Abstract
Introduction
Onychomycosis is a fungal infection of the nails that can be challenging to treat. Here, matrix-assisted laser desorption ionization–Fourier transform ion cyclotron resonance (MALDI-FTICR) imaging was applied to the quantitative analysis of the penetration profile of the antifungal compound, amorolfine, in human mycotic toenails. The amorolfine profile was compared with those of three other antifungals, ciclopirox, naftifine, and tioconazole.
Methods
Antifungal compounds (amorolfine 5% lacquer, ciclopirox 8% lacquer, naftifine 1% solution, and tioconazole 28% solution) were applied to mycotic nails (n = 42). Nail sections were prepared, and MALDI-FTICR analysis was performed on the sections at a spatial resolution of 70 μm to compare the distribution profiles. Based on the minimum inhibitory concentrations of the four test compounds needed to kill 90% (MIC90) of the fungal organism, Trichophyton rubrum, the fold differences between the MIC90 and the antifungal concentrations in the nails (termed the multiplicity of the MIC90) were calculated for each.
Results
The penetration profiles indicated higher concentrations of amorolfine and ciclopirox in the deeper layers of the nails 3 h after treatment, compared with naftifine and tioconazole. The mean concentrations across the entire nail sections at 3 h were significantly different among the four antifungals: amorolfine, 2.46 mM; ciclopirox, 0.95 mM; naftifine, 0.63 mM; and tioconazole, 1.36 mM (p = 0.016; n = 8 per compound). The median multiplicity of the MIC90 at 3 h was 191-fold for amorolfine, tenfold for ciclopirox, 52-fold for naftifine, and 208-fold for tioconazole.
Conclusion
In this study, MALDI-FTICR was successfully applied to the quantitative analysis of antifungal distribution in human mycotic nails. The findings suggest that amorolfine penetrates deeper layers of the nail and accumulates at concentrations far exceeding the MIC needed to exert antimycotic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Imaging methods to determine the penetration of topical antifungal agents through mycotic nails can provide useful insights into whether drug distribution is sufficient to result in treatment success. |
We applied a quantitative method, termed matrix-assisted laser desorption ionization mass spectrometry imaging–Fourier transform ion cyclotron resonance (MALDI-FTICR) imaging, to describe the penetration profile of amorolfine within the nail compared with three other antifungal compounds (ciclopirox, naftifine, and tioconazole). |
What was learned from the study? |
Although amorolfine, ciclopirox, naftifine, and tioconazole had similar distribution profiles in the nail, these antifungals reached different multiples of their respective minimum inhibitory concentrations toward Trichophyton rubrum. |
MALDI-FTICR is a valid method to evaluate penetration of the nail plate by different antifungal compounds, and this technique will, therefore, assist in the development of new formulations to treat onychomycosis. |
Clinical studies are required to determine whether the effects observed ex vivo translate to differences in treatment success. |
Introduction
Onychomycosis is a fungal infection of the nails that can range in clinical presentation from a superficial infection of the upper layers of the nail plate to total dystrophic onychomycosis involving the entire nail [1, 2]. The condition can be difficult to treat topically, as the dense keratinous matrix in the upper layers of the nail plate acts as a barrier against the permeation of effective antifungal agents into the deeper layers of the nail.
Matrix-assisted laser desorption ionization–mass spectrometry imaging (MALDI-MSI) provides a label-free approach for identifying and mapping molecular localization [3], and it is widely used to visualize the penetration of pharmaceutical compounds in three-dimensional cell cultures [4, 5], as well as in tissue sections [6,7,8,9].
Our previous study used MALDI-MSI for the first time to visualize the penetration of antifungal compounds through nail tissue [10]. Qualitative differences were observed between the penetration profiles of three topical antifungal compounds, amorolfine, ciclopirox, and naftifine hydrochloride. Ciclopirox (8% nail lacquer) and naftifine (1% solution) showed highly localized distribution in the uppermost layer of the nail plate at 6 h following application, whereas amorolfine (5% nail lacquer) had diffused to deeper layers of the nail plate within this period and continued diffusing toward the deepest layer of the nail over the remainder of the 24-h observation.
In the present study, we applied MALDI–Fourier transform ion cyclotron resonance (MALDI-FTICR) imaging [11] to allow quantitative MALDI-MSI analysis of amorolfine penetration in mycotic toenails and to compare its penetration profile to that of ciclopirox, naftifine, and tioconazole.
Methods
Samples
The samples used in these studies were sourced and deidentified via Tissue Solutions Ltd., a subsidiary of BioIVT LLC which is accredited for the collection, storage, and commercial distribution of biospecimens by the Office for Human Research Protections (OHRP) of the United States Department of Health. The use of publicly available biospecimens from an accredited supplier is not considered to fall under research in human subjects, and as such, institutional review board (IRB) approval or exemption was not required. Prior to use, the toenails (n = 42), which had been frozen for storage, were thawed at room temperature for 10 min, immersed in 70% (v/v) ethanol for 1 min, and then washed twice by vortexing in deionized water. The four antifungal compounds tested were amorolfine (Loceryl® 5% lacquer), ciclopirox (Ciclopoli® 8% lacquer), naftifine (Exoderil® 1% solution), and tioconazole (Trosyd® 28% solution). Before treatment, the thickness of each toenail sample was measured with an Oditest caliper (Kroeplin GmbH, Schlüchtern, Germany). Five measurements were performed on different areas of each toenail and the mean of these measurements recorded.
Sample Treatment
For treatment, each toenail was laid on gauze moistened with sterile water in a petri dish. One antifungal was applied per toenail within a premarked zone of 1 cm2 on the nail surface at a dose of 10 µL/cm2. Nails were then allowed to air-dry for 30 min at 24 °C in a humid cell culture incubator, after which the surface of each toenail was cleaned with five cotton swabs wetted with acetone. The samples were then stored at −80 °C.
Sample Sectioning and Mounting
Nails stored at −80 °C were placed inside a cryostat maintained at −20 °C, and 10-µm nail sections were prepared for each sample. The midline cross sections of the toenails (region of interest) were mounted onto adhesive tape and placed on a MALDI plate. The mounted nail sections were then placed in a desiccator prior to matrix deposition to ensure the sections were dry. An optical image of the plate was acquired with a scanner to synchronize the positions of the nail sections with the laser target.
Preparation of Antifungal Dilution Series
A stock solution of 10 mM amorolfine was prepared in 100% (v/v) dimethyl sulfoxide (DMSO), and stock solutions of 10 mM ciclopirox, naftifine, and tioconazole were prepared in 100% (v/v) ethanol. A dilution series of each antifungal was then prepared by diluting the stock solutions in a 1:1 (v:v) solution of ethanol and water; 1.0 μL of each dilution was spotted directly onto adhesive tape and placed in a desiccator for 15 min before MALDI matrix deposition. A spot of pure solvent was included as a negative control. The spotted calibration series was later placed onto the slides near the sections for image calibration.
MALDI-FTICR Analysis
For MALDI-FTCIR analysis, 2,5-dihydroxybenzoic acid matrix (DHB; 40 mg/mL in 1:1 methanol/water + 1% trifluoroacetic acid) was sprayed over the toenail sections with an automatic sprayer system (TM-Sprayer; HTX Technologies, Chapel Hill, NC, USA). The nail sections were then analyzed with a 7 T MALDI-FTICR instrument (SolariX; Bruker, Billerica, MA, USA) in the continuous accumulation of selected ions (CASI) positive mode centered on the targeted compound m/z at a spatial resolution of either 70 μm to cover the entire nail section or 200 μm for the dilution series to generate the calibration curves. All MSI acquisitions were performed with data reduction set between 0.30 and 0.50 (this was kept consistent among images of nails treated with the same antifungal), and one image was acquired per nail.
For the kinetic study of amorolfine penetration, the distribution profile of amorolfine was assessed 3, 6, 9, and 24 h after treatment in eight nails (n = 2 at each time point), and one control sample was used as a reference. For comparison of the amorolfine, ciclopirox, naftifine, and tioconazole distribution profiles after 3 h, 32 nails (n = 8 per antifungal agent) were evaluated, and one control sample was used as a reference.
The control nails were used to evaluate the tissue extinction coefficients (TECs) [12] of the antifungals for normalization. For this purpose, each antifungal was sprayed at a concentration of 5 μM on top of the control nail sections and next to the nail sections (directly onto the adhesive tape). Signals detected in the nail sections were compared with the signals beside the nail sections to determine the TECs.
Data Analysis
The MALDI-FTICR data were analyzed with the flexImaging v.5.0 (Bruker), DataAnalysis v.5.0 (Bruker), and Multimaging v1.2 (Aliri, Loos, France) software packages. When evaluating antifungal distribution, the image intensity scale was adjusted to eliminate the background noise (by increasing the lower signal threshold) and enable optimal visualization (by decreasing the upper signal threshold). Convolution of the signal distributions was performed on the original images using a normalized uniform kernel that averaged the values around a position. The kernel size was manually optimized for the analysis to minimize background noise.
For absolute quantification of the antifungals within the nail sections, the MSI datasets obtained from the nail sections and dilution series were used. The TEC-based method in the Multimaging software was applied to these data to correct the signal in each region of interest and obtain the antifungal concentration in μg/g of tissue and the concentration in μM.
To quantitatively evaluate penetration of the antifungals through the nail samples, penetration profiles were generated for each treated nail section. The methodology was based on the following workflow: (1) the molecular distribution of the antifungal was first obtained by MSI as described above; (2) the region of interest for the penetration profile was selected (this was generally the entire region of acquisition, unless undesirable features present, such as contamination, poor histology, or folding of the sections, would significantly impact the results); (3) each pixel in the region of interest was re-aligned to define a common 0-μm position at the top of the nail section; (4) the mean concentration per raw pixel was calculated according to the quantitative MSI results and the depth was determined based on the spatial resolution for the acquisition (70 μm for each pixel); and (5) penetration profiles were then generated using the quantification and depth data.
Based on published data on the minimum inhibitory concentrations (MIC) of the four test compounds needed to inhibit 50% and 90% (MIC50 and MIC90) of Trichophyton rubrum, the fold differences between the MIC and the antifungal concentrations in the nails (termed the multiplicity of MIC) were calculated for each. The published MIC50 and MIC90 values (in µM) used for these calculations were 0.79 and 12.6 for amorolfine [13], 9.65 and 77.19 for ciclopirox [13], 0.39 and 12.35 for naftifine [13], and 1.29 and 2.58 for tioconazole [14], respectively.
Statistical Analysis
The Shapiro–Wilk test was used to check the assumption of normality of data within groups. The Kruskal–Wallis H test was used to test for statistically significant differences across groups. When testing for statistically significant differences between groups two by two, the Student t test was used where data were normally distributed, and the Mann–Whitney U test was used where data were not normally distributed. A p value of < 0.05 was considered statistically significant. Python v3.8 (Python Software Foundation, Beaverton, OR, USA) was used for all statistical analyses.
Results
Kinetic Study of Amorolfine Penetration
No significant differences were observed between the mean concentrations of amorolfine attained in the nail at 3, 6, or 9 h following treatment, indicating that the drug penetrates the tissue quickly (Fig. 1). At 24 h, the concentration of amorolfine in the nails was approximately twice the concentration at 3 h. Based on the greater signal homogeneity and reproducibility observed at 3 h following treatment, this time point was selected for subsequent comparisons of the penetration profiles of the four antifungal compounds.
Penetration of mycotic nails by amorolfine as detected by MALDI-FTICR. The mean intensity (± standard deviation) of amorolfine in the nail sections (n = 2) at various time points following treatment is indicated in arbitrary units (a.u.). MALDI-FTICR matrix-assisted laser desorption ionization–Fourier transform ion cyclotron resonance
Distribution and Penetration of Amorolfine, Ciclopirox, Naftifine, and Tioconazole
As shown by MALDI-FTICR, amorolfine, ciclopirox, and naftifine penetrated the entire section of the toenail from the top to the nail bed within 3 h of application (Fig. 2). The penetration profiles indicated a higher concentration of amorolfine and ciclopirox in the deep layers of the nails compared with naftifine and tioconazole. A nail showing amorolfine nail penetration is shown in Fig. 3. In contrast, tioconazole did not fully penetrate the nail, nor did it reach the nail bed within 3 h.
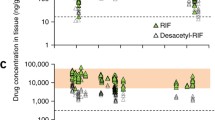
Mean concentrations of amorolfine, ciclopirox, naftifine, and tioconazole in mycotic human nail sections 3 h following treatment (n = 8 per group) as determined by MALDI-FTICR. a Full penetration profile of each antifungal agent across all concentrations detected in the nail. b Penetration profile for each antifungal agent, focused on concentrations of up to 1000 µM. MALDI-FTICR matrix-assisted laser desorption ionization–Fourier transform ion cyclotron resonance. *Complete penetration of the nail section observed
Not accounting for the different concentrations of the lacquers/solutions of each antifungal tested, the highest mean concentration within the toenail tissues at 3 h was observed for amorolfine, and the lowest mean concentration was observed for ciclopirox: amorolfine, 2.46 mM (equivalent to 780.4 μg/g of tissue; coefficient of variation [CV] = 49%; 5% lacquer applied); tioconazole, 1.36 mM (equivalent to 528.5 μg/g; CV = 143%; 28% solution applied); naftifine, 0.63 mM (equivalent to 204.2 μg/g; CV = 53%; 1% solution applied); and ciclopirox, 0.95 mM (equivalent to 197.1 μg/g; CV = 63%; 8% lacquer applied). The differences among the mean concentrations of the four compounds were found to be significant (p = 0.016). Pairwise comparisons indicated that the mean concentration of amorolfine was significantly higher than that of ciclopirox and naftifine (p = 0.010 vs ciclopirox; p = 0.004 vs naftifine), with no other significant differences observed.
Multiplicity of MIC90 of Amorolfine, Ciclopirox, Naftifine, and Tioconazole in the Nail Following Treatment
Relative to their MIC50 and MIC90 values, the mean concentration of each antifungal agent in the nail at 3 h following treatment was 3111- and 195-fold for amorolfine, 99- and 12-fold for ciclopirox, 1822- and 58-fold for naftifine, and 1057- and 528-fold for tioconazole, respectively, and the differences among the mean multiplicities of the four compounds were significant (MIC50 values, p = 0002; MIC90 values, p = 0.0001). Pairwise comparisons of the mean multiplicity of MIC90 found significant differences for amorolfine versus ciclopirox (p = 0.001) and naftifine (p = 0.005; Fig. 4). The median tissue exposure after 3 h to each antifungal relative to their MIC90 was 191-fold for amorolfine versus tenfold for ciclopirox, 52-fold for naftifine, and 208-fold for tioconazole (Fig. 4).
Nail tissue exposure 3 h after treatment with the topical antifungals amorolfine, ciclopirox, naftifine, or tioconazole (n = 8 sections each), based on the fold difference between the concentration of each antifungal reached in the nail and its MIC90. The difference in the multiplicity of MIC90 was also statistically significant for ciclopirox vs. naftifine (p = 0.004) and ciclopirox vs. tioconazole (p = 0.003); no other significant differences were found. For tioconazole, there was an outlier value of 2261 (not shown on graph), which was included in the calculation of the boxplot values illustrated here. Boxplots indicate the median (horizontal bars), interquartile range (25th to 75th percentile), and minimum and maximum (whiskers). MIC90, minimum inhibitory concentration needed to kill 90% (MIC90) of the fungal organism
Discussion
Many factors can influence the penetration and effective concentration of topical antifungals in mycotic nails, including the physicochemical properties of the drug (size, shape, charge, and hydrophobicity), nail properties (extent of disease, hydration, and thickness), and characteristics of the drug formulation (e.g., concentration, pH, vehicle used, and the use of chemical penetration enhancers) [1, 2]. For this reason, the quantification of antifungal distribution and penetration are key to understanding the performance of these agents. In this study, we evaluated the ex vivo toenail drug penetration of amorolfine, a broad-spectrum topical antimycotic that is typically applied once or twice weekly as a nail lacquer [15,16,17], as well as naftifine and ciclopirox, for which daily application is recommended [18, 19], and tioconazole, which is administered twice daily [20].These different compounds have been chosen as representatives of four classes of antifungal agents: morpholine, pyridine, allylamine and azole [21].
Various methods have been used to evaluate nail penetration by antifungals, including spectroscopic techniques such as photothermal beam deflection spectroscopy [22], laser scanning confocal microscopy [23, 24], and zonal inhibition of T. rubrum growth on agar plates by solubilized treated nails [25]. MALDI-FTICR has been widely used for quantitative MALDI-MSI analysis of drug distribution in various tissues [9] due to its high resolving power and mass accuracy [11]. To our knowledge, our study is the first report of MALDI-FTICR being applied to quantitative analysis in nail permeation studies.
Amorolfine was observed to reach the nail bed and achieve a fungicidal concentration within 3 h (first time point measured). The penetration profiles of the four antifungals indicated a higher concentration of amorolfine and ciclopirox in deep layers of the nail compared with naftifine and tioconazole. These findings are consistent with those of our previous, qualitative MALDI-MSI study, which showed ciclopirox (8% nail lacquer) and naftifine (1% solution) localized in the uppermost layer of the nail at 6 h following application, whereas amorolfine (5% nail lacquer) had penetrated the deeper layers of the nail within this time period [10]. Further, considering the ratio of the concentration of each compound to its MIC90, amorolfine achieved a similar multiple of this parameter compared with tioconazole and a higher multiple than that of ciclopirox and naftifine, indicating tissue exposure above pharmacologically active concentrations following treatment.
An early report on the penetration kinetics of topical amorolfine (5% nail lacquer) similarly demonstrated rapid penetration of human nails, with the concentration in the deeper layers of the nail being sufficient to inhibit T. rubrum growth within 24 h of application [26]. Further, a recent pilot ex vivo study showed that amorolfine (5% nail lacquer) penetrated human nails at a sufficient concentration to inhibit T. rubrum following a single topical application, whereas the comparators (ciclopirox 8% nail lacquer, naftifine 1% nail solution, and terbinafine 10% nail solution) did not [25]. Both studies evaluated efficacy based on the zones of inhibition surrounding solubilized nail samples on agar plates seeded with T. rubrum.
Employing a similar method to evaluate antimycotic activity, Ghannoum et al. also generated zonal inhibition reference curves for amorolfine, ciclopirox, naftifine, and terbinafine using a dilution series of each prepared in DMSO [25]. With amorolfine, there was an almost linear relationship between antifungal concentration and the size of the zone of inhibition. Amorolfine had the lowest concentration necessary to yield a zone of inhibition (0.095 µg/mL) compared with terbinafine (24.4 µg/mL), ciclopirox (156 µg/mL), and naftifine (625 µg/mL), and it produced the largest zone of inhibition of the four antifungals when each was applied undiluted. As a possible explanation for these findings, the authors of this pilot suggested that amorolfine may have higher potency against T. rubrum than the other three antifungals.
Our group previously found the DHB matrix (40 mg/mL in 1:1 methanol/water + 1% trifluoroacetic acid) to be suitable for the MALDI-based detection of amorolfine and ciclopirox (data not shown). For the detection of naftifine and tioconazole, two matrices were tested here: DHB in the positive ion mode and 1,5-diaminonaphthalene (10 mg/mL in acetonitrile/water 1:1 [v:v]) in the negative ion mode. The DHB matrix was found to be optimal (data not shown), and this matrix was, therefore, used for the analysis of all four antifungal agents.
One limitation of this study was the low number of replicates (n = 2) used for the kinetic analysis. Further, while every attempt was made to standardize the protocol, variation in the quantity of compound removed from the nail samples during the cleaning step may limit interpretation of the results. These data are ex vivo, and outcomes during clinical treatment will be impacted by additional factors. In addition, we acknowledge that not all parameters that may affect drug permeability were standardized; two of the formulations were solutions and the other two were lacquers. Further, amorolfine has lower affinity toward keratin than antifungals such as ciclopirox [27, 28]; however, since keratin-binding ratios were not available for all the agents tested in our study, we have not accommodated these differences here.
Conclusion
This is the first report to describe the use of MALDI-FTICR for the quantitative analysis of antifungal distribution in nails. Ex vivo, amorolfine achieved a higher fungicidal concentration in the nail than ciclopirox, naftifine, or tioconazole within 3 h of application, suggesting that this antifungal compound may offer greater antifungal efficacy. Further studies are required to evaluate the clinical application of amorolfine.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Baswan S, Kasting GB, Li SK, et al. Understanding the formidable nail barrier: a review of the nail microstructure, composition and diseases. Mycoses. 2017;60(5):284–95.
Murdan S. Drug delivery to the nail following topical application. Int J Pharm. 2002;236:1–26.
Porta Siegel T, Hamm G, Bunch J, Cappell J, Fletcher JS, Schwamborn K. Mass spectrometry imaging and integration with other imaging modalities for greater molecular understanding of biological tissues. Mol Imaging Biol. 2018;20(6):888–901.
LaBonia GJ, Lockwood SY, Heller AA, Spence DM, Hummon AB. Drug penetration and metabolism in 3-dimensional cell cultures treated in a 3D printed fluidic device: assessment of irinotecan via MALDI imaging mass spectrometry. Proteomics. 2016;16(11–12):1814–21.
Russo C, Lewis EEL, Flint L, Clench MR. Mass spectrometry imaging of 3D tissue models. Proteomics. 2018;18(14):1700462.
Reyzer ML, Chaurand P, Angel PM, Caprioli RM. Direct molecular analysis of whole-body animal tissue sections by MALDI imaging mass spectrometry. Methods Mol Biol. 2010;656:285–301.
Jove M, Spencer J, Clench M, Loadman PM, Twelves C. Precision pharmacology: mass spectrometry imaging and pharmacokinetic drug resistance. Crit Rev Oncol Hematol. 2019;141:153–62.
Granborg JR, Handler AM, Janfelt C. Mass spectrometry imaging in drug distribution and drug metabolism studies—principles, applications and perspectives. Trends Anal Chem. 2022;146: 116482.
Schulz S, Becker M, Groseclose MR, Schadt S, Hopf C. Advanced MALDI mass spectrometry imaging in pharmaceutical research and drug development. Curr Opin Biotechnol. 2019;55:51–9.
Pinto F, Bagger C, Kunze G, et al. Visualisation of penetration of topical antifungal drug substances through mycosis-infected nails by matrix-assisted laser desorption ionisation mass spectrometry imaging. Mycoses. 2020;63(8):869–75.
Bowman AP, Blakney GT, Hendrickson CL, Ellis SR, Heeren RMA, Smith DF. Ultra-high mass resolving power, mass accuracy, and dynamic range MALDI mass spectrometry imaging by 21-T FT-ICR MS. Anal Chem. 2020;92(4):3133–42.
Hamm G, Bonnel D, Legouffe R, et al. Quantitative mass spectrometry imaging of propranolol and olanzapine using tissue extinction calculation as normalization factor. J Proteomics. 2012;75(16):4952–61.
Adimi P, Hashemi SJ, Mahmoudi M, et al. In-vitro activity of 10 antifungal agents against 320 dermatophyte strains using microdilution method in Tehran, Iran. J Pharm Res. 2013;12(3):537–45.
Fachin A, Maffei C, Martinez-Rossi N. In vitro susceptibility of Trichophyton rubrum isolates to griseofulvin and tioconazole. Induction and isolation of a resistant mutant to both antimycotic drugs: mutant of Trichophyton rubrum resistant to griseofulvin and tioconazole. Mycopathologia. 1996;135(3):141–3.
Pittrof F, Gerhards J, Erni W, Klecak G. Loceryl nail lacquer–realization of a new galenical approach to onychomycosis therapy. Clin Exp Dermatol. 1992;17:26–8.
Reinel D, Clarke C. Comparative efficacy and safety of amorolfine nail lacquer 5% in onychomycosis, once-weekly versus twice-weekly. Clin Exp Dermatol. 1992;17:44–9.
Drugbank Online. Amorolfine 2021. Available from: https://go.drugbank.com/drugs/DB09056. Accessed 5 Mar 2024.
Food and Drug Administration. Penlac nail lacquer (ciclopirox) topical solution, 8%: summary of product characteristics. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21022s004lbl.pdf. Accessed 5 Mar 2024.
Food and Drug Administration. Naftin (naftifine hydrochloride) gel, for topical use: summary of product characteristics. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204286s004lbl.pdf. Accessed 5 Mar 2024.
Clissold SP, Heel RC. Tioconazole: a review of its antimicrobial activity and therapeutic use in superficial mycoses. Drugs. 1986;31:29–51.
Hay RJ. Antifungal drugs. In: Katsambas AD, Lotti TM, Dessinioti C, D’Erme AM, editors. European handbook of dermatological treatments. Cham: Springer International Publishing; 2023. p. 1543–54.
Gotter B, Faubel W, Neubert R. Photothermal imaging in 3D surface analysis of membrane drug delivery. Eur J Pharm Biopharm. 2010;74(1):26–32.
Dutet J, Delgado-Charro M. Assessment of iontophoretic and passive ungual penetration by laser scanning confocal microscopy. Pharm Res. 2012;29(12):3464–74.
Flores F, Chiu W, Beck R, da Silva C, Delgado-Charro M. Enhancement of tioconazole ungual delivery: combining nanocapsule formulation and nail portion approaches. Int J Pharm. 2018;535(1–2):237–44.
Ghannoum M, Long L, Kunze G, Sarkany M, Osman-Ponchet H. A pilot, layerwise, ex vivo evaluation of the antifungal efficacy of amorolfine 5% nail lacquer vs other topical antifungal nail formulations in healthy toenails. Mycoses. 2019;62(6):494–501.
Polak A. Kinetics of amorolfine in human nails. Mycoses. 1993;36(3–4):101–3.
Matsuda Y, Sugiura K, Hashimoto T, Ueda A, Konno Y, Tatsumi Y. Efficacy coefficients determined using nail permeability and antifungal activity in keratin-containing media are useful for predicting clinical efficacies of topical drugs for onychomycosis. PLoS One. 2016;11(7): e0159661.
Plaum S, Verma A, Fleischer ABJ, Olayinka B, Hardas B. Detection and relevance of naftifine hydrochloride in the stratum corneum up to four weeks following the last application of naftifine cream and gel, 2%. J Drugs Dermatol. 2013;12(9):1004–8.
Acknowledgements
Medical writing and editorial support for the preparation of this manuscript, under the guidance of the authors, was provided by Natasha Beeton-Kempen, PhD, of ApotheCom, UK, and was funded by Galderma SA, Lausanne, Switzerland, in accordance with Good Publication Practice (GPP) standards (Ann Intern Med 2022;175:1298–1304).
Authorship.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study and the journal’s Rapid Service Fee were sponsored by Galderma SA, Lausanne, Switzerland.
Author information
Authors and Affiliations
Contributions
Nicolas Joly-Tonetti was responsible for and involved in the study conception and design. Raphael Legouffe, Aurore Tomezyk, Clémence Gumez, Mathieu Gaudin, and David Bonnel were responsible for the acquisition of the cadaver samples and data. All authors were involved in the data analysis and interpretation. All authors also reviewed the manuscript and approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of Interest
Nicolas Joly-Tonetti is an employee of Galderma SA, Lausanne, Switzerland. Clémence Gumez, Aurore Tomezyk, Raphael Legouffe, Mathieu Gaudin, and David Bonnel are employees of Aliri (formerly ImaBiotech), Loos, France. Martin Schaller is an advisory board member for Galderma and Marpinion; a speaker for AbbVie, Bayer Healthcare, Galderma, and La Roche-Posay; a recipient of research grants from Galderma and Bayer HealthCare; and an investigator for GSK and Galderma.
Ethical Approval
The samples used in these studies were sourced and deidentified via Tissue Solutions Ltd., a subsidiary of BioIVT LLC which is accredited for the collection, storage, and commercial distribution of biospecimens by the Office for Human Research Protections (OHRP) of the United States Department of Health. The use of publicly available biospecimens from an accredited supplier is not considered to fall under research in human subjects, and as such, IRB approval or exemption was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior presentation: Part of the data in this manuscript were presented at the 31st European Academy of Dermatology and Venereology (EADV) Congress in Milan, Italy, on September 7–10, 2022.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Joly-Tonetti, N., Legouffe, R., Tomezyk, A. et al. Penetration Profiles of Four Topical Antifungals in Mycotic Human Toenails Quantified by Matrix-Assisted Laser Desorption Ionization–Fourier Transform Ion Cyclotron Resonance Imaging. Infect Dis Ther 13, 1269–1279 (2024). https://doi.org/10.1007/s40121-024-00978-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00978-3