Abstract
Introduction
This study aimed to estimate and compare the lifetime clinical and economic burden of invasive pneumococcal diseases (IPD) attributable to the serotypes contained in a new 21-valent pneumococcal conjugate vaccine (V116) vs. the 20-valent pneumococcal conjugate vaccine (PCV20) among adults aged 18 years and above in the USA.
Methods
A state-transition Markov model was used to track IPD cases and deaths as well as the associated direct medical costs (in 2023 US dollars) from a US healthcare payer perspective at 3% annual discount rate. The results were summarized for V116, PCV20, and eight unique serotypes contained in V116. A sensitivity analysis was conducted to determine the most influential inputs on the overall total direct lifetime cost.
Results
For the total population of US adults aged 18 years and above in 2021 (approx. 258 million residents), the estimated lifetime numbers of cases of IPD, post-meningitis sequelae (PMS), and IPD-related deaths attributable to the serotypes contained in V116 were approximately 1.4 million, 17,608, and 186,200, respectively, with a total discounted lifetime direct cost of $32.6 billion. A substantial proportion (approx. 31%) of those were attributable to the unique eight serotypes. The corresponding estimates for PCV20 were approximately 35% lower—934,000, 11,500, and 120,000, respectively—with a total discounted direct lifetime cost of $21.9 billion.
Conclusion
These results show that V116 serotypes (compared to PCV20) are associated with substantially higher clinical and economic burden of IPD. The addition of V116 to vaccination recommendations can help to reduce the residual burden of IPD in US adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Invasive pneumococcal diseaes (IPD) is associated with significant morbility, mortality, and costs among adults in the USA. |
V116 is a 21-valent pneumococcal conjugate vaccine (PCV) specifically designed for adults that contains eight unique serotypes (i.e., not found in any currently licensed vaccine). |
In this study, we estimated and compared the epidemiological and economic burden of IPD attributable to V116 serotypes vs. the 20-valent pneumococcal conjugate vaccine (PCV20) serotypes among adults in the USA. |
What was learned from the study? |
Our results show that V116 serotypes (compared to PCV20) are associated with substantially higher clinical and economic burden of IPD. |
The addition of V116 to vaccination recommendations can help to reduce the residual burden of IPD in US adults. |
Introduction
Associated with substantial morbidity and mortality worldwide (with highest burden in children < 5 years and elderly adults), Streptococcus pneumoniae continues to be the main cause of vaccine-preventable pneumococcal diseases (PDs) globally [1]. S. pneumoniae is transmitted primarily by human contact via respiratory droplets. PDs range in severity from mucosal noninvasive infections, such as otitis media, and other respiratory tract infections (e.g., community acquired pneumonia), to severe and life-threatening infections. Invasive or bacteremic pneumococcal disease (IPD) is the most serious manifestation, requiring hospitalization in some cases [2, 3]. IPD includes pneumococcal pneumonia, bacterial meningitis, and bacteremia [2, 3]. Moreover, patients contracting meningitis can develop post-meningitis sequelae (PMS), such as neurological impairment or hearing loss. In adults, non-invasive PDs, commonly known as non-bacteremic pneumococcal pneumonia (NBPP), account for approximately 75% of cases of pneumococcal pneumonia [4]. In addition, the presence of comorbid conditions is associated with a higher risk of PD [5, 6].
Despite the remarkable positive impact that pneumococcal conjugate vaccines (PCVs) have had on the burden of PDs in several countries with established national immunization programs (NIP) [1], there remains substantial PD burden among adults primarily attributable to the non-vaccine-serotypes (NVTs). After the approval of the two new vaccines in 2021, the United States (US) Advisory Committee on Immunization Practices (ACIP) recommended using either the 20-valent pneumococcal conjugate vaccine (PCV20 or Prevnar 20™, Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc.) alone or PCV15 (Vaxneuvance™, Merck & Co., Inc., Rahway, NJ, USA) in sequence with PPSV23 (Pneumovax23™, Merck & Co., Inc., Rahway, NJ, USA) for all adults aged ≥ 65 as well as those aged 19–64 years who have certain underlying medical conditions or risk factors [7].
Merck & Co., Inc., Rahway, NJ, USA is developing a 21-valent pneumococcal conjugate vaccine (PCV) specifically designed for adults that contains eight unique serotypes (i.e., not found in any currently licensed vaccine): 15A, 15C [generated from deOAc-15B], 16F, 23A, 23B, 24F, 31 and 35B) in addition to 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 17F, 19A, 20A, 22F, and 33F [8]. Recent IPD data suggests that the unique eight serotypes in V116 account for a substantial proportion of the residual IPD burden in adults [9, 10]. For example, according to the Centers for Disease Control and Prevention (CDC) data from 2018 to 2021, the serotypes covered by V116 are responsible for approximately 85% of invasive pneumococcal disease (IPD) in individuals aged 65+, and the unique eight serotypes were responsible for approximately 30% [11].
Objectives
Given the serotype profile of the new 21-valent vaccine (V116), it is important to estimate the magnitude of the health and economic burden of PD attributable to the serotypes it contains. Although NBPP constitutes a large proportion of PDs, this study only focuses on the burden of IPD. The study objectives include:
-
1.
Estimate and compare the total lifetime number of IPD cases and deaths attributable to the serotypes contained in V116 (including the unique eight) vs. PCV20 among US adults aged 18 years and above.
-
2.
Estimate and compare the total lifetime direct costs associated with the IPD cases attributable to the serotypes contained in V116 (including the unique eight) vs. PCV20 among US adults aged 18 years and above.
These estimates can help to assess and compare the magnitude of the burden of IPD among US adults attributable to the serotypes contained in V116 vs. PCV20.
Methods
Analytical Approach
A state-transition Markov model was developed to conduct this analysis. The model follows adults 18 years and above until death or 100 years of age and tracks the health and economic outcomes associated with the corresponding serotypes (V116, PCV20, and the unique eight). The study population was stratified into three mutually exclusive risk groups: low-risk (LR), at-risk (AR), and high-risk (HR) adults based on the age group specific proportions and classifications from Pelton et al. [5].
The health outcomes that were tracked over the time horizon were the number of cases of IPD, post-meningitis sequelae (PMS), and IPD-related deaths. Cost outcomes were determined by assigning the respective direct costs to each case of IPD and PMS. Half-cycle correction is applied to account for the fact that events and transitions can occur at any point during the cycle, not necessarily at the start or end of each cycle. It is applied to all outcomes except for clinical outcomes. All costs were discounted at 3% annual rate and, where needed, were inflated to reflect 2023 US dollars using the medical care component of the Consumer Price Index (CPI) obtained from US Bureau of Labor Statistics [12]. A deterministic sensitivity analysis was conducted to show the 15 most influential inputs on the total lifetime direct costs of IPD and PMS cases attributable to the V116 serotypes based on the plausible ranges used. The model and the analysis were developed and conducted in Microsoft Excel 365® (Microsoft Corporation, Redmon, WA, USA).
Model Structure
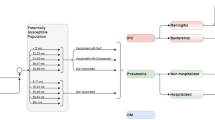
Figure 1 is a schematic diagram of the model. The model has four states: no IPD, IPD, PMS, and death. Among these, three are long-term states (no IPD, PMS, and death) where the cohorts can stay for more than 1 year/cycle and IPD is a short-term state where the cohorts stay for just one cycle. In other words, it is assumed that all IPD manifestations resolve within a year (one cycle). All cohorts start from the no IPD state and are at risk of infection. In each cycle, a proportion of those are infected with IPD. This is achieved by applying the annual incidence rates to the number in the no IPD state. Those with IPD may either have non-meningitis or meningitis IPD which is determined by applying the reported proportion of IPD cases that are meningitis, and the remaining are non-meningitis.
A proportion of those with meningitis progress to PMS which is determined by the reported percentage of meningitis cases that develop PMS. In this study, PMS is modeled as a long-term disease. In other words, PMS lasts for the rest of the lifetime/time horizon. An annual treatment cost for PMS occurs in each model cycle until death or end of the time horizon. On the other hand, patients with PMS are still at risk of non-meningitis IPD, but not meningitis IPD because data on meningitis reinfection for patients with PMS is lacking. In addition, as a result of lack of data, the risk of patients with PMS developing non-meningitis PMS was the same as that for the general population (i.e., those in the no IPD state). Finally, background (all-cause) death is applied to all states in the model to account for death from all other causes using the annual age-specific mortality rates. In addition, IPD individuals are at increased risk of death on top of the all-cause mortality. The case fatality rates are used to estimate the number of deaths associated with IPD cases.
To account for immunosenescence (age-associated immune system decline), risk transitioning was implemented in the model as individuals aged. Specifically, low-risk individuals can move to at-risk or high-risk, and at-risk can move to high-risk in each cycle as depicted in the no IPD and PMS states (Fig. 1). This was achieved via the annual transition probabilities among LR, AR, and HR that were derived from the risk distribution and classification data reported by Pelton et al. [5] (Table 1) in the long-term states (no IPD and PMS). It is assumed that the individuals’ risk profiles stay the same in the short-term state (IPD).
Model Inputs
This article does not contain any new studies with human participants or animals performed by any of the authors. The inputs for this study were obtained from the published literature, publicly available reports, and databases. Population size by single year of age from 2020 to 2021 was obtained from US Census Bureau (Table S1 in the supplementary material) [13]. Mortality in 2019 was obtained from the US National Vital Statistics Reports (Table S1 in the supplementary material) [14]. Initial risk distribution (Pelton et al. [5]) and the derived annual risk transition probabilities are presented in Table 1.
Table 2 shows the base case values for all the epidemiological and economic parameters IPD incidence, case fatality rates, proportion of meningitis in IPD, IPD disease coverage for serotypes included in V116, PCV20, and the unique eight, as well as the direct treatment costs for IPD and PMS. In this study, PMS is modeled via two specific sequalae: neurological disorder and hearing loss. The percentage of neurological disorder and hearing loss resulting from meningitis cases was 13% and 7%, respectively (Rubin et al. [15]).
Lower and upper bounds used for all parameters included in the deterministic sensitivity analysis (DSA) can be found in Table S2 in the supplementary material.
Model Validation
To ensure that the model was performing as expected and in line with the designed objective, all the calculations were verified through several tests. In particular, tests were used to check the three major types of mathematical disease modelling errors: logic, mechanical, and omission [16]. For example, to verify the model logic, the total number of persons in each age cohort and risk group equals the size of the population for that age group, after accounting for deaths.
Given the large number of inputs used in the calculations, systematic tests were implemented to confirm that the right inputs (from the right table cells—rows and/or columns) were used in each calculation. Tests for omissions were used to ensure that the theoretical model structure and its component states (as presented in Fig. 1) were accurately represented in the calculations [16]. Finally, the model was cross-validated quantitatively and qualitatively with another version of the same Markov model developed in Mathematica® 13.1 (Wolfram Research, Champaign, IL).
Results
Clinical Outcomes
For the total population of US adults aged 18 years and above in 2021 (approx. 258 million residents), the estimated lifetime numbers of cases of IPD, PMS, and IPD-related deaths attributable to the serotypes contained in V116 were 1.44 million, 17,628 and 186,198, respectively (Figs. 2, 3, 4 & Table S3). Of the total estimated IPD cases/deaths and PMS, approximately 31% were attributable to the unique eight serotypes in V116 (Figs. 2, 3, 4 and Table S3). On the other hand, the estimated lifetime numbers of cases of IPD, PMS, and IPD-related deaths attributable to the serotypes contained in PCV20 for the total population of US adults were approximately 35% lower: 933,986, 11,464, and 120,001, respectively (Figs. 2, 3, 4 and Table S3).
Among the age-groups analyzed, the 18–49 age group had the highest number of IPD cases and deaths followed by the 50–64, and then the 65+ (Figs. 2, 4 and Table S3).Consistently, within each age group, the at-risk population had the highest number of IPD cases and deaths followed by the high-risk group, and then the low-risk group (Table S3).
Economic Outcomes
Figure 5 and Table S3 shows the direct lifetime costs associated with clinical outcomes for each age group and risk category. For the total US adult population, the estimated discounted lifetime direct costs associated with IPD and PMS cases that were attributable to the serotypes contained in V116 were $32.6 billion. Approximately 28% of the total estimated direct lifetime costs associated with the IPD and PMS cases attributable to the serotypes in V116 were attributable to the unique eight serotypes in V116. On the other hand, the estimated lifetime direct cost of IPD and PMS that were attributable to the serotypes contained in PCV20 was $21.9 billion. Similar to the clinical outcomes, the 18–49 age group had the highest associated total direct lifetime costs followed by the 50–64, and then the 65+. Within each age group, the high-risk population had the highest associated total direct lifetime costs, followed by the at-risk group for the 18–49 and 50–64 age groups. The 65+ age group followed similar pattern as the clinical outcomes: at-risk had the highest direct lifetime cost followed by the high-risk and then the low-risk group.
Sensitivity Analysis
A summary of the one-way sensitvity analysis results depicted in decreasing order of the top 15 inputs’ influence on the total discounted direct lifetime cost is presented in Fig. 6 as a tornado diagram. On the basis of the ranges used, the most influential parameters are IPD incidence and direct treatment costs among the 50–64 HR and AR groups (Fig. 6). Overall, the top 15 influential inputs were associated with the at-risk and high-risk groups. However, discounting rate was the most influential input. The direct costs associated with the lower bounds (0%) and upper bounds (5%) of the discounting rates were $64 billion and $23.2 billion, respectively. These were excluded from the diagram because of their relatively large size making the others invisible.
Discussion
In this study, a state-transition Markov model was developed to estimate and compare the lifetime number of IPD cases/deaths (and associated direct costs) attributable to the serotypes contained in V116 vs. PCV20, as well as those attributable to the unique eight serotypes in V116 among US adults aged 18 years and above. The results indicated that the estimated lifetime numbers of cases of IPD, PMS, and IPD-related deaths that were attributable to the serotypes contained in V116 were approximately 1.4 million, 17,600, and 186,200, respectively, with a total discounted lifetime direct cost of $32.6 billion. In addition, a substantial proportion—about a third—of them was attributable to the unique eight serotypes contained in V116. The results also indicated that the lifetime numbers of cases of IPD, PMS, and IPD-related deaths that were attributable to the serotypes contained in PCV20 were approximately 35% lower than those estimated for the V116 serotypes—934,000, 11,500, and 120,000, respectively—with a total discounted direct lifetime cost of $21.9 billion.
The clinical and economic results showed that among the age groups, the 18–49 age group had the highest number of IPD cases and deaths followed by the 50–64, and then the 65+. This increasing order in the magnitude of the clinical and cost outcomes for the age groups is primarily because of two factors: population size and the tracking period. Although the 18–49 age group started with the lowest incidence among the three age groups, the 18–49 make up more than half of the 18 years and above population and they were tracked for the longest time period. The same is true for the 50–64 when compared to the 65+.
As expected, within each age group, the low-risk group had the lowest number of cases and costs compared to the at-risk and high-risk groups. This is also because of two major factors: risk of IPD and population size. The low-risk group had the lowest incidence rates within each age group and their population size diminished over time as a result of immunosenescense (transitioning to at-risk and high-risk states as they age through the model).
In general, the estimated burden of IPD cases and deaths found in this study are consistent with published estimates for other low-valent PCVs [17, 18]. While Huang et al. [18] assessed the health and economic burden of IPD attributable to the serotypes in PCV20, they limited their burden estimates to 1 year—annual clinical and cost burden. Owusu-Edusei et al. [17] examined the lifetime health and economic burden of IPD. However, their study focused on IPD cases that were attributable to the serotypes in PCV15 [17]. Because there are no studies that estimated the lifetime health and economic burden of IPD attributable to the serotypes in V116, a direct comparison of the magnitude of the estimates reported in this study is not possible.
Strengths
This study estimated the health and economic burden of a new 21-valent pneumococcal conjugate vaccine (V116) and compared it to the current highest-valent pneumococcal conjugate vaccine (PCV20). To our knowledge, this is the first study that examined the health and economic burden of the new 21-valent vaccine. Given that the objective of this study was to determine the burden of IPD for the entire US adult population, the model type used was a multi-cohort (rather than single-cohort) analysis which is more appropriate for accounting population heterogeneity and capturing all the population-level burden (health and costs) as indicated in the studies by Stoecker et al. [19] and Chen et al. [20]. In addition, the model used in this study accounted for risk groups as well as immunosenescence—changing risk profile as the cohorts aging through the model. This feature is critical as the at-risk and high-risk categories are associated with higher IPD risk and costs [5, 6] and therefore enables the model to provide a more truthful estimate on the outcomes.
Limitations
The limitations of this study are outlined below. First, the model assumed constant IPD epidemiology. This is unrealistic as the risk of IPD is expected to change over time with changing vaccine (and vaccine recommendations) in the USA. However, this assumption is typical for Markov/static models [3, 17,18,19, 21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. In addition, the comprehensive one-way sensitivity analysis addressed this limitation to some extent. Another major limitation of this study is that it focused on IPD burden only, although NBPP accounts for a large majority (approx. 75%) of pneumococcal pneumonia [4]. This implies that the estimates of the health and economic burden attributable to the serotypes in V116 and PCV20 reported in this study are underestimated considerably. Had the NBPP health and economic burden attributable to the serotypes in V116 and PCV20 been included, their respective estimates would be substantially higher. Finally, the economic estimates are conservative because only direct costs were included. Had indirect costs been included, the economic burden estimates would have been substantially higher.
Conclusion
This study estimated and demonstrated the substantial lifetime cases of IPD/PMS and IPD-related deaths among adults in the USA attributable to V116 serotypes, and to the unique eight serotypes contained in V116. The study also showed that these clinical outcomes are remarkably lower (approx. 35%) for the serotypes attributable to PCV20. These results suggest that the addition of V116 to vaccination recommendations can help to reduce the clinical and economic burden of invasive pneumococcal disease further among US adults.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Global Burden of Disease 2016. Lower respiratory infections collaborators, estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210.
Centers for Disease Control and Prevention. Chapter 11: Pneumococcal, manual for the surveillance of vaccine-preventable diseases. 2020. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt11-pneumo.html. Accessed 2023 May.
Ogilvie I, Khoury AE, Cui Y, et al. Cost-effectiveness of pneumococcal polysaccharide vaccination in adults: a systematic review of conclusions and assumptions. Vaccine. 2009;27(36):4891–904.
Said MA, Johnson HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS ONE. 2013;8(4):e60273.
Pelton SI, Bornheimer R, Doroff R, et al. Decline in pneumococcal disease attenuated in older adults and those with comorbidities following universal childhood PCV13 immunization. Clin Infect Dis. 2019;68(11):1831–8.
Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015;70(10):984–9.
Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the advisory committee on immunization practices-United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):109–17.
Merck & Co., Inc., Rahway, NJ, USA. Safety and immunogenicity of V116 in pneumococcal vaccine-naïve adults (V116-003, STRIDE-3). 2022. https://classic.clinicaltrials.gov/show/NCT05425732. Accessed Mar 2024.
Centers for Disease Control and Prevention. Active bacterial core surveillance report, emerging infections program network, Streptococcus pneumoniae, 2020. 2020. Atlanta, GA: Centers for Disease Control and Prevention.
Centers for Disease Control and Prevention. Active bacterial core surveillance report, emerging infections program network, Streptococcus pneumoniae, 2019. 2019. Atlanta, GA: Centers for Disease Control and Prevention.
Centers for Disease Control and Prevention. 2016–2021 serotype data for invasive pneumococcal disease cases by age group from active bacterial core surveillance. 2023. Atlanta, GA: Centers for Disease Control and Prevention.
Bureau of Labor Statistics. CPI for all urban consumers (CPI-U): medical care in U.S. city average, all urban consumers, not seasonally adjusted. 2023. Bureau of Labor Statistics.
United States Census Bureau. Annual estimates of the resident population by single year of age and sex for the United States: April 1, 2020 to July 1, 2021. 2022; https://www.census.gov/data/datasets/time-series/demo/popest/2020s-national-detail.html. Accessed Mar 2024.
Arias E, Xu JQ. United States life table, 2019, in National Vital Statistics Report. 2022. Hyattsville, MD: National Center for Health Statistics (USA).
Balsells E, Dagan R, Yildirim I, et al. The relative invasive disease potential of Streptococcus pneumoniae among children after PCV introduction: a systematic review and meta-analysis. J Infect. 2018;77(5):368–78.
Dasbach EJ, Elbasha EH. Verification of decision-analytic models for health economic evaluations: an overview. Pharmacoeconomics. 2017;35(7):673–83.
Owusu-Edusei K, Deb A, Johnson KD. Estimates of the health and economic burden of pneumococcal infections attributable to the 15-valent pneumococcal conjugate vaccine serotypes in the USA. Infect Dis Ther. 2022. https://doi.org/10.1007/s40121-022-00588-x.
Huang LP, Wasserman M, Grant L, et al. Burden of pneumococcal disease due to serotypes covered by the 13valent and new higher-valent pneumococcal conjugate vaccines in the United States. Vaccine. 2022;40(33):4700–8.
Stoecker C, Kim L, Gierke R, Pilishvili T. Incremental cost-effectiveness of 13-valent pneumococcal conjugate vaccine for adults age 50 years and older in the United States. J Gen Intern Med. 2016;31(8):901–8.
Chen C, Wood J, Beutels P, et al. The role of timeliness in the cost-effectiveness of older adult vaccination: a case study of pneumococcal conjugate vaccine in Australia. Vaccine. 2018;36(10):1265–71.
Leidner A. Summary of three economic models assessing pneumococcal vaccines in US adults. Atlanta, GA: Advisory Committee on Immunization; 2021.
Leidner AJ, Murthy N, Chesson HW, et al. Cost-effectiveness of adult vaccinations: a systematic review. Vaccine. 2019;37(2):226–34.
Treskova M, Scholz SM, Kuhlmann A. Cost effectiveness of elderly pneumococcal vaccination in presence of higher-valent pneumococcal conjugate childhood vaccination: systematic literature review with focus on methods and assumptions. PharmacoEconomics. 2019;37(9):1093–127.
Deb A, Guggisberg P, Mutschler T, et al. Cost-effectiveness of the 15-valent pneumococcal conjugate vaccine for high-risk adults in Switzerland. Expert Rev Vaccines. 2022;21(5):711–22.
Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011–21.
Stoecker, C. Economic analysis of sustaining the current recommendation for PCV13 use among adults 65 years or older in the context of continued indirect effects from the pediatric PCV13 program. Presented at the Advisory Committee on Immunization Practices. Meeting (2018 October 24 : Atlanta, GA). October 24, 2018. https://stacks.cdc.gov/view/cdc/61452. Accessed on March 2024.
Stoecker C. Economic analysis of sustaining the current recommendation for PCV13 use among adults 65 years or older in the context of continued indirect effects from the pediatric PCV13 program. 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-10/Pneumococcal-05-Stoecker-508.pdf. Accessed 2019 June.
Stoecker C. Economic assessment of PCV15 & PCV20. Presentation to the ACIP June 25, 2021. 2021. Centers for Disease Control and Prevention.
Stoecker C. Economic assessment of PCV20 for adults vaccinated with PCV13. Presented at ACIP, October 19, 2022. 2022. Centers for Disease Control and Prevention.
Stoecker C, Hampton LM, Link-Gelles R, et al. Cost-effectiveness of using 2 vs 3 primary doses of 13-valent pneumococcal conjugate vaccine. Pediatrics. 2013;132(2):e324–32.
Stoecker C, Hampton LM, Moore MR. 7-Valent pneumococcal conjugate vaccine and otitis media: effectiveness of a 2-dose versus 3-dose primary series. Vaccine. 2012;30(44):6256–62.
Stoecker C, Kobayashi M, Matanock A, Cho BH, Pilishvili T. Cost-effectiveness of continuing pneumococcal conjugate vaccination at age 65 in the context of indirect effects from the childhood immunization program. Vaccine. 2020;38(7):1770–7.
Wateska AR, Nowalk MP, Lin CJ, et al. Cost-effectiveness of adult pneumococcal vaccination policies in underserved minorities aged 50–64 years compared to the US general population. Vaccine. 2019;37(14):2026–33.
Wateska AR, Nowalk MP, Lin CJ, et al. Cost-effectiveness of pneumococcal vaccination policies and uptake programs in us older populations. J Am Geriatr Soc. 2020;68(6):1271–8.
Wateska AR, Nowalk MP, Lin CJ, et al. Pneumococcal vaccination in adults aged >/=65 years: cost-effectiveness and health impact in U.S. populations. Am J Prev Med. 2020;58(4):487–95.
Wateska AR, Nowalk MP, Lin CJ, et al. Cost-effectiveness of pneumococcal vaccination and uptake improvement programs in underserved and general population adults aged < 65 Years. J Community Health. 2020;45(1):111–20.
Wateska AR, Nowalk MP, Lin CJ, et al. Cost-effectiveness of an in-development adult-formulated pneumococcal vaccine in older US adults. Vaccine. 2023;41(30):4431–7.
Wateska AR, Nowalk MP, Zimmerman RK, Smith KJ, Lin CJ. Cost-effectiveness of increasing vaccination in high-risk adults aged 18–64 Years: a model-based decision analysis. BMC Infect Dis. 2018;18(1):52.
Wateska AR, PatriciaNowalk M, Lin CJ, et al. Cost-effectiveness of revised US pneumococcal vaccination recommendations in underserved minority adults < 65-years-old. Vaccine. 2022;40(50):7312–20.
Stoecker C. Economic assessment of PCV15 & PCV20. Presented at ACIP. June 15, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-Pneumococcal-Stoecker-508.pdf. Accessed Mar 2024.
Rubin JL, McGarry LJ, Strutton DR, et al. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine. 2010;28(48):7634–43.
Medical Writing, Editorial, and Other Assistance
None.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study and the manuscript publication fee was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author information
Authors and Affiliations
Contributions
Zinan Yi, Kelly D. Johnson and Kwame Owusu-Edusei conceptualized and determined the scope of the study. Zinan Yi performed the study analyses. Zinan Yi, Kelly D. Johnson and Kwame Owusu-Edusei reviewed the inputs, results and the interpretation of the results. Zinan Yi developed the first draft of the manuscript. Zinan Yi, Kelly D. Johnson and Kwame Owusu-Edusei critically reviewed and revised the manuscript for intellectual content. All the authors approved submission of this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
This study was performed by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. V116 is being developed by Merck & Co., Inc., Rahway, NJ, USA. Zinan Yi, Kwame Owusu-Edusei and Kelly D. Johnson are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Ethical Approval
This article does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yi, Z., Johnson, K.D. & Owusu-Edusei, K. Lifetime Health and Economic Burden of Invasive Pneumococcal Diseases Attributable to V116 Serotypes Among Adults in the United States. Infect Dis Ther 13, 1501–1514 (2024). https://doi.org/10.1007/s40121-024-00988-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00988-1