Abstract
Introduction
Ropeginterferon alfa-2b is a novel mono-pegylated proline-interferon. This clinical study aimed to evaluate its antiviral efficacy of ropeginterferon alfa-2b against SARS-CoV-2 infection.
Methods
This is a multicenter, randomized, open-label study. Adult patients with confirmed SARS-CoV-2 infection with initial cycle threshold (Ct) value < 30 and symptom onset within 4 days were enrolled. Eligible patients were randomized in a 2:1 ratio to receive a single 250-µg dose of ropeginterferon alfa-2b subcutaneously plus standard of care (SOC) or to receive SOC alone. The primary endpoint was the proportion of patients with a negative RT-PCR result for SARS-CoV-2 or discharged from the hospital before Day 8. Change in clinical status based on the World Health Organization (WHO) clinical progression scale and pulmonary infiltrations through chest radiograph were also evaluated.
Results
A total of 132 patients were enrolled and treated with study medication. Higher percentages of patients who achieved Ct ≥ 30 or were discharged from the hospital were observed on Day 8 and every other time point of assessment, i.e., Days 5, 11, 15, and 22, in the ropeginterferon alfa-2b group compared to the SOC alone group. However, the difference was statistically significant on Day 11 but not on Day 8. The primary endpoint was not met. The ropeginterferon alfa-2b group showed a higher improvement rate in lung infiltration on Day 5 (27.6% vs. 0.0%, p = 0.0087) and a higher improvement rate in WHO clinical progression scores on Day 8 (69.4% vs. 35.3%, p = 0.03) than those in the SOC group. No ropeginterferon alfa-2b-related serious adverse event was observed.
Conclusion
Our data show that ropeginterferon alfa-2b with SOC shortened the duration of SARS-CoV-2 shedding compared with SOC alone. In addition, ropeginterferon alfa-2b as an additional therapy could be beneficial by improving lung infiltration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study |
To date, administration of antiviral agents against SARS-CoV-2 infection remains limited due to drug–drug interaction, contraindication for patients with impaired liver or renal function, and clinical capacities in intravenous administration. |
Ropeginterferon alfa-2b is a novel mono-pegylated proline-interferon, which was proposed as an additional therapy for SARS-CoV-2 infection. This phase 3 clinical study aimed to evaluate the efficacy and safety of ropeginterferon alfa-2b against SARS-CoV-2 infection. |
What was learned from the study |
Statistically significant higher percentages of patients who achieved Ct ≥ 30 or were discharged from the hospital were observed at Day 11 after the treatment of ropeginterferon alfa-2b plus SOC compared to the SOC alone group. The ropeginterferon alfa-2b group showed a higher improvement rate in lung infiltration on Day 5 and a higher improvement rate in WHO clinical progression scores on Day 8 than those in the SOC group. |
Ropeginterferon alfa-2b with SOC shortened the duration of SARS-CoV-2 shedding compared with SOC alone. In addition, ropeginterferon alfa-2b as an additional therapy could be beneficial by improving lung infiltration. |
Introduction
Coronavirus disease 2019 (COVID-19), or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, have continued to spread worldwide since 2019. COVID-19 has a tremendous impact on human health leading to social burdens and economic loss. Massive vaccination has been implemented on a global basis and proved to decrease COVID-19-related morbidity and mortality. Anti-viral agents including remdesivir, nirmatrelvir/ritonavir, and molnupiravir have been widely administered among patients with comorbidities or severe disease. However, the implementation has been limited due to drug–drug interaction, contraindication for patients with impaired liver or renal function, and clinical capacities in intravenous administration [1,2,3]. Monoclonal antibodies including bamlanivimab/etesevimab, casirivimab/imdevimab, and tixagevimab/cilgavimab were introduced for both treatment and prophylaxis. However, the decrease of the neutralized activity was reported against new SARS-CoV-2 variants [4]. Therefore, the development of novel treatments with alternative mechanisms is required.
Type I interferons (IFN) was proposed to be a treatment option against SARS-CoV-2 infection by activating both cellular and humoral immunities. [5,6,7,8,9] In vitro and cohort clinical studies with small case numbers showed potential benefits with type I IFN treatment [6, 9,10,11]. Ropeginterferon alfa-2b is a site-selective PEGylated proline-IFN alfa-2b [12,13,14,15]. It was approved as the first IFN-based therapy for adult patients with polycythemia vera in Europe and the US, and is currently under clinical development for viral infection, including hepatitis B and C [16,17,18,19,20,21,22]. As for SARS-CoV-2 infection, a retrospective study by Chen et al. demonstrated that ropeginterferon alfa-2b in combination with standard of care (SOC) was associated with a more rapid viral clearance than SOC alone in patients with moderate SARS-CoV-2 infection [7]. In this multi-centered, open-labeled, randomized, phase 3 study, we aimed to evaluate the efficacy and safety of ropeginterferon alfa-2b for the treatment of patients with moderate SARS-CoV-2 infection.
Methods
Study Design and Patient Population
This multi-centered, open-labeled, randomized, phase 3 study was conducted at eight clinical centers in Taiwan to assess the efficacy and safety of ropeginterferon alfa-2b for the treatment of moderate SARS-CoV-2 infection. Moderate SARS-CoV-2 infection was defined as individuals who showed evidence of lower respiratory disease during clinical assessment or imaging and who ha d an oxygen saturation measured by pulse oximetry (SpO2) ≥ 94% on room air at sea level [8]. Patients aged more than 20 years with real-time polymerase chain reaction (RT-PCR)-confirmed SARS-CoV-2 infection were enrolled between April, 2022 and January, 2023, when the B.1.1.529 (Omicron) variant dominated the epidemics. Only those with initial cycle threshold (Ct) values of RT-PCR less than 30 and initial symptom onset within 4 days were included. Patients with Child–Pugh C liver cirrhosis, chronic kidney disease stage III–V (defined as an estimated glomerular filtration rate < 60 mL/min/1.73 m [2]), initial oxygen saturation < 93% when breathing ambient air, or receiving concurrent systemic corticosteroid therapy were excluded.
The study was conducted in accordance with the Helsinki Declaration of 2008. The study protocol and informed consent form (ICF) were approved by the Institutional Review Boards (IRB) of National Taiwan University Hospital, Taipei Medical University, and all other participating hospitals. ICFs including the consents of study participation and publication wer obtained from all patients prior to their participation. The IRB approval numbers and the number of patients enrolled in each clinical center are provided in supplemental Table 1. This study was registered at ClinicalTrials.gov (NCT05770466).
Treatments
Eligible patients were randomized at study Day 1 in a ratio of 2:1 to receive a single 250-µg dose of ropeginterferon alfa-2b (BESREMI®; PharmaEssentia) subcutaneously plus SOC or SOC alone. Patients were randomized centrally using the Interactive Web Response Systems system, stratified by study site and age, i.e., ≥ 65 years or < 65 years. SOC included anti-viral agents, i.e., molnupiravir, remdesivir, and nirmatrelvir/ritonavir, and infection prevention and control measures, as well as supportive care measures by the physicians’ judgement [8]. For patients randomized to the ropeginterferon alfa-2b group, ropeginterferon alfa-2b was given immediately after the randomization at Day 1. Patients randomized to the SOC group received SOC alone during the whole study period. The enrolled patients were followed for 29 days after randomization until death, discharge, or loss to follow-up, whichever occurred first.
Outcomes
As preliminary clinical data previously suggested that ropeginterferon alfa-2b therapy led to a shortened the SARS-CoV-2 RT-PCR conversion time of 7 days compared to 14 days in SOC alone [7], the primary endpoint was to evaluate the proportion of patients with a negative RT-PCR result of SARS-CoV-2 (defined as Ct value ≥ 30) on Day 8 or fulfilling the criteria of discharge before Day 8 (defined as defervescence for at least 24 h and improvement of respiratory symptoms). Secondary outcomes included the proportion of patients with a negative RT-PCR result for SARS-CoV-2 or fulfilling the criteria of discharge before Day 5 or Day 11, the change of clinical status over time evaluated as the proportion of patients with an improvement in at least one category based on the World Health Organization (WHO) clinical progression scale [23] at Day 5, Day 8, and Day 11, mean change from baseline on the WHO clinical progression scale, change in oxygen saturation overtime, interval between symptom onset and resolution, development of severe or critical disease that required oxygen therapy or mechanical ventilation, and mortality. The change of pulmonary infiltrations through chest radiograph was evaluated as the proportion of patients with an improvement in at least one category in the lung consolidation score [24].
The safety of ropeginterferon alfa-2b was assessed based on treatment-emergent adverse events (TEAEs), clinical laboratory evaluations including hemogram, biochemistry and inflammatory markers, chest radiograph or computed tomography scan, 12-lead electrocardiogram measurements, vital signs, and physical examinations. TEAEs were those adverse events (AEs) which happened at any time from the dose initiation (randomization) until the last contact in the study or the end of follow-up. For each TEAE, the investigator provided an assessment of the causal relationship with study treatment and graded the severity as mild, moderate, or severe.
Sample Size Estimation
Sample size estimation was calculated based on the primary endpoint to show non-inferiority for study group (ropeginterferon alfa-2b treatment and SOC) versus control group (SOC). The mean time to hospital discharge (± SD) for the study group was assumed to be 16.71 (± 4.23) days [7], while that for the control group was assumed to be 12.37 (± 8.96) days [25]. A non-inferiority margin of 10 was pre-defined based on 1.25σ. With 95% power, two-sided alpha of 5%, allocation ratio of 2:1 and 6% attrition rate, the estimated sample size was 100 for the ropeginterferon alfa-2b group and 50 for the SOC group [26, 27].
Statistics
For the categorical data, Chi-square test or the Cochran–Mantel–Haenszel test was used for treatment group comparisons as appropriate. Student’s t test was used for continuous data to compare groups. The safety analysis dataset included all patients who received treatment. The efficacy analysis dataset included patients who received either ropeginterferon alfa-2b + SOC or SOC alone as the safety analysis set. There was no imputation of efficacy data for analyses in this study. Unrecorded values were treated as missing and were be imputed except for the severity and the relationship to the study drug of AEs or where otherwise specified. If the severity or the relationship to the study drug were missing, the AE was regarded as severe or suspected, respectively.
Results
Patients Characteristics
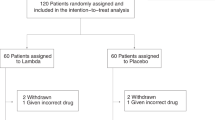
A total of 134 hospitalized patients with confirmed COVID-19 were enrolled in the study (Fig. 1). Enrollment was stopped when 134 patients were actually enrolled due to the changes of hospital admission policies, which caused difficulty for continuing to enroll patients. Two patients were excluded from the efficacy and safety analyses in the ropeginterferon alfa-2b group due to withdrawal of consent before the administration of the study drug. Of 132 patients included in the efficacy and safety analysis sets, four patients had major protocol deviations, including three lacking baseline chest radiograph and one who developed a critical disease upon trial drug administration. In all, 87 (65.9%) patients were randomized to receive a single 250-μg dose of ropeginterferon alfa-2b at Day 1 plus SOC treatment and 45 (34.1%) received SOC treatment alone (Fig. 1).
Table 1 shows the clinical characteristics of the 132 patients in the efficacy and safety analysis sets. The mean age was 63 years, while 71 (53.8%) were ≥ 65 years. Baseline characteristics including age, gender, baseline BMI, Ct value of SARS-CoV-2 RT-PCR, high-risk comorbidities associated with developing severe or critical disease were similar between the two groups. The most common high-risk comorbidity was hypertension (50.0%), followed by diabetes mellitus (31.1%) and coronary artery disease (12.1%). There were 100 enrolled patients receiving anti-viral agents, including 46 with nirmatrelvir/ritonavir, 28 with molnupiravir, and 29 with remdesivir, respectively. Twenty patients, including ten in each group, received steroids during the study period. None of the enrolled patients received tocilizumab or baricitinib. There was no statistically significant difference between the two groups in treatments, including antiviral agents and other immunosuppressants (Table 2).
Efficacy
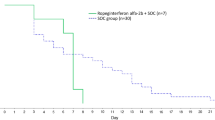
Seventy-four (85.1%) of 87 patients of the ropeginterferon alfa-2b and SOC groups and 36 (80.0%) of 45 patients of the SOC group had a negative RT-PCR result of SARS-CoV-2 or fulfilled the criteria of discharge on Day 8. However, there were no statistically significant differences (p = 0.46). In addition, more patients of the ropeginterferon alfa-2b and SOC groups had a negative RT-PCR result or were discharged throughout the observation period (i.e., Days 5, 11, 15, and 22) compared with those of SOC group. Statistically significant difference was only found on Day 11 (p = 0.04) (Fig. 2).
The percentage of patients with negative results in RT-PCR (defined as CT value ≥ 30) or discharge from hospital in all patients (A) or in a subgroup population excluding patients who received oxygen therapies before study medication (B) are presented. Time from randomization to PCR negative or discharge, which ever came first (C), is also presented. SOC standard of care
Forty-eight (36.4%) of the 132 patients received oxygen therapies before the administration of the study drugs. All these patients received oxygen therapy via a nasal cannula. These patients were kept in the study due to only mild oxygen desaturation being noted and as a potential clinical benefit by the investigator’s judgement. A subgroup analysis after excluding these patients was performed. Similar to the results in the primary analysis, a higher percentage of patients in the ropeginterferon alfa-2b group achieved a negative RT-PCR result or was discharged on Days 5, 8, 11, and 15. A statistically significant difference was also found on Day 11 (p = 0.04) (Fig. 2B). We also performed subgroup analyses by gender (supplemental Figure A, B), age (supplemental Figure C, D), WHO clinical progression scale (supplemental Figure E, F), and the use of other anti-viral agents (supplemental Figure G to J). The ropeginterferon alfa-2b group showed a higher response rate on Day 11 compared to the SOC group in the subgroup of patients who received any SARS-CoV-2 agents and in the subgroup of patients who received molnupiravir. We also performed a Kaplan–Meier analysis to evaluate the time from randomization to the date of SARS-CoV-2 seroconversion (Ct ≧30) or discharge due to remission, which ever came first. However, no statistically significant difference was observed between the ropeginterferon alfa-2b and SOC groups (Fig. 2C).
As shown in Fig. 3A and supplemental Table 2, patients in the ropeginterferon alfa-2b group showed a higher improvement rate of lung infiltration on Day 5 than those in the SOC alone group (27.6% vs. 0%, p = 0.0087). A higher rate of patients with improved lung infiltration was also observed on Day 8 in the ropeginterferon alfa-2b group, though the difference between the two groups was not statistically significant (35.3% vs. 7.7%, p = 0.08). All patients receiving ropeginterferon alfa-2b had an improved or stable status of lung infiltration compared to baseline during the study period, while two patients receiving SOC alone had a progression on lung filtration from < 25% to between 25 and 50%. Moreover, a higher rate of patients had improvements based on the WHO clinical progression scale on Day 8 in the ropeginterferon alfa-2b treatment group than in the SOC alone group (69.4% vs. 35.3%, p = 0.03; Fig. 3B and supplemental Table 2). The ropeginterferon alfa-2b group also showed a better improvement on Day 8 when evaluating the mean change from baseline on the WHO clinical progression scale compared to the SOC group (supplemental Table 2; − 1.78 vs. − 0.35, p = 0.001). There were three (6.8%) patients who developed a critical disease that required intubation and mechanical ventilation in the SOC group while none in the ropeginterferon alfa-2b group developed a critical disease (Table 3; p = 0.02). Two patients died of sepsis in the ropeginterferon alfa-2b group while one patient of the SOC group died of pulmonary hemorrhage (2.4% vs. 2.3%, p = 0.97).
Safety
Most of the AEs were mild or moderate (Table 3). The most common AE in the ropeginterferon alfa-2b group was constipation (9.8%), followed by diarrhea (9.1%). No ropeginterferon alfa-2b-related serious AE was observed and no patient withdrew from the study because of ropeginterferon alfa-2b-related AEs. Two fatal patients in the ropeginterferon alfa-2b group were considered as unrelated to study medication. No unexpected AEs were observed following ropeginterferon alfa-2b treatment. Most hematology, biochemistry, and urinalysis values were normal or abnormal without clinical significance throughout the study. No ropeginterferon alfa-2b-related hematocrit, leukocyte, or platelet decrease was observed. In addition, there is no ropeginterferon alfa-2b-related aspartate transaminase (AST) and alanine aminotransferase (ALT) increases in the study. Most patients had normal or no-clinically significant abnormal vital signs, electrocardiogram, and physical examinations throughout the study. No drug-related abnormalities in chest X-rays were reported.
Discussion
Ropeginterferon alfa-2b is a site-selective PEGylated proline-IFN alfa-2b [12,13,14,15]. It was approved as the first IFN-based therapy for adult patients with polycythemia vera (PV) in Europe and the US. Several studies have also demonstrated its clinical activities against chronic hepatitis B or C viral infections. The treatment with ropeginterferon alfa-2b notably decreased or cleared the viral load in patients with HBV or HCV [20,21,22]. As for SARS-CoV-2 infection, preliminary data suggested that a single injection of ropeginterferon alfa-2b in combination with SOC contributed to a faster viral clearance than SOC treatment alone in patients with moderate disease [7]. In this multi-centered, randomized, phase 3 clinical trial, our data show that a single 250-μg dose of ropeginterferon alfa-2b is a safe additional treatment for moderate COVID-19, by accelerating virus clearance and improving pulmonary infiltrations. To date, treatment options of antiviral agents approved for SARS-CoV-2 infection remain limited. Application of corticosteroid and other immunosuppressants should be cautious due to potential complications [28]. Development of an adjuvant treatment for SARS-CoV-2 infection is essential. Preclinical studies showed that the Omicron variant was less effective than the Delta viruses in antagonizing the interferon response, which make it highly sensitive to interferon therapy [29, 30]. While monoclonal antibody and some of the antiviral agents showed suboptimal activity against new variants of concerns, IFN may remains less influenced and continues to be effective in the era of new variants. [3]. [1]
Type I IFN-alpha (α) and -beta (β), and type III IFN-lambda are known to play essential roles in protecting against SARS-CoV-2 infections. Type I IFNs bind to the IFN-α/β receptor, while type III IFN binds to the IFN-lambda receptor [32]. Their signaling leads to the activation of overlapping IFN-stimulated genes (ISGs) for antiviral responses. SARS-Cov-2 is highly pathogenic, partly because of its ability to evade and inhibit the IFN signaling pathway [33]. Clinical data have shown a correlation between the inhibition of IFNs and poor outcomes of COVID-19. The concentration of IFN-α is upregulated in survivors, but not in non-survivors [34]. ISG expression is relatively higher, lower, and lowest in mild/moderate, severe, and critical patients, respectively. [35]
Clinical studies had demonstrated that early intervention with IFN showed notable clinical efficacy against SARS-CoV-2 infection. Bhushan et al. demonstrated that a single dose of pegylated-IFN α-2b induced a better clinical improvement and faster viral clearance than in the SOC alone group in a trial with 250 patients with moderate COVID-19 [6]. Our study showed a similar trend in viral clearance. Nearly 40% of the enrolled patients received oxygen treatment on the same day of study drug administration, which might impact the efficacy analysis of ropeginterferon alfa-2b. In addition, the concomitant medication of the other anti-SARS-CoV-2 agents including molnupiravir, remdesivir, and nirmatrelvir plus ritonavir in 75.8% of patients might also affect the efficacy of ropeginterferon alfa-2b. We therefore conducted subgroup analyses to evaluate the efficacy of ropeginterferon alfa-2b in those patients receiving other anti-SARS-CoV-2 agents. In the subgroup of patients who received molnupiravir, a statistically significant higher response rate, a Ct value ≥ 30, or discharge due to symptom remission, was observed on Day 11 in the ropeginterferon alfa-2b group compared to the SOC group (supplemental Fig. 1H). Our result implied that there might be a synergistic effect between IFN and molnupiravir, which was also observed in Bojkova’s study [30]. Further analysis will be needed to study the combination between ropeginterferon alfa-2b and molnupiravir. Since the case number was relatively small and the mortality rate in our study was low, the efficacy of ropeginterferon alfa-2b against COVID-19-related mortality could not be evaluated. Moreover, a retrospective study including patients having received off-label treatment with ropeginterferonalfa-2b during the outbreak of the B.1.1.7 variant of SARS-CoV-2 demonstrated that ropeginterferon alfa-2b treatment was associated with a shortened time to viral clearance [7]. This randomized clinical study showed promising results of ropeginterferon alfa-2b in the era of the B.1.1.529 variant.
Previous clinical studies revealed that pegylated IFN-lambda also showed a good efficacy in COVID-19 treatment. Feld et al. enrolled 60 COVID-19 patients with mild to moderate disease. Their study indicated that a higher percentage of patients had an undetectable viral load at Day 7 after receiving pegylated IFN-lambda compared to the SOC control group [36]. In addition, Reis et al. enrolled 2,617 patients with COVID-19 and found that early intervention with a single dose of pegylated IFN-lambda led to a lower rate of COVID-19-associated hospitalization and death [9]. Our clinical data suggested that the efficacy of ropeginterferon alfa-2b for COVID-19 treatment was similar to that of pegylated IFN-lambda. While pegylated IFN-lambda is still an investigational agent. ropeginterferon alfa-2b is an approved medicine for patients with PV. Vast amounts of clinical data have demonstrated its safety in human use.
Most AEs were mild or moderate in our study, and no unexpected AE was observed. Long-term use of IFN-based therapies may lead to increases of liver enzymes ALT and AST. However, no study drug-related ALT or AST increase was observed in the study, which implies a very low risk for triggering abnormal liver function when receiving a single dose of ropeginterferon alfa-2b at 250 μg in COVID-19 patients. No drug-related leukocytopenia was observed in the study. Two deaths due to sepsis observed in the ropeginterferon alfa-2b group did not appear to be due to an immunosuppressive effect. Per investigator’s judgement, these two deaths was related to disease progression but not to study treatment. Therefore, a single dose of ropeginterferon alfa-2b is safe and tolerable in patients with moderate SARS-CoV-2 infection.
There are some limitations in this study. First, the patient enrollment was discontinued before the target enrollment number was met, due to limited capacity of hospitalization during the pandemic. As the study proceeded, hospitals changed their policies from admission of all patients with COVID-19 to high-risk individuals and those with severe or critical disease. Second, we did not follow the RT-PCR of SARS-CoV-2 of those who was discharged, which might overestimate the efficacy of viral clearance. Third, 37.5% of the enrolled patients received oxygen therapies before study medications. Despite the oxygen supplement being relatively subtle, the disease severity might dampen the efficacy of ropeginterferon alfa-2b. A similar trend of viral clearance was found in the subgroup analysis (Fig. 2B).
Conclusions
Ropeginterferon alfa-2b showed promising clinical activities in the treatment of moderate COVID-19. Patients who received a single 250-µg dose of ropeginterferon alfa-2b with SOC showed improvement of lung infiltration and WHO clinical progression status compared to those who received SOC alone. Ropeginterferon alfa-2b was well-tolerated in patients with moderate COVID-19. Although our study provides valuable clinical data, further clinical studies are needed to fully validate the effectiveness of ropeginterferon alfa-2b treatment in patients with COVID-19.
Data Availability
The datasets generated during and/or analyzed in the current study are available from the corresponding author on reasonable request.
References
Moghadasi SA, Heilmann E, Khalil AM, Nnabuife C, Kearns FL, Ye C, et al. Transmissible SARS-CoV-2 variants with resistance to clinical protease inhibitors. Sci Adv 2023;9:eade8778.
Vitiello A. Sars-Cov-2 and risk of antiviral drug resistance. Ir J Med Sci. 2022;191:2367–8.
Rahmah L, Abarikwu SO, Arero AG, Essouma M, Jibril AT, Fal A, et al. Oral antiviral treatments for COVID-19: opportunities and challenges. Pharmacol Rep. 2022;74:1255–78.
Hoffmann M, Hofmann-Winkler H, Krüger N, Kempf A, Nehlmeier I, Graichen L, et al. SARS-CoV-2 variant B.1.617 is resistant to bamlanivimab and evades antibodies induced by infection and vaccination. Cell Rep 2021;36:109415.
King C, Sprent J. Dual Nature of Type I Interferons in SARS-CoV-2-Induced Inflammation. Trends Immunol. 2021;42:312–22.
Bhushan BLS, Wanve S, Koradia P, Bhomia V, Soni P, Chakraborty S, et al. Efficacy and safety of pegylated interferon-α2b in moderate COVID-19: a phase 3, randomized, comparator-controlled, open-label study. Int J Infect Dis. 2021;111:281–7.
Chen KY, Lee KY, Qin A, Luo CS, Yeh YK, Zheng JQ, et al. Clinical experience with ropeginterferon alfa-2b in the off-label use for the treatment of COVID-19 patients in Taiwan. Adv Ther. 2022;39:910–22.
National Institutes of Health. COVID-19 treatment guidelines. Last Updated: March 6, 2023. Available on: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
Reis G, Moreira Silva EAS, Medeiros Silva DC, Thabane L, Campos VHS, Ferreira TS, et al. Early treatment with pegylated interferon lambda for COVID-19. N Engl J Med. 2023;388:518–28.
Lokugamage KG, Hage A, deVries M, Valero-Jimenez AM, Schindewolf C, Dittmann M, et al. Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. J Virol. 2020;94:e01410-e1420.
Mantlo E, Bukreyeva N, Maruyama J, Paessler S, Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 2020;179: 104811.
Miyachi N, Zagrijtschuk O, Kang L, Yonezu K, Qin A. Pharmacokinetics and pharmacodynamics of ropeginterferon alfa-2b in healthy Japanese and Caucasian subjects after single subcutaneous administration. Clin Drug Investig. 2021;41:391–404.
Huang YW, Qin A, Fang J, Wang TF, Tsai CW, Lin KC, et al. Novel long-acting ropeginterferon alfa-2b: pharmacokinetics, pharmacodynamics, and safety in a phase I clinical trial. Br J Clin Pharmacol. 2022;88:2396–407.
Huang YW, Tsai CY, Tsai CW, Wang W, Zhang JJ, Qin A, et al. Pharmacokinetics and pharmacodynamics of novel long-acting ropeginterferon alpha-2b in healthy Chinese subjects. Adv Ther. 2021;38:4756–70.
Huang YW, Qin A, Tsai CY, Chen PJ. Novel pegylated interferon for the treatment of chronic viral hepatitis. Viruses. 2022;14:1128.
Chen CY, Chuang WL, Qin A, Zhang WH, Zhu LY, Zhang GQ, et al. A phase 3 clinical trial validating the potency and safety of an innovative, extra-long-acting interferon in chronic hepatitis C. JGH Open. 2022;6:782–91.
Qin A, Urbansky RW, Yu L, Ahmed T, Mascrenhas J. An alternative dosing strategy for ropeginterferon alfa-2b may help improve outcomes in myeloproliferative neoplasms: an overview of previous and ongoing studies with perspectives on the future. Front Oncol. 2023;13:1109866.
Jin J, Qin A, Zhang L, Shen WH, Wang W, Zhang JJ, et al. A phase 2 trial to assess the efficacy and safety of ropeginterferon alfa-2b in Chinese patients with polycythemia vera. Future Oncol 2023;Online ahead of print.
Verstovsek S, Komatsu N, Gill H, Jin J, Lee SE, Hou HA, et al. SURPASS-ET: phase III study of ropeginterferon alfa-2b versus anagrelide as second-line therapy in essential thrombocythemia. Future Oncol. 2022;18:2999–3009.
Lin HH, Hsu SJ, Lu SN, Chuang WL, Hsu CW, Chien RN, et al. Ropeginterferon alfa-2b in patients with genotype 1 chronic hepatitis C: pharmacokinetics, safety, and preliminary efficacy. JGH Open. 2021;5:929–40.
Hsu SJ, Yu ML, Su CW, Peng CY, Chien RN, Lin HH, et al. Ropeginterferon alfa-2b administered every two weeks for patients with genotype 2 chronic hepatitis C. J Formos Med Assoc. 2021;120:956–64.
Huang YW, Hsu CW, Lu SN, Yu ML, Su CW, Su WW, et al. Ropeginterferon alfa-2b every 2 weeks as a novel pegylated interferon for patients with chronic hepatitis B. Hepatol Int. 2020;14:997–1008.
WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020 Aug;20(8):e192-e197.
Martínez Chamorro E, Díez Tascón A, Ibáñez Sanz L, Ossaba Vélez S, Borruel Nacenta S. Radiologic diagnosis of patients with COVID-19. Radiologia (Engl Ed). 2021 Jan-Feb;63(1):56–73.
Abd-Elsalam S, Salama M, Soliman S, Naguib AM, Ibrahim IS, Torky M, et al. Remdesivir efficacy in COVID-19 treatment: a randomized controlled trial. Am J Trop Med Hyg. 2021;106:886–90. https://doi.org/10.4269/ajtmh.21-0606.
Shih W, Aisner J. Statistical design and analysis of clinical trials: principles and methods. 1st ed. Chapman & Hall/CRC Biostatistics Series; 2015.
Chow SC, Shao J, Wang HS, Lokhnygina Y. Sample Size calculations in clinical research 3rd ed. Chapman & Hall/CRC Biostatistics Series; 2017.
Liu WD, Wang JT, Shih MC, Chen KH, Huang ST, Huang CF, et al. Effect of early dexamethasone on outcomes of COVID-19: A quasi-experimental study using propensity score matching. J Microbiol Immunol Infect. 2024;S1684–1182(24):00039–42.
Bojkova D, Widera M, Ciesek S, Wass MN, Michaelis M, Cinatl J Jr. Reduced interferon antagonism but similar drug sensitivity in omicron variant compared to delta variant of SARS-CoV-2 isolates. Cell Res. 2022;32:319–21.
Bojkova D, Rothenburger T, Ciesek S, Wass MN, Michaelis M, Cinatl J Jr. SARS-CoV-2 omicron variant virus isolates are highly sensitive to interferon treatment. Cell Discov. 2022;8:42.
Vitiello A, Zovi A, Rezza G. New emerging SARS-CoV-2 variants and antiviral agents. Drug Resist Updat. 2023;70: 100986.
Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity. 2019;50:907–23.
Park A, Iwasaki A. Type I and type III interferons- induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870–8.
Contoli M, Papi A, Tomassetti L, Rizzo P, Vieceli Dalla Sega F, Fortini F, et al. Blood interferon-α levels and severity, outcomes, and inflammatory profiles in hospitalized COVID-19 patients. Front Immunol 2021;12:648004.
Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–24.
Feld JJ, Kandel C, Biondi MJ, Kozak RA, Zahoor MA, Lemieux C, et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med. 2021;9:498–510.
Acknowledgements
We are grateful to the patients and their families. We would like to thank all the participants as well as the study coordinators and nurses including Ching-Mei Chen, Ya-Chun Fang, Pu-Chi He, Hui-Ruo Heng, Hsiao-Ling Liu, Jing-En Dai, Shin-Jiun Tsai, Chu-Chun Lu, Chen-En Hsieh, Ying-Lan Li, Ya-Chun Tang, Hui-Ling Tang, and Hsin-Hui Lai.
Medical Writing/Editorial Assistance
The authors worked on all the tasks of medical writing and editing. There’s no medical writing or editorial assistance received during the writing of this article.
Funding
This work was supported by PharmaEssentia Corporation that also provided the drug used in the study. The Rapid Service Fee was also supported by PharmaEssentia Corporation.
Author information
Authors and Affiliations
Contributions
The study was designed by Wang-Huei Sheng, Kang-Yun Lee, Po-Hao Feng, Wang-Da Liu, Albert Qin, and PharmaEssentia clinical team. We would like to thank Jason Liao and Sheena Lin for their help with the statistical design and method. Wang-Da Liu, Po-Hao Feng, Chien-Yu Cheng, Chun-Liang Chou, Chih-Hsin Lee, Min-Chi Lu, Po-Yu Liu, Mei-Hui Lee, Chun-Hsing Liao, Mei-Chuan Chen, Cheng-Pin Chen, Shang-Fu Hsu, Yu-Tien Tzeng, Yi-Chun Lin, Tsong-Yih Ou, Kang-Yun Lee, and Wang-Huei Sheng recruited the patients and collected the data. Chan-Yen Tsai, Albert Qin, Weichung Joe Shih, Po-Hao Feng, Wang-Da Liu, Kang-Yun Lee, and Wang-Huei Sheng analyzed the data and wrote the initial manuscript. All authors interpreted the data and were involved in writing, reviewing, and approving the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
Albert Qin and Chan-Yen Tsai work for PharmaEssentia Corporation. Wang-Huei Sheng serves as a consultant of PharmaEssentia Corporation. The other authors do not have conflict of interest to declare.
Ethical Approval
The study was conducted in accordance with the Helsinki Declaration of 2008. The study protocol and informed consent form (ICF) were approved by the Institutional Review Boards of National Taiwan University Hospital, Taipei Medical University, and all other participating hospitals. The committee names and the approval numbers are provided in supplemental Table 1. ICF including the consents of study participation and publication was obtained from all patients prior to their participation. This study was registered at ClinicalTrials.gov (NCT05770466).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Liu, WD., Feng, PH., Cheng, CY. et al. A Phase 3, Randomized, Controlled Trial Evaluating the Efficacy and Safety of Ropeginterferon Alfa-2b in Patients with Moderate COVID-19. Infect Dis Ther (2024). https://doi.org/10.1007/s40121-024-00992-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40121-024-00992-5