presented separately in Supplemental Tables 2 and 3.
Study Characteristics
Publications comprised 12 retrospective cohort studies [23,24,25,26,27,28,29,30,31,32,33,34], ten retrospective comparative/case–control studies [22, 35,36,37,38,39,40,41,42,43], and two prospective registry studies [44, 45] (Table 1, Fig. 2A). Most studies were 2020 onwards and were from China [22, 26, 30, 31, 33, 39, 41, 43] and Spain [23, 25, 32, 36, 42, 45] (Fig. 2B and C).
Of a total of 24 studies, 13 included patients with infection caused by K. pneumoniae, comprising six studies focused on CR-K. pneumoniae (CRKP, mechanism of resistance not reported), five studies focused on KPC- K. pneumoniae, and two studies focused on OXA-48K. pneumoniae. Six studies included patients with infections caused by more than one Enterobacterales comprising three studies focused on carbapenemase-producing Enterobacterales (CPE) and three studies focused on CRE. Two studies included patients with infection caused by P. aeruginosa (MDR/extensive drug-resistant, CR), and three studies with mixed pathogens.
Outcomes were not segregated based on the type of CAZ-AVI treatment (mono/combination) for bacteremia and nosocomial pneumonia in the included studies, hence outcomes extracted for the CAZ-AVI group include data from patients treated with either monotherapy, combination therapy, or both. We could extract separate outcomes for patients with bacteremia in 16/24 studies, patients with nosocomial pneumonia in 2/24 studies, and for both bacteremia and nosocomial pneumonia in 6/24 studies.
Patients with Bacteremia
Qualitative Synthesis
Mortality was reported in 18/22 studies which enrolled patients with bacteremia (Table 2). Overall, 30-day mortality was 24% (n = 217/904) in patients treated with CAZ-AVI and 40.12% (n = 199/496) in patients treated with other antibiotics.
Clinical cure was evaluated in 11/22 studies. Overall, clinical cure rates were 80.33% (n = 196/244) in patients treated with CAZ-AVI and 54.15% (n = 124/229) in patients treated with other antibiotics.
Microbiological cure occurred in 58.33% (n = 42/72) of patients in the CAZ-AVI group and 35.90% (n = 14/39) of patients in the comparator group, which was in a single study (Table 2) [42]. Resistance to CAZ-AVI was observed in 2.89% (n = 19/656, repeat susceptibility data not available) patients during the therapy, reported across five studies [24, 28, 29, 36, 44].
Sources of bacteremia were reported in six studies, but outcomes could not be differentiated by source. Overall, 10.80–99.30% of patients had bacteremia secondary to a pulmonary source sites [22, 33, 35, 36, 40, 43].
Quantitative Synthesis (Meta-analysis)
Of the total eight studies included in the quantitative synthesis, there was no statistical heterogeneity among included studies (I2 = 0%; P = 0.72–0.99), and therefore a common effect model was applied for all outcomes.
Mortality
All-cause 30-day mortality was evaluated in all eight studies (Fig. 3A). Overall, observed 30-day mortality was 15.38% (n = 32/208) in the CAZ-AVI group and 40.12% (n = 199/496) in the comparator group. Study heterogeneity was low (I2 = 0%, P = 0.99). Lower odds of 30-day mortality were evident among patients treated with CAZ-AVI versus comparators (Odds ratio [OR] = 0.30, 95% CI 0.19–0.46) (Fig. 3A) [22, 35,36,37,38, 40, 42, 43].
Meta-analysis of studies reporting 30-day mortality and clinical cure outcomes in patients with bacteremia. I2 significance of heterogeneity, CI confidence interval, OR odds ratio, Te number of events observed in the treatment group, TN total number of patients in the treatment group, Ce number of events observed in the comparator group, CN total number of patients in the comparator group
Clinical Cure
Clinical cure was evaluated in five studies (Fig. 3B) [36,37,38, 40, 42]. Overall, the clinical cure rates were 89.19% (n = 132/148) in the CAZ-AVI group and 54.15% (n = 124/229) in the comparator group (Fig. 3B). Study heterogeneity was low (I2 = 0%, P = 0.72). Higher clinical cure rates were evident among patients treated with CAZ-AVI vs. comparators (OR = 4.90, 95% CI 2.60–9.23).
Patients with Nosocomial Pneumonia
Qualitative Analysis
Of a total of eight studies that reported outcomes of patients with nosocomial pneumonia, mortality was reported in 6/8 studies (Table 2). Overall, all-cause 30-day mortality rates were 32.17% (n = 46/143) in patients treated with CAZ-AVI based therapy and 34.62% (n = 27/78) in patients treated with other antibiotics. Clinical cure was evaluated in 5/8 studies. Overall, clinical cure rates were 61.16% (n = 63/103) in patients treated with CAZ-AVI and 34.62% (n = 27/78) in patients treated with other antibiotics.
Microbiological cure was evaluated in five studies with overall rates of 63.21% (n = 67/106) in patients treated with CAZ-AVI and 30.77% (n = 24/78) in the patients treated with other antibiotics. Resistance was not reported in any of the studies.
Quantitative Synthesis (Meta-analysis)
Mortality and Clinical Cure
All cause 30-day mortality and clinical cure was evaluated in two studies (Fig. 4) [39, 42]. The 30-day mortality was 27.27% (n = 18/66) in the CAZ-AVI and 34.62% (n = 27/78) in the comparator group (Table 2). Study heterogeneity was low (I2 = 0%, P = 0.47). However, since data was available from two studies with high confidence interval (OR = 0.73, 95% CI 0.35–1.49), no definitive conclusions can be made (Fig. 4A). Clinical cure rates were 65.15% (n = 43/66) in the CAZ-AVI and 34.62% (n = 27/78) in the comparator group. Study heterogeneity was low (I2 = 28%, P = 0.24). Higher clinical cure rates were evident among patients treated with CAZ-AVI versus comparators (OR = 3.20, 95% CI 1.55–6.60) (Fig. 4B).
Meta-analysis of studies reporting 30-day mortality, clinical cure, and microbiological cure in patients with nosocomial pneumonia. I2 significance of heterogeneity, CI confidence interval, OR odds ratio, Te number of events observed in the treatment group, TN total number of patients in the treatment group, Ce number of events observed in the comparator group, CN total number of patients in the comparator group
Overall, in patients with nosocomial pneumonia higher rates of clinical cure were noted among patients treated with CAZ-AVI versus the comparator group; however, no differences were noted for mortality.
Microbiological Cure
Microbiological cure was 63.63% (n = 42/66) in the CAZ-AVI and 30.76% (n = 24/78) in the comparator group, also evaluated in the above two studies [39, 42]. Study heterogeneity was low (I2 = 0%, P = 0.55). Higher microbiological cure rates were evident among patients treated with CAZ-AVI versus comparators (OR = 4.95, 95% CI 2.34–10.46) (Fig. 4C).
Renal Failure at Baseline
Renal failure at baseline in patients with bacteremia was present in 26.50% of patients with CAZ-AVI (n = 159/600, four studies) and 19.51% in patients received other antibiotics (n = 16/82, two studies [28, 29, 36, 42]. The 30-day mortality in these studies was 22.17% in the CAZ-AVI and 28.40% in other antibiotics group; clinical cure was 93.65% in the CAZ-AVI and 75.45% for other antibiotics group. For nosocomial pneumonia, renal failure at baseline was reported in a single study (CAZ-AVI: 26.10%, other antibiotics: 12.5%) [42]; 30-day mortality was 21.70% in the CAZ-AVI and 37.50% in other antibiotics group; clinical cure was 91.30% in the CAZ-AVI and 56.20% for other antibiotics group.
Mortality Predictors
Mortality predictors were reported in 19 studies, of which 17 used multivariate analysis [22,23,24, 26, 28, 29, 31,32,33,34,35,36, 38, 41,42,43, 45] and one each used univariate [44] and bivariate analysis [40]. Mortality predictors reported in ≥ 3 studies were Pitt score (median: 3.0–4.0) [26, 33, 40, 43], INCREMENT-CPE score (> 7) [28, 32, 42, 45], neutropenia (absolute neutrophil count < 500) [26, 28, 29, 40], Charlson Comorbidity Index (≥ 2) [29, 41, 44], age [22, 35, 36], and septic shock [28, 29, 40]. Other mortality predictors reported in ≤ 2 studies are presented in Supplemental Fig. 1 and listed in Table 2 in detail. Thirteen mortality predictors, including time from blood culture collection to appropriate therapy, were individually reported in one study each and presented under the ‘other’ category.
Survival Predictors
Of a total of nine studies that reported survival predictors [22, 28, 29, 31, 33, 38, 41, 43, 44], eight reported CAZ-AVI as one of the survival predictors comprising two studies with CAZ-AVI monotherapy, five studies with CAZ-AVI combination therapy, and one with administration of CAZ-AVI via prolonged infusion (lasting ≥ 3 h) [22, 28, 29, 31, 33, 38, 41, 44]. Lower sequential organ failure assessment (SOFA) score at infection onset [38], source control of infection, appropriate empirical therapy [43], length of hospital stay after CRKP infection, creatinine clearance [41], and central venous catheterization [22] were reported in one study each and are presented under ‘other’ category (Supplemental Fig. 1, Table 2).
Adverse Events
Adverse events (AEs) were reported across the infection types and were not separated by type of indication for patients with bacteremia or nosocomial pneumonia. AEs were reported in 12/24 studies (Table 2). CAZ-AVI related AEs were mild diarrhea [25]; encephalopathy [36]; mild blood urea and creatinine increase, ALT and AST increase, ALP, GGT and total bilirubin increase; and thrombocytosis (no severe AEs) [30]; and decrease in creatinine levels [38]. Renal failure unrelated to CAZ-AVI was reported in two and three patients in two studies, respectively [32, 42] and nephrotoxicity in one patient in one study [23]. Significantly fewer AEs were reported in CAZ-AVI group than comparator (CAZ-AVI: 11/189, 5.8% vs. BAT: 30/150, 20%; P = 0.001) [42].
Renal adjustment for CAZ-AVI was reported in four (40%) patients in Chen et al., 2020 study [30]. King et al., 2017 and Sousa et al., 2018, also reported renal adjustment of CAZ-AVI in 33/60 (14/33 of those patients received RRT) and 20/57 (no treatment interruption) of total patients across infection type, respectively [27, 45]. In King et al., 2017, patients trended towards high in-hospital mortality (42% vs. 19% without renal adjustment, P = 0.057) with no drug-related AEs [27].
Quality Assessment
The quality assessment of included studies is presented in Supplemental Table 4. Ten studies assessed treatment with CAZ-AVI and a comparator scored 16–20/24 [22, 35,36,37,38,39,40,41,42,43] and 14 studies assessed treatment with CAZ-AVI without a comparator scored 8–14/16 [23,24,25,26,27,28,29,30,31,32,33,34, 44, 45]. The overall risk of bias in the studies was moderate to high. No exclusions were made based on the quality score.
Discussion
This SLR and meta-analysis provides comprehensive insights into the real-world use of CAZ-AVI for the treatment of patients with bacteremia or nosocomial pneumonia. From an analysis of 24 real-world studies (retrospective: 22, prospective: 2), compared to non-CAZ-AVI treatment regimens, CAZ-AVI or CAZ-AVI based regimens demonstrated lower all-cause 30-day mortality and improved clinical cure rates among patients with bacteremia and improved clinical cure rates among patients with nosocomial pneumonia.
The majority of studies that were assessed included patients with severe infections and concomitant conditions/diseases including hematological malignancies, solid organ transplantation, COVID-19, etc. Key infection-causing pathogens were CRE, including CPE, and MDR P. aeruginosa; however, the data for patients infected with P. aeruginosa is limited. A large proportion of reported cases treated with CAZ-AVI also included combinations with other antibiotics.
We performed a meta-analysis to synthesize results from studies with a comparator arm that evaluated patients with bacteremia or nosocomial pneumonia. Our analysis suggested that treatment with CAZ-AVI resulted in lower mortality and improved cure rates compared to non-CAZ-AVI regimens in patients with bacteremia specifically. Furthermore, since the chance of fatality is high in bacteremia [1, 5], receipt of appropriate initial therapy is critical, and thus, patients at high risk for infections due to CRE or MDR P. aeruginosa may be considered candidates for empiric therapy with CAZ-AVI. Clinical cure rates were also improved in our meta-analysis for patients with bacteremia. These findings are in line with prior meta-analyses showing that CAZ-AVI improved 30-day mortality and clinical cure rate when compared with other antibiotics (carbapenems, colistin, etc.); however, those analyses were pathogen-focused rather than indication-focused [16, 17, 46, 47]. Similarly, improved clinical cure rates were also observed in patients with nosocomial pneumonia; however, since data were available from few studies, results should be interpreted with caution. Additionally, a few studies were available to assess other outcomes, including mortality and microbiologic relapse/resistance.
The IDSA panel recommends CAZ-AVI as a preferred treatment option along with other antibiotics for the treatment of complicated urinary tract infection, pyelonephritis, and infections outside of the urinary tract caused by CRE [48]. In addition, if an OXA-48 enzyme is identified, CAZ-AVI is preferred. The current analysis is supportive of IDSA panel recommendation and suggests that CAZ-AVI may be considered as one of the initial treatment options in patients with known or suspected CRE bacteremia.
This review provides information on the real-world use of CAZ-AVI, and this quantitative and qualitative synthesis of available evidence may be useful for clinicians and caregivers in treating such patients. Our results should be considered in light of some limitations. Most included studies (22/24) were retrospective, which are subject to confounding and bias given their nature. Additionally, the outcomes assessed by different studies were not always uniform. While some studies reported mixed data for monotherapy and combination therapy, few reported clinical cure, and others clinical success. Considering the heterogeneity of studies, direct comparisons across studies (except studies included in the meta-analysis) were not possible due to variability in definitions, methods, and outcomes analyzed. Moreover, individual patient-level data were not available, which limits our ability to control for confounders and draw more definitive conclusions. Our analysis was also limited by grouping together patients who received CAZ-AVI alone or in combination with other agents. Moreover, our comparator group included various agents (Table 1), which reflects real-world practice. Finally, outcomes for P. aeruginosa were limited due to low number of studies and therefore the generalizability of these findings is limited.
This meta-analysis also has a number of strengths. First, a focused literature search strategy was applied to identify studies with data on bacteremia and nosocomial pneumonia, and a sufficient number of studies was included. Second, the outcomes used (mortality and clinical cure) can be considered significant in clinical practice and are useful in evaluating the effectiveness of an antibiotic regimen in the treatment of an infection. Third, no between-study heterogeneity was observed for meta-analysis. Heterogeneity is a potential problem in interpreting the results of any meta-analysis and, the absence of heterogeneity demonstrates the reliability of the quantitative results obtained.
Taken together, there are several key insights from this review. Information on the effectiveness of CAZ-AVI in the treatment of severe infections, which are not typically represented in homogenous populations in clinical trials was synthesized. Appropriate use of CAZ-AVI was one of the survival predictors reported in eight studies and on the other hand Pitt score, INCREMENT score (> 7), and neutropenia were prominent mortality predictors. RWE is especially useful to evaluate the changing rates of drug resistance to advanced combination antibiotics such as CAZ-AVI. Ideally these data should be evaluated systematically across studies and determine the impact of antimicrobial stewardship efforts to minimize rates of treatment-emergent resistance. Some studies have used CAZ-AVI as empiric treatment while others were reserving its use for targeted treatment. Choosing to treat aggressively early for better outcomes in patients rather than waiting for culture tests and susceptibility results must be balanced, however, waiting for the targeted treatment might result in worsening clinical outcomes in patients at high risk of death. In vitro surveillance of antimicrobial resistance trends is being monitored by various databases such as ATLAS, however, they are not based on patient outcomes. Clinical outcomes from RWE can provide useful indicators to prescribe appropriate antibiotics. Real-world data are required to fill in this gap, though, there is high heterogeneity in the use of different comparators and differences in outcomes based on which drug or drug combinations CAZ-AVI are being compared with and the conclusion should be interpreted with caution. Future studies are needed to compare CAZ-AVI with other novel β-lactam/β-lactamase inhibitors used to treat CRE and/or MDR P. aeruginosa.
Conclusions
This SLR and meta-analysis synthesized clinical outcome data and presents quantitative and qualitative evidence that may be considered while using CAZ-AVI for the treatment of patients with bacteremia and nosocomial pneumonia caused by non-MBL producing CRE and/or MDR P. aeruginosa. Nevertheless, there is a need for continued robust evidence generation to further fortify the role CAZ-AVI play in improving patients’ outcomes.
Data Availability
This article is based on published literature. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mancuso G, Midiri A, Gerace E, Biondo C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens. 2021;10:1310.
Wang Y, Wang J, Wang R, Cai Y. Resistance to ceftazidime-avibactam and underlying mechanisms. J Glob Antimicrob Resist. 2020;22:18–27. https://doi.org/10.1016/j.jgar.2019.12.009.
World Health Organization. Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis. 2017. https://apps.who.int/iris/handle/10665/311820 Accessed Jan 16, 2023.
Centers for Disease Control and Prevention. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE)—November 2015 update CRE toolkit. 2015. https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed Jan 16, 2023.
Breijyeh Z, Jubeh B, Karaman R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020. https://doi.org/10.3390/molecules25061340.
Centers for Disease Control and Prevention. Healthcare-Associated Infections (HAIs). CRE Technical Information. https://www.cdc.gov/hai/organisms/cre/technical-info.html#Risk. Accessed Jan 16, 2023.
Potter RF, D’Souza AW, Dantas G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist Updates. 2016;29:30–46. https://doi.org/10.1016/j.drup.2016.09.002.
Daikos GL, da Cunha CA, Rossolini GM, Stone GG, Baillon-Plot N, Tawadrous M, et al. Review of ceftazidime-avibactam for the treatment of infections caused by Pseudomonas aeruginosa. Antibiotics. 2021;10:1126.
Tabah A, Buetti N, Staiquly Q, Ruckly S, Akova M, Aslan AT, et al. Epidemiology and outcomes of hospital-acquired bloodstream infections in intensive care unit patients: the EUROBACT-2 international cohort study. Intensive Care Med. 2023;49:178–90. https://doi.org/10.1007/s00134-022-06944-2.
Patolia S, Abate G, Patel N, Patolia S, Frey S. Risk factors and outcomes for multidrug-resistant Gram-negative bacilli bacteremia. Ther Adv Infect Dis. 2018;5:11–8. https://doi.org/10.1177/2049936117727497.
Lin TL, Chang PH, Chen IL, Lai WH, Chen YJ, Li WF, et al. Risk factors and mortality associated with multi-drug-resistant Gram-negative bacterial infection in adult patients following abdominal surgery. J Hosp Infect. 2022;119:22–32. https://doi.org/10.1016/j.jhin.2021.09.021.
Shirley M. Ceftazidime-avibactam: a review in the treatment of serious Gram-negative bacterial infections. Drugs. 2018;78:675–92. https://doi.org/10.1007/s40265-018-0902-x.
U.S. Food and Drug Administration. NDA multi-disciplinary review and evaluation—NDA 206494 supplements 005 and 006 AVYCAZ (ceftazidime/avibactam) for injection. 2018. https://www.fda.gov/media/124307/download. Accessed Jan 19, 2023.
European Medicines Agency (EMA). European public assessment report (EPAR) for Zavicefta. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/004027/WC500210237.pdf. Accessed Jan 17, 2023.
U.S. Department of Health and Human Services UFaDA, Center for Drug Evaluation and Research (CDER). Guidance for industry. Hospital acquired bacterial pneumonia and ventilator-associated bacterial pneumonia: developing drugs for treatment. 2020. https://www.fda.gov/downloads/drugs/guidances/ucm234907.pdf. Accessed Jan 17, 2023.
Chen Y, Huang HB, Peng JM, Weng L, Du B. Efficacy and safety of ceftazidime-avibactam for the treatment of carbapenem-resistant Enterobacterales bloodstream infection: a systematic review and meta-analysis. Microbiol Spectr. 2022;10: e0260321. https://doi.org/10.1128/spectrum.02603-21.
Fiore M, Alfieri A, Di Franco S, Pace MC, Simeon V, Ingoglia G, et al. Ceftazidime-avibactam combination therapy compared to ceftazidime-avibactam monotherapy for the treatment of severe infections due to carbapenem-resistant pathogens: a systematic review and network meta-analysis. Antibiotics. 2020;9:1–12. https://doi.org/10.3390/antibiotics9070388.
Soriano A, Carmeli Y, Omrani AS, Moore LSP, Tawadrous M, Irani P. Ceftazidime-avibactam for the treatment of serious Gram-negative infections with limited treatment options: a systematic literature review. Infect Dis Ther. 2021;10:1989–2034. https://doi.org/10.1007/s40121-021-00507-6.
PRISMA. PRISMA: Transparent Reporting of Systematic Reviews and Meta-Analyses. https://www.prisma-statement.org/.
Shweta Kamat PMI, Margaret T, Ryan S, Juan Pablo H, Tobias W. Ceftazidime-avibactam in patients with bacteremia or pneumonia: a systematic literature review and meta-analysis. PROSPERO 2022 CRD42022362856. 2022. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022362856.
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. https://doi.org/10.1046/j.1445-2197.2003.02748.x.
Chen J, Liang Q, Chen X, Wu J, Wu Y, Teng G, et al. Ceftazidime/avibactam versus polymyxin B in the challenge of carbapenem-resistant Pseudomonas aeruginosa infection. Infect Drug Resist. 2022;15:655–67. https://doi.org/10.2147/idr.S350976.
Corbella L, Boán J, San-Juan R, Fernández-Ruiz M, Carretero O, Lora D, et al. Effectiveness of ceftazidime-avibactam for the treatment of infections due to Pseudomonas aeruginosa. Int J Antimicrob Agents. 2022;59: 106517. https://doi.org/10.1016/j.ijantimicag.2021.106517.
Vena A, Giacobbe DR, Castaldo N, Cattelan A, Mussini C, Luzzati R, et al. Clinical experience with ceftazidime-avibactam for the treatment of infections due to multidrug-resistant Gram-negative bacteria other than carbapenem-resistant Enterobacterales. Antibiotics. 2020. https://doi.org/10.3390/antibiotics9020071.
De la Calle C, Rodríguez O, Morata L, Marco F, Cardozo C, García-Vidal C, et al. Clinical characteristics and prognosis of infections caused by OXA-48 carbapenemase-producing Enterobacteriaceae in patients treated with ceftazidime-avibactam. Int J Antimicrob Agents. 2019;53:520–4. https://doi.org/10.1016/j.ijantimicag.2018.11.015.
Meng H, Han L, Niu M, Xu L, Xu M, An Q, et al. Risk factors for mortality and outcomes in hematological malignancy patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Infect Drug Resist. 2022;15:4241–51. https://doi.org/10.2147/idr.S374904.
King M, Heil E, Kuriakose S, Bias T, Huang V, El-Beyrouty C, et al. Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2017. https://doi.org/10.1128/AAC.00449-17.
Tumbarello M, Raffaelli F, Giannella M, Mantengoli E, Mularoni A, Venditti M, et al. Ceftazidime-avibactam use for klebsiella pneumoniae carbapenemase-producing K. pneumoniae infections: a retrospective observational multicenter study. Clin Infect Dis. 2021;73:1664–76. https://doi.org/10.1093/cid/ciab176.
Tumbarello M, Trecarichi EM, Corona A, De Rosa FG, Bassetti M, Mussini C, et al. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis. 2019;68:355–64. https://doi.org/10.1093/cid/ciy492.
Chen W, Sun L, Guo L, Cao B, Liu Y, Zhao L, et al. Clinical outcomes of ceftazidime-avibactam in lung transplant recipients with infections caused by extensively drug-resistant Gram-negative bacilli. Ann Transl Med. 2020;8:39. https://doi.org/10.21037/atm.2019.10.40.
Chen Y, Ying S, Jiang L, Dong S, Dai J, Jin X, et al. A novel nomogram for predicting risk factors and outcomes in bloodstream infections caused by Klebsiella pneumoniae. Infect Drug Resist. 2022;15:1317–28. https://doi.org/10.2147/idr.S349236.
Castón JJ, Gallo M, García M, Cano A, Escribano A, Machuca I, et al. Ceftazidime-avibactam in the treatment of infections caused by KPC-producing Klebsiella pneumoniae: factors associated with clinical efficacy in a single-center cohort. Int J Antimicrob Agents. 2020;56: 106075. https://doi.org/10.1016/j.ijantimicag.2020.106075.
Shen L, Lian C, Zhu B, Yao Y, Yang Q, Zhou J, et al. Bloodstream infections due to carbapenem-resistant Klebsiella pneumoniae: a single-center retrospective study on risk factors and therapy options. Microb Drug Resist. 2021;27:227–33. https://doi.org/10.1089/mdr.2019.0455.
Micozzi A, Gentile G, Santilli S, Minotti C, Capria S, Moleti ML, et al. Reduced mortality from KPC-K. pneumoniae bloodstream infection in high-risk patients with hematological malignancies colonized by KPC-K. pneumoniae. BMC Infect Dis. 2021. https://doi.org/10.1186/s12879-021-06747-8.
Falcone M, Bassetti M, Tiseo G, Giordano C, Nencini E, Russo A, et al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit Care. 2020;24:29. https://doi.org/10.1186/s13054-020-2742-9.
Lima O, Sousa A, Longueira-Suárez R, Filgueira A, Taboada-Martínez C, Portela-Pino C, et al. Ceftazidime–avibactam treatment in bacteremia caused by OXA-48 carbapenemase-producing Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis. 2022. https://doi.org/10.1007/s10096-022-04482-9.
Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2017. https://doi.org/10.1128/aac.00883-17.
Tsolaki V, Mantzarlis K, Mpakalis A, Malli E, Tsimpoukas F, Tsirogianni A, et al. Ceftazidime-avibactam to treat life-threatening infections by carbapenem-resistant pathogens in critically ill mechanically ventilated patients. Antimicrob Agents Chemother. 2020. https://doi.org/10.1128/aac.02320-19.
Shi Y, Hu J, Liu P, Wang T, Wang H, Liu Y, et al. Ceftazidime-avibactam-based versus tigecycline-based regimen for the treatment of carbapenem-resistant Klebsiella pneumoniae-induced pneumonia in critically ill patients. Infect Dis Ther. 2021. https://doi.org/10.1007/s40121-021-00542-3.
Castón JJ, Lacort-Peralta I, Martín-Dávila P, Loeches B, Tabares S, Temkin L, et al. Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing Enterobacteriaceae in hematologic patients. Int J Infect Dis. 2017;59:118–23. https://doi.org/10.1016/j.ijid.2017.03.021.
Fang J, Li H, Zhang M, Shi G, Liu M, Wang Y, et al. Efficacy of ceftazidime-avibactam versus polymyxin B and risk factors affecting clinical outcomes in patients with carbapenem-resistant Klebsiella pneumoniae infections a retrospective study. Front Pharmacol. 2021;12: 780940. https://doi.org/10.3389/fphar.2021.780940.
Castón JJ, Cano A, Pérez-Camacho I, Aguado JM, Carratalá J, Ramasco F, et al. Impact of ceftazidime/avibactam versus best available therapy on mortality from infections caused by carbapenemase-producing Enterobacterales (CAVICOR study). J Antimicrob Chemother. 2022;77:1452–60. https://doi.org/10.1093/jac/dkac049.
Chen L, Han X, Li Y, Li M. Assessment of mortality-related risk factors and effective antimicrobial regimens for treatment of bloodstream infections caused by carbapenem-resistant Enterobacterales. Antimicrob Agents Chemother. 2021;65: e0069821. https://doi.org/10.1128/aac.00698-21.
Karaiskos I, Daikos GL, Gkoufa A, Adamis G, Stefos A, Symbardi S, et al. Ceftazidime/avibactam in the era of carbapenemase-producing Klebsiella pneumoniae: experience from a national registry study. J Antimicrob Chemother. 2021;76:775–83. https://doi.org/10.1093/jac/dkaa503.
Sousa A, Pérez-Rodríguez MT, Soto A, Rodríguez L, Pérez-Landeiro A, Martínez-Lamas L, et al. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2018;73:3170–5. https://doi.org/10.1093/jac/dky295.
Zhong H, Zhao XY, Zhang ZL, Gu ZC, Zhang C, Gao Y, et al. Evaluation of the efficacy and safety of ceftazidime/avibactam in the treatment of Gram-negative bacterial infections: a systematic review and meta-analysis. Int J Antimicrob Agents. 2018;52:443–50. https://doi.org/10.1016/j.ijantimicag.2018.07.004.
Onorato L, Di Caprio G, Signoriello S, Coppola N. Efficacy of ceftazidime/avibactam in monotherapy or combination therapy against carbapenem-resistant Gram-negative bacteria: a meta-analysis. Int J Antimicrob Agents. 2019;54:735–40. https://doi.org/10.1016/j.ijantimicag.2019.08.025.
Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin Infect Dis. 2020;72:e169-e83. https://doi.org/10.1093/cid/ciaa1478.
Medical Writing, Editorial, and Other Assistance
Under the direction of the authors, Varkha Agrawal (PhD, CMPP™, CMD) provided data analysis and medical writing support; Sandeep Patil and Prajakta Hardas (MS) performed the literature search and data extraction; Kripa Madnani (PhD, CMPP™, CMD) provided review and Kanchan Bhati (MS) provided editorial assistance; we acknowledge Zubdahe Noor (PhD) for providing statistical support for the meta-analysis (all employees of Pfizer).
Funding
Sponsorship for this study and the journal’s Rapid Service Fee was funded by Pfizer Inc.
Author information
Authors and Affiliations
Contributions
Ryan K. Shields, Shweta Kamat, Paurus M. Irani, Margaret Tawadrous and Tobias Welte: Conceptualization and investigation; Shweta Kamat: data curation, funding, project administration, resources; Shweta Kamat and Tobias Welte: supervision; Ryan K. Shields, Shweta Kamat, Paurus M. Irani, Margaret Tawadrous, Tobias Welte and Juan P. Horcajada: Methodology; Shweta Kamat, Tobias Welte and Juan P. Horcajada: validation; Ryan K. Shields, Tobias Welte and Juan P. Horcajada: visualization; Ryan K. Shields and Margaret Tawadrous: writing original draft; All authors: Writing-critical review and editing; approval of final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Shweta Kamat and Paurus M. Irani are current employees of Pfizer and may hold stock/stock options with Pfizer. Margaret Tawadrous was an employee of Pfizer when this analysis was conducted. Ryan K. Shields is an employee of University of Pittsburgh and has served as a consultant for Allergan, Cidara, Entasis, GlaxoSmithKline, Melinta, Menarini, Merck, Pfizer, Shionogi, Utility, and Venatorx, and has received investigator-initiated funding from Merck, Melinta, Roche, Shionogi, and Venatorx; Juan P. Horcajada is an employee of Hospital del Mar and University of Pompeu Fabra, Barcelona, Spain and has received honoraria for advisory activities and served as a speaker for Pharma companies; Tobias Welte is an employee of Hannover School of Medicine and has received fees for lectures and served in advisory board for AstraZeneca and Pfizer.
Ethical Approval
This article is based on published literature and does not contain any previously unreported studies with human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tobias Welte: Deceased 10 March 2024.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shields, R.K., Horcajada, J.P., Kamat, S. et al. Ceftazidime-Avibactam in the Treatment of Patients with Bacteremia or Nosocomial Pneumonia: A Systematic Review and Meta-analysis. Infect Dis Ther 13, 1639–1664 (2024). https://doi.org/10.1007/s40121-024-00999-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00999-y