Abstract
Introduction
The delivery of COVID-19 vaccines was successful in reducing hospitalizations and mortality. However, emergence of the Omicron variant resulted in increased virus transmissibility. Consequently, booster vaccination programs were initiated to decrease the risk of severe disease and death among vulnerable members of the population. This study aimed to estimate the effects of the booster program and alternative vaccination strategies on morbidity and mortality due to COVID-19 in the UK.
Method
A Susceptible-Exposed-Infectious-Recovered (SEIR) model was used to assess the impact of several vaccination strategies on severe outcomes associated with COVID-19, including hospitalizations, mortality, National Health Service (NHS) capacity quantified by hospital general ward and intensive care unit (ICU) bed days, and patient productivity. The model accounted for age-, risk- and immunity-based stratification of the UK population. Outcomes were evaluated over a 48-week time horizon from September 2022 to August 2023 considering the actual UK autumn 2022/spring 2023 booster campaigns and six counterfactual strategies.
Results
The model estimated that the autumn 2022/spring 2023 booster campaign resulted in a reduction of 18,921 hospitalizations and 1463 deaths, compared with a no booster scenario. Utilization of hospital bed days due to COVID-19 decreased after the autumn 2022/spring 2023 booster campaign. Expanding the booster eligibility criteria and improving uptake improved all outcomes, including averting twice as many ICU admissions, preventing more than 20% additional deaths, and a sevenfold reduction in long COVID, compared with the autumn 2022/spring 2023 booster campaign. The number of productive days lost was reduced by fivefold indicating that vaccinating a wider population has a beneficial impact on the morbidities associated with COVID-19.
Conclusion
Our modelling demonstrates that the autumn 2022/spring 2023 booster campaign reduced COVID-19-associated morbidity and mortality. Booster campaigns with alternative eligibility criteria warrant consideration in the UK, given their potential to further reduce morbidity and mortality as future variants emerge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study |
COVID-19 booster vaccination programs have been successful in reducing hospitalization and deaths associated with COVID-19 during the Delta and Omicron periods. |
Many modelling analyses do not consider the full complexity of COVID-19 transmission dynamics, such as booster vaccinations, vaccine waning and hybrid immunity. |
This study used the most recently available data to assess the impact of the autumn 2022 and spring 2023 booster vaccinations on public health outcomes including hospitalizations, NHS occupancy (a key determinant of bed capacity), patient productivity, and mortality across several scenarios. |
What was learned from this study |
The model estimated that the autumn 2022 and spring 2023 vaccination campaign caused a 14% and 16% reduction in general ward and ICU admissions respectively, when compared with a no booster scenario. Similar reductions were observed in general ward and ICU bed days (14% and 16% reduction respectively). Patient productivity was partially restored with the number of paid and unpaid productive days lost reduced by 3% and 7%. Overall mortality decreased by 15%. |
Further benefits in health and productivity outcomes were observed in additional scenarios with increased eligibility and uptake, with greatest clinical benefit predicted when coverage was highest in both autumn and spring booster campaigns with a 64% further reduction in hospitalizations and approximately twice as many ICU admissions prevented. |
Expanding the eligibility for the COVID-19 booster vaccine programs has the potential to substantially reduce mortality, reduce general ward and ICU bed days and further alleviate the healthcare burden due to COVID-19. |
Introduction
Current evidence suggests that the rapid development and implementation of the COVID-19 vaccination program in the UK in December 2020 achieved a substantial reduction in the number of clinically significant cases [1], the number of serious complications such as long COVID [2], hospitalizations associated with the disease [3], and ultimately COVID-19 mortality rates [3]. Primary vaccine courses were followed by booster campaigns against the Delta variant in 2021/2022 [4]. The Omicron variant emerged in late 2021, and was associated with higher rates of transmission, but less severe outcomes [5]. However, vaccines were less effective against the Omicron variant and vaccine effectiveness (VE) was observed to wane more rapidly [6, 7]. The VE against symptomatic disease from the Omicron variant was reported to drop to 8.8% after 25 weeks post-immunization whereas the VE against the Delta variant at the same timepoint was reported at 43.5% [7].
An updated bivalent vaccine booster program was initiated in autumn 2022 [8,9,10], and continued in spring 2023 [11] targeting vulnerable individuals at risk of developing severe COVID-19 or those in contact with individuals at risk of developing severe COVID-19, such as frontline health or social care workers. The emergence of the Omicron lineage of SARS-CoV-2 was hallmarked by an increase in infection rates, partly due to the variant’s ability to evade pre-existing immunity mechanisms [12, 13]. As the disease changes to a more endemic state, analyses accounting for the role of hybrid immunity from previous COVID-19 infections and vaccinations, vaccine waning and differences in vaccine efficacy against new variants are needed to help inform public health strategies for controlling SARS-CoV-2.
The aim of this study was to quantify the public health impact of the autumn 2022 and spring 2023 booster vaccination program in the UK over a 48-week time horizon, from September 2022 to August 2023, and to explore the impact of alternative strategies with extended eligibility and/or improved uptake. Exploring the impact of expanding the eligibility criteria or increasing booster vaccine uptake rates can help inform the design of future booster campaigns. This study can provide valuable insight into the benefits of vaccination programs in both the current eligible population and broader eligibility populations.
Methods
Overview
A previously described age- and risk-structured deterministic compartmental Susceptible-Exposed-Infectious-Recovered (SEIR)-type model [14, 15] was adapted and extended to evaluate the public health impact of six strategies for the autumn 2022/spring 2023 booster vaccination campaign in the UK over a 48-week time horizon as presented in Table 1.
Model Overview
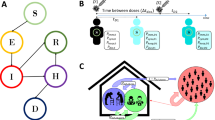
The SEIR model of SARS-CoV-2 transmission [14] was expanded using R® [16] to include general hospital ward and intensive care unit (ICU) compartments, and COVID-19 deaths within the dynamic transmission system (Fig. 1).
Model schematic. A SEIRD compartmental model was developed where subscript k = vaccination status; a = stratification by age. Parameters in blue indicate fixed/calculated parameters and those in red show calibrated parameters. S: susceptible; Vk=0,1,2,3,4: susceptible, vaccinated; Ea,k: exposed; Iaa,k: infected asymptomatic; Isa,k: infected symptomatic; Ta,k: treatment; Ha,k: hospital general ward; ICa,k: intensive care unit; Ra,k: recovered; Da: covid death. Hybrid immunity (HI1–4) replaces Ra,k in Vk=0
The UK population was stratified into age groups in line with contemporaneous vaccine recommendations from the Joint Committee on Vaccination and Immunisation (JCVI; Supplementary Table 12) [9], as well as two currently ineligible pediatric age compartments; ≤ 0.5 years and 0.5–4 years. Each age group was further stratified into high- and low-risk cohorts with clinical risk identified by the UK Health and Security Agency (UKHSA) and defined as those at heightened risk of experiencing severe illness—a considerable 24.4% of the UK population [17]. The population was also stratified by vaccination class to include unvaccinated, protected by vaccination with one dose, two doses or a booster dose and waned from booster protection. Booster doses and waned booster populations were further compartmentalized into pre-autumn 2022 boosters and autumn 2022/spring 2023 boosters.
A hybrid immunity (HI) compartment was included for each vaccination strata, containing vaccinated individuals who have recovered from prior infection, or previously infected individuals who have since been vaccinated. Notably, it was assumed that the combined effect of infection- and vaccine-derived immunity for individuals in the HI compartment was greater against infection, but was the same against hospitalization as protection from vaccine-induced immunity alone [18]. The starting population in the HI compartment for Vk=1–Vk=3 (Fig. 1) was 10.5%. The proportion of hybrid immunity per age group is provided in Supplementary Table 14. The waning immunity rate from HI was assumed to be the same as for individuals with only infection- or vaccine-induced immunity.
Model Inputs
Baseline inter-age contact rates in the population were calculated from contact matrices reported in the POLYMOD study [19]. Baseline age-specific probabilities of hospitalization from infection, ICU admission from infection and death from severe infection requiring hospitalization (Supplementary Table 5) were sourced from an England-based analysis conducted at the beginning of the pandemic [20], and assumed these were representative of the UK. Age-stratified severity scaling factors were calibrated to account for changes in severity between clinical metrics from the beginning of the pandemic and those relevant to the study period during Omicron dominance (Supplementary Table 6). VE against infection, symptomatic disease, hospitalization, ICU admission and death for each vaccination status and age group are taken from UKHSA data (Supplementary Table 1), assuming 100% Omicron variant distribution.
The model assumed 12-week cycles for vaccination campaigns. In the model, the population in the compartments other than symptomatic infected, inpatient, and death were eligible for booster vaccinations following the criteria of each campaign. For factual strategies, weekly vaccination uptake rates were estimated from observed uptake rates from UKHSA data (Supplementary Table 2). Uptake rates for high-risk individuals, including immunosuppressed patients, outside of the eligible age groups were derived from the Department for Health and Social Care (DHSC) autumn booster impact assessment [21]. Age-stratified uptake rates were obtained from the UKHSA reports (Supplementary Table 2). Assumptions and sources for counterfactual strategies are presented in Supplementary Table 3.
The number of long COVID cases was estimated using two conditional probabilities: the probability of experiencing persistent symptoms 4–12 weeks after the initial onset of infection, termed ‘ongoing COVID symptomatic’ and the probability of experiencing symptoms 12 weeks or more after the initial onset of infection, termed ‘post COVID syndrome’ (Supplementary Table 7). In both cases, the conditional probabilities were stratified by age (< 18 or ≥ 18) and by the level of care received, either receiving outpatient care, receiving inpatient care in the general ward or receiving inpatient care in the ICU [22].
The input parameters were estimated under uncertainty and the sensitivity was analysed via a deterministic sensitivity analysis (DSA). The model outputs are subjected thus to parameter, methodological, and structural uncertainty.
Model Analysis and Outputs
The public health impact of various alternative vaccination strategies was quantified on the basis of the changes in the following outputs:
-
Number of infections, calculated on the basis of the relative incidence of infection to both asymptomatic and symptomatic compartments.
-
General ward and ICU admissions, calculated from the compartmental occupancy of hospitalisation (H) and ICU (IC).
-
Corresponding general ward and ICU bed days, using the average number of days of hospital and ICU stay per admission.
-
Long COVID cases, which were calculated on the basis of the number of symptomatic infections, inpatient cases, and probabilities of long COVID obtained from literature. Double counting was mitigated by accounting for outpatient and inpatient cases separately.
-
The associated number of productive days lost as a result of hospitalization, for those in outpatient care, and as a result of fatal COVID infection. The hospitalization length of stay was assumed to directly correlate with reduced patient productivity. Patient productivity loss was conservatively calculated on the basis of ONS estimates [23, 24] and published estimates [25, 26] of paid and unpaid productive time. Additionally, ONS estimates were used to calculate premature deaths associated with COVID infection [27].
-
The number needed to vaccinate (NNV) to avoid one hospitalization (NNVh), one ICU admission (NNVi), and one death (NNVd) were also estimated by dividing the number of additional vaccinations required by the predicted number of averted hospitalizations or deaths over the prediction period.
The impact of each vaccination strategy was considered through the incremental difference of these outcomes compared to the counterfactual no booster strategy.
To explore whether the booster campaign could be optimized, several different strategies were considered, as presented in Table 1. These strategies considered the benefit of the current campaign compared to a no booster strategy, or a strategy where only one booster is administered per year. They then expand on the current strategy to consider the impact of increasing the current eligibility criteria and how increasing the uptake of the booster would affect hospitalizations, deaths, and wider societal benefits, such as reduced productivity loss. Improved uptake was defined by the maximum observed uptake rate among all in eligible age groups in the corresponding campaign. By modelling these strategies with a broader population or greater uptake, it is hoped that policy makers can identify areas in the current strategy that could be modified to ensure that future booster campaigns are as beneficial as possible.
Sensitivity Analysis
Sensitivity analyses were carried out to determine how the range of key parameters within the model impact key outcomes. Uncertainty around the clinical parameters such as relative infectiousness of symptomatic versus asymptomatic cases, duration of immunity (infection, vaccine, and hybrid), duration of vaccine protection, risk ratios, and length of stay for hospitalization and ICU were tested via DSA (Supplementary Table 8). Parameter ranges for sensitivity analysis were sourced from the literature (Supplementary Table 8).
Calibration
The model was calibrated over a 36-week period from January to August 2022 against three calibration targets: PCR-positivity estimates [28], hospital admissions [29], and COVID-19 mortality [30]. A simultaneous, non-trivial parallel flow approach was taken to estimate PCR positivity from modelled infections, per existing literature [31]. A full description of this approach is outlined in the Supplementary Material (Parallel flow). All three calibration targets were scaled, using the L-BFGS-B method in optim function in R® [32], and equally weighted to minimize the negative log-likelihood function or equivalently, maximize the log-likelihood of recovering input data. Further description of model calibration and results are presented in the Supplementary Material (Model calibration).
Statement of Ethics Compliance
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Base Case
The impact of vaccination during the autumn 2022/spring 2023 booster period, hereafter referred to as autumn/spring, was modelled for several health and societal outcomes. Outcomes were compared against a counterfactual scenario where no booster was administered during autumn/spring, hereafter referred to as ‘no booster.’ When compared to a counterfactual no booster scenario, the model estimated that hospitalizations, categorized by general ward and ICU admissions, decreased by 17,785 (14% reduction; NNVh 1357), and 1136 (16% reduction; NNVi 21,247; Tables 2 and 4), respectively. General ward and ICU bed days (i.e. occupancy) were decreased by 237,253 (14%) and 16,184 (16%), respectively, and the number of paid and unpaid productive days lost decreased by 3.3 million (3%) and 0.5 million (7%), respectively (Table 3). An estimated 1463 (15%; NNVd 16,492) deaths were averted in the autumn/spring booster strategy compared with no booster strategy.
Given the considerable benefit of the autumn/spring booster on clinical outcomes, several strategies were considered where the eligibility and uptake of the vaccine were increased to determine how changing these factors could further enhance the benefit of the booster. The absolute results for each scenario are presented in Supplementary Tables 15 and 16.
Mortality
Those at highest risk of the most severe outcomes, including death, were vaccinated under the current eligibility criteria. When counterfactual scenarios were modelled whereby the eligibility of the booster campaign was expanded to include all adults and children over the age of 12, or vaccine uptake was increased to the highest uptake observed in any eligible age group during the actual autumn 2022/spring 2023 campaign (Supplementary Table 2) additional benefits in deaths averted were observed. The strategy that provided the greatest protection from mortality was expanding eligibility criteria of autumn and spring with improved uptake levels. In this strategy, the booster campaign was estimated to avoid 1877 (19%; NNVd 36,261) deaths compared to the no booster strategy (Tables 2 and 4).
Figure 2 demonstrates additional insights into the patterns of COVID-19 deaths associated with the different vaccination strategies. All booster strategies reduced the number of deaths compared with a no booster strategy. Notably, the smallest reduction in deaths averted occurred where the booster was given in autumn 2022 only with 990 deaths averted (10% reduction; NNVd 20,047; Tables 2 and 4), compared with a no booster strategy, demonstrating that a biannual campaign is required for prevention of deaths due to vaccine waning.
Number of COVID-19 deaths averted against time for the whole population for each strategy. Strategy I: factual autumn 22/spring 23 booster campaign. Strategy II: autumn 22 booster only. Strategy III: autumn 2022/spring 2023 with improved uptake. Strategy IV: expanded autumn and expanded spring. Strategy V: expanded autumn and expanded spring with improved uptake. Strategy II had increased infections and thus deaths within the first 24 weeks of the autumn booster, due to a higher number of individuals in the susceptible and waned booster compartments
Hospitalizations
When compared with the no booster scenario, increasing uptake rates for the autumn and spring campaigns with eligibility remaining the same led to an estimated reduction in general ward and ICU admissions by 18,013 (15%; NNVh 1428) and 1322 (18%; NNVi 20,877; Tables 2 and 4), respectively. The number of general ward admissions averted increased to 23,894 (20%; NNVh 1960; Fig. 3) with ICU admissions averted increased to 1840 (25%; NNVi 27,423; Fig. 4) when eligibility was expanded for both the autumn and spring campaigns, and uptake rates remaining the same. The greatest benefit was observed when eligibility was expanded and uptake rates increased in both autumn and spring campaigns to prevent 27,371 (23%; NNVh 2299) general ward and 2236 (31%; NNVi 30,441) ICU admissions compared with the no booster campaign. When considering hospital bed days, expanding the eligibility criteria and increasing uptake of both the autumn and spring campaigns had the greatest impact, reducing time spent in the general ward by 390,030 days (23%) and reducing time spent in the ICU by 31,867 days (31%).
Number of general ward admissions averted against time for the whole population for each strategy. Strategy I: factual autumn 22/spring 23 booster campaign. Strategy II: autumn 22 booster only. Strategy III: autumn 2022/spring 2023 with improved uptake. Strategy IV: expanded autumn and expanded spring. Strategy V: expanded autumn and expanded spring with improved uptake
Number of ICU admissions averted against time for the whole population for each strategy. Strategy I: factual autumn 22/spring 23 booster campaign. Strategy II: autumn 22 booster only. Strategy III: autumn 2022/spring 2023 with improved uptake. Strategy IV: expanded autumn and expanded spring. Strategy V: expanded autumn and expanded spring with improved uptake
Conversely, when comparing the no booster strategy to the autumn only strategy, reductions in general ward admissions of 11,501 (10%; NNVh 1598) and ICU admissions of 919 (13%; NNVi 21,596) were observed (Tables 2 and 4), substantially less than the factual observed campaign of autumn 2022/spring 2023, demonstrating that a biannual campaign is required for a reduction in clinical outcomes.
Long COVID
The autumn/spring booster campaigns were estimated to prevent 22,293 (2%) long COVID cases (Table 2). However, improved uptake levels in autumn and spring had the potential to prevent 28,838 (3%) cases compared to no booster strategy, which increased to 103,701 (11%) when the eligibility was expanded in the autumn and the spring, but uptake remained at the observed rates. The greatest reduction in long COVID cases was observed when the eligibility of both the autumn and spring strategies was expanded to include all adults and children over the age of 12, and uptake rates were increased, which was estimated to prevent 152,243 (16%) long COVID cases compared to a no booster strategy.
Productivity
When the wider impact of vaccination on individual productivity was considered, increasing the eligibility and/or increasing the vaccine uptake rates of the COVID-19 booster had the potential to substantially reduce productivity loss. This was observed in the strategy considering increased uptake rates for the autumn and spring booster, where productivity loss was estimated to decrease by over 4.9 million (5%) days averted (Table 3). This benefit was increased when eligibility was increased in the autumn and spring booster campaigns, which averted 13.7 (13%) million productive days lost compared to a no booster strategy. The greatest decrease in productivity loss was observed in the strategy considering expanded eligibility for both the autumn and spring boosters with improved uptake which averted 19.6 million (18%) productive days being lost. Similar trends were observed in productivity loss averted associated with deaths due to COVID infection. Expanding eligibility criteria in autumn and spring boosters would have resulted in 4.2 million (20%) productive days lost averted when compared with a no booster strategy. A further breakdown of averted paid and unpaid productive days lost is described in Table 3. This reduction in productivity lost could have substantial impact on the economic and societal burden of COVID-19.
Deterministic Sensitivity Analysis
One-way sensitivity of model outputs to certain parameters were selected for DSA, as shown in Fig. 5. The relative infectiousness of symptomatic and asymptomatic cases had the greatest influence across all clinical outcomes, followed by the mean duration of recovery for symptomatic and asymptomatic infection and risk ratios for hospitalization, ICU, and deaths. When the relative infectiousness of symptomatic versus asymptomatic cases was varied by 2.1–7.2 times the base case value, the number of averted hospitalizations ranged from 2.2 in the upper limit and 0.6 in the lower limit. Similar results were observed for averted ICU admissions and deaths which were increased by 2.1-fold and reduced by 0.6-fold of the base case results (Fig. 5).
Discussion
Given the evolving landscape of COVID-19 in the UK and globally, where new variants will continue to emerge and the profile of population immunity will shift over time, it remains crucial to account for the role of hybrid immunity and consider the benefit of adapting future vaccination campaigns. Since the emergence of SARS-CoV-2, studies have used mathematical modelling to investigate population dynamics and the effectiveness of public health interventions to control the COVID-19 pandemic [33,34,35,36,37]. While the effect of vaccination on COVID-19 transmission has been modelled in the UK before [14, 15], this study builds on the dynamics of the disease and vaccination strategy by additionally considering hybrid immunity to COVID-19. A growing body of evidence strongly suggests that hybrid population immunity profiles, acquired from both prior infection and vaccination, are potentially more protective against surges in COVID-19 infections and healthcare demand [38,39,40,41].
This study provides novel insights into the impact of the autumn 2022 booster campaign in the UK, explicitly accounting for the role of hybrid immunity in the population. Beyond the impact on averting infections and hospital admissions, this study quantified the impact of boosters in averting long COVID and patient productivity—two outcomes that are considered to have serious clinical and societal implications [42, 43]. The model assessed the impact of vaccination across several health outcomes for six vaccination strategies, compared to a counterfactual no booster campaign. Under conservative assumptions, the most beneficial results across all health outcomes were observed when coverage was expanded to all adults and children aged 12 and over, during both the autumn 2022 and spring 2023 campaigns with improved vaccine uptake rates observed during the campaigns. Enhanced uptake may be achieved by public health information campaigns driven by government and public health authorities [44].
Previous studies indicate booster vaccination has been consistently advantageous, with an estimated 100,000 hospitalizations and 23,000 deaths due to COVID-19 averted in England between October 2021 and December 2022 when compared with a counterfactual no booster scenario [45]. This study looked at a different time horizon, focusing on the current landscape of the COVID-19 pandemic dominated by the Omicron lineage of subvariants. Published evidence has shown that initial doses of the vaccine provided high levels of protection from severe disease, which is now diminished via waning and immune escape of the Omicron variant. Administration of a booster vaccine developed against the Omicron variant versus the ancestral vaccine designed for previous strains has shown that booster vaccines targeting specific and current circulating variants result in substantially more reductions in the number of severe outcomes associated with disease, demonstrating the long-term applicability of COVID booster vaccines for public health [46].
Current JCVI eligibility criteria recommend booster vaccinations for those at highest risk of developing severe COVID-19 or those with a higher risk of contracting and transmitting SARS-CoV-2 through occupational exposure, such as healthcare workers [47]. Expanding vaccine eligibility criteria to all adults and children over the age of 12 may indirectly protect those of advanced age and at risk of severe complications from COVID-19, which aligns with previous studies [48, 49]. Therefore, our study supports a growing argument that expanding the eligibility for COVID-19 booster vaccination would provide direct and indirect protection against serious COVID-19-related outcomes in the most vulnerable members of society, as well as the wider population [50].
COVID-19 is associated with reduced productivity [51, 52], which has widespread effects such as workplace absenteeism or inability to care for others. A recent study demonstrated that vaccinated individuals with COVID-19 had lower absenteeism rates compared to unvaccinated individuals (45.6% versus 65.0%) [51]. By increasing eligibility criteria to include standard risk adults for the autumn and spring booster campaigns, this study predicts a substantial reduction in productivity loss associated with COVID-19, which could result in significant economic benefits.
Further benefits of the booster campaign on long COVID have been modelled here. Long COVID has a considerable detriment to productivity and has a significant societal burden with 3.6% of individuals in the UK self-reporting as experiencing long COVID. Over half (57.0%) of those individuals reported that the condition negatively affected their well-being, and one-third reported that long COVID impacted their work [53]. Long COVID has a substantial clinical burden with an increased cost of £23.4 million attributed to primary care consultations per year in the UK [54]. Self-reported long COVID has been described to affect people across all age groups, with women most susceptible to the condition [53, 55]. Further, there is a 25% lower risk of long COVID in those aged > 70 years, and 6.0% lower risk in those aged 30–39 compared with those aged 18–30, indicating that long COVID may disproportionately affect younger generations [56]. In a study of 672 individuals with long COVID, defined as experiencing symptoms 12 months post-infection, a reduction in work ability scores was reported compared with those without long COVID symptoms [57]. Here, expansion of the eligibility criteria to include the wider population and increasing uptake rates for the 2022 autumn and 2023 spring booster campaign was estimated to substantially offset long COVID cases and could further restore productivity. Further analyses are required to examine this full productivity gain and the wider societal benefits associated with booster vaccination.
A reduction in hospital bed days by the administration of the autumn and spring booster demonstrates the potential benefit that vaccination may have on healthcare resources and bed capacity. Even small changes in occupancy translate to large differences in real-world hospital settings, particularly in the post-pandemic era. The NHS has experienced overwhelming pressures since the COVID-19 pandemic [58] and measures to alleviate such pressures, particularly during a winter season, could have substantial benefits. The potential to save 18,921 admissions over winter may have enhanced value, given the significant bed capacity limits of NHS during this time [59,60,61,62,63]. Vaccination reduces severe consequences of COVID-19 that result in hospitalization and mortality [64], and so, vaccination strategies that directly and indirectly protect those at risk may have substantial benefits for the NHS.
Our NNV estimates are aligned with those estimated by the UKHSA for the autumn 2023 COVID-19 booster campaign [65]. Our analysis shows that increasing the eligibility of the COVID-19 booster campaign has the potential to reduce the NNV to avoid one symptomatic case from 64 to 34, demonstrating that the impact of the booster campaign could be improved by widening the current eligibility criteria.
The model presented here accounts for real world dynamics and complexities of disease transmission. As with any modelling analysis, there are some limitations. Firstly, it should be noted that the model does not account for the various sublineages of the Omicron strain and variants that may influence transmission and hospitalizations. This is reflected using the transmission parameters obtained during the calibration period for predictions. As the model was calibrated to data from the 9 months prior to the prediction period, projections over time may be impacted as fewer people have severe reactions to COVID-19 owing to the combined effect of vaccine and prior infection, and a higher number of people would have been infected with different subvariants of Omicron with different risk profiles and waned protection over time, which may not be reflected in the calibrated parameters. The observed hospital admissions (until March 2023) and deaths (until July 2023) were 133,623 and 13,144 respectively [66, 67]. Using the point estimates of parameters and conservative assumptions for VE, the model underpredicts both hospitalizations (109,460) and deaths (8344). Further, any disparities in disease transmission due to differences in the microenvironment, such as hospital or community care, are not explicitly modelled.
There is no specific vaccine considered in the model, nor are the differences between vaccines investigated between outcomes. Productivity gains due to prevention of long COVID by vaccination have not been accounted for in this model; therefore, the societal benefits of the booster campaign are underestimated. We have not modelled adverse events because of the rarity of severe adverse events after vaccination [68, 69]. Adverse events after COVID-19 vaccination are mostly mild and their risk is largely outweighed by the benefit of vaccination in reducing the risk of severe disease [68, 69]. Guidance from the NHS supports seasonal vaccination to protect against severe COVID-19 [70] with the Medicines and Healthcare products Regulatory Agency (MHRA) continually reviewing suspected adverse events associated with vaccination via the Yellow Card reporting scheme. Reviews following the autumn 2022 booster campaign did not acknowledge any new safety concerns associated with vaccination [71]. A full description of model limitations is provided in the Supplementary Material.
Conclusion
This study estimates the impact of the autumn 2022/spring 2023 booster campaigns on COVID-19 morbidity and mortality and demonstrates the considerable public health benefit the booster campaigns had on healthcare and societal outcomes by reducing pressures on NHS bed occupancy and restoring patient productivity. Additional hypothetical vaccination strategies such as expanding the eligibility criteria and/or promoting an increase in vaccine uptake would considerably boost protection against severe outcomes, resulting in substantial social, economic and public health benefits.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Singanayagam A, Hakki S, Dunning J, et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2022;22(2):183–95.
Notarte KI, Catahay JA, Velasco JV, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. eClinicalMedicine. 2022;53:101624.
Public Health England. COVID-19 vaccine surveillance report published. 2021. https://www.gov.uk/government/news/covid-19-vaccine-surveillance-report-published. Accessed 05 Sep 2023.
GOV.UK. JCVI statement regarding a COVID-19 booster vaccine programme for winter 2021 to 2022. 2021. https://www.gov.uk/government/publications/jcvi-statement-september-2021-covid-19-booster-vaccine-programme-for-winter-2021-to-2022/jcvi-statement-regarding-a-covid-19-booster-vaccine-programme-for-winter-2021-to-2022. Accessed 28 June 2023.
Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–12.
Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–44.
Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–46.
GOV.UK. COVID-19 vaccines for autumn 2022: JCVI advice, 15 August 2022 (updated 3 September 2022). 2022. https://www.gov.uk/government/publications/covid-19-vaccines-for-autumn-2022-jcvi-advice-15-august-2022. Accessed 04 Jan 2023.
GOV.UK. JCVI statement on the COVID-19 vaccination programme for 2023: 8 Novemeber 2022. 2023. https://www.gov.uk/government/publications/covid-19-vaccination-programme-for-2023-jcvi-interim-advice-8-november-2022/jcvi-statement-on-the-covid-19-vaccination-programme-for-2023-8-november-2022. Accessed 28 June 2023.
GOV.UK. JCVI updated statement on the COVID-19 vaccination programme for autumn 2022. 2022. https://www.gov.uk/government/publications/jcvi-updated-statement-on-the-covid-19-vaccination-programme-for-autumn-2022. Accessed 28 June 2023.
GOV.UK. A guide to the COVID-19 spring booster 2023. 2023. https://www.gov.uk/government/publications/covid-19-vaccination-spring-booster-resources/a-guide-to-the-covid-19-spring-booster-2023. Accessed 08 Nov 2023.
Suzuki R, Yamasoba D, Kimura I, et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature. 2022;603(7902):700–5.
Meng B, Abdullahi A, Ferreira IATM, et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature. 2022;603(7902):706–14.
Mendes D, Chapman R, Aruffo E, et al. Public health impact of UK COVID-19 booster vaccination programs during Omicron predominance. Expert Rev Vaccines. 2023;22(1):90–103.
Mendes D, Chapman R, Gal P, et al. Public health impact of booster vaccination against COVID-19 in the UK during Delta variant dominance in autumn 2021. J Med Econ. 2022;25(1):1039–50.
The R Project for Statistical Computing. https://www.r-project.org/. Accessed 25 July 2023.
Walker JL, Grint DJ, Strongman H, et al. UK prevalence of underlying conditions which increase the risk of severe COVID-19 disease: a point prevalence study using electronic health records. BMC Public Health. 2021;21(1):484.
Bobrovitz N, Ware H, Ma X, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023;23(5):556–67.
Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5(3): e74.
Knock ES, Whittles LK, Lees JA, et al. Key epidemiological drivers and impact of interventions in the 2020 SARS-CoV-2 epidemic in England. Sci Transl Med. 2021;13(602):eabg4262.
Department of Health and Social Care. COVID-19 autumn 2023 booster programme impact assessment. June 2023. Report No. 9604. https://assets.publishing.service.gov.uk/media/650ade0f52e73c001254dc08/covid-19-autumn-2023-impact-assessment.pdf. Accessed 25 July 2023.
Global Burden of Disease Long COVID Collaborators. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604–15.
Office for National Statistics. Leisure time in the UK: 2015 (2017). https://www.ons.gov.uk/economy/nationalaccounts/satelliteaccounts/articles/leisuretimeintheuk/2015. Accessed 25 July 2023.
Office for National Statistics. Employment, unemployment and economic inactivity by age group (seasonally adjusted). 2023. https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/datasets/employmentunemploymentandeconomicinactivitybyagegroupseasonallyadjusteda05sa. Accessed 25 July 2023.
Soare I-A, Ansari W, Nguyen JL, et al. Health-related quality of life in mild-to-moderate COVID-19 in the UK: a cross-sectional study from pre- to post-infection. Health Qual Life Outcomes. 2024;22(1):12.
Atchison CJ, Davies B, Cooper E, et al. Long-term health impacts of COVID-19 among 242,712 adults in England. Nat Commun. 2023;14(1):6588.
Office for National Statistics. National life tables: UK. 2018–2020 (2024). https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables. Accessed 12 Jan 2024.
Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/7july2022. Accessed 17 Aug 2023.
Office for National Statistics. Coronavirus (COVID-19) latest insights: Hospitals. 2023; Updated 28/03/2023. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/hospitals#hospital-admissions-by-age. Accessed 09 Jan 2024.
Office for National Statistics. Single year of age and average age of death of people whose death was due to or involved COVID-19. 2023; Updated 21/07/2023. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/singleyearofageandaverageageofdeathofpeoplewhosedeathwasduetoorinvolvedcovid19. Accessed 04 Aug 2023.
Baguelin M, Flasche S, Camacho A, et al. Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med. 2013;10(10):e1001527.
RDocumentation.org. optim: General-purpose Optimization. 2023. https://www.rdocumentation.org/packages/stats/versions/3.6.2/topics/optim. Accessed 09 Jan 2024.
MacIntyre CR, Costantino V, Trent M. Modelling of COVID-19 vaccination strategies and herd immunity, in scenarios of limited and full vaccine supply in NSW, Australia. Vaccine. 2022;40(17):2506–13.
Ferreira LS, de Almeida GB, Borges ME, et al. Modelling optimal vaccination strategies against COVID-19 in a context of Gamma variant predominance in Brazil. Vaccine. 2022;40(46):6616–24.
LaJoie Z, Usherwood T, Sampath S, Srivastava V. A COVID-19 model incorporating variants, vaccination, waning immunity, and population behavior. Sci Rep. 2022;12(1):20377.
Mak HY, Dai T, Tang CS. Managing two-dose COVID-19 vaccine rollouts with limited supply: operations strategies for distributing time-sensitive resources. Prod Oper Manag. 2022. https://doi.org/10.1111/poms.13862.
Matrajt L, Eaton J, Leung T, Brown ER. Vaccine optimization for COVID-19: who to vaccinate first? Sci Adv. 2020;7(6):eabf1374.
Suryawanshi R, Ott M. SARS-CoV-2 hybrid immunity: silver bullet or silver lining? Nat Rev Immunol. 2022;22(10):591–2.
The Lancet Infectious Diseases. Why hybrid immunity is so triggering. Lancet Infect Dis. 2022;22(12):1649.
Suryawanshi RK, Chen IP, Ma T, et al. Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. Nature. 2022;607(7918):351–5.
Perez-Guzman PN, Knock E, Imai N, et al. Epidemiological drivers of transmissibility and severity of SARS-CoV-2 in England. Nat Commun. 2023;14(1):4279.
O'Mahoney LL, Routen A, Gillies C, et al. The prevalence and long-term health effects of long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. eClinicalMedicine. 2023;55:101762.
Schnitzler L, Janssen LMM, Evers SMAA, et al. The broader societal impacts of COVID-19 and the growing importance of capturing these in health economic analyses. Int J Technol Assess Health Care. 2021;37(1):e43.
GOV.UK. New vaccine marketing campaign urges millions to boost their immunity this winter. 2022; Updated 23/10/2022. https://www.gov.uk/government/news/new-vaccine-marketing-campaign-urges-millions-to-boost-their-immunity-this-winter. Accessed 09 Jan 2024.
Barnard RC, Davies NG, Munday JD, et al. Modelling the medium-term dynamics of SARS-CoV-2 transmission in England in the Omicron era. Nat Commun. 2022;13(1):4879.
Hogan AB, Doohan P, Wu SL, et al. Estimating long-term vaccine effectiveness against SARS-CoV-2 variants: a model-based approach. Nat Commun. 2023;14(1):4325.
Department of Health and Social Care. JCVI statement on the COVID-19 vaccination programme for autumn 2023, 26 May 2023. 2023. https://www.gov.uk/government/publications/covid-19-autumn-2023-vaccination-programme-jcvi-advice-26-may-2023/jcvi-statement-on-the-covid-19-vaccination-programme-for-autumn-2023-26-may-2023. Accessed 17 Aug 2023.
Bubar KM, Reinholt K, Kissler SM, et al. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021;371(6532):916–21.
Fitzpatrick MC, Galvani AP. Optimizing age-specific vaccination. Science. 2021;371(6532):890–1.
The UK government needs to expand covid-19 vaccination this winter. BMJ. 2023;382:p2006.
Di Fusco M, Sun X, Moran MM, et al. Impact of COVID-19 and effects of BNT162b2 on patient-reported outcomes: quality of life, symptoms, and work productivity among US adult outpatients. J Patient Rep Outcomes. 2022;6(1):123.
Yaghoubi M, Salimi M, Meskarpour-Amiri M. Systematic review of productivity loss among healthcare workers due to Covid-19. Int J Health Plann Manage. 2022;37(1):94–111.
GOV.UK. Coronavirus and the social impacts of 'long COVID' on people's lives in Great Britain: 7 April to 13 June 2021. 2021; Updated 21/07/2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronavirusandthesocialimpactsoflongcovidonpeopleslivesingreatbritain/7aprilto13june2021. Accessed 08 Nov 2023.
Tufts J, Guan N, Zemedikun DT, et al. The cost of primary care consultations associated with long COVID in non-hospitalised adults: a retrospective cohort study using UK primary care data. BMC Primary Care. 2023;24(1):245.
Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 1 April 2021. 2021; Updated 01/04/2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021. Accessed 08 Nov 2023.
Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706–14.
Kerksieck P, Ballouz T, Haile SR, et al. Post COVID-19 condition, work ability and occupational changes in a population-based cohort. The Lancet Regional Health – Europe. 2023;31:100671.
Alderwick H. Is the NHS overwhelmed? BMJ. 2022;376: o51.
NHS Confederation. Latest urgent and emergency care situation report shows rising winter virus levels. 2023; Updated 07/12/2023. https://www.nhsconfed.org/news/latest-urgent-and-emergency-care-situation-report-shows-rising-winter-virus-levels. Accessed 09 Jan 2024.
NHS England. COVID-19 Hospital Activity. 2023. https://www.england.nhs.uk/statistics/statistical-work-areas/covid-19-hospital-activity/. Accessed 09 Jan 2024.
NHS Providers. NHS Winter Watch 2023/2024 Week 1 27 November - 03 December. 2023. https://nhsproviders.org/nhs-winter-watch-202324/week-1#:~:text=Measures%20are%20in%20place%20to,remains%20concerningly%20high%20at%2094.4%25. Accessed 09 Jan 2024.
O’Dowd A. Hospital bed occupancy rates in England reach dangerously high levels. BMJ. 2021;374: n2079.
NHS England. NHS sets out plans for winter with new measures to help speed up discharge for patients and improve care. 2023; Updated 27/07/2023. https://www.england.nhs.uk/2023/07/nhs-sets-out-plans-for-winter-with-new-measures-to-help-speed-up-discharge-for-patients-and-improve-care/. Accessed 09 Jan 2024.
GOV.UK. Press release: COVID-19 vaccines have prevented 7.2 million infections and 27,000 deaths. 2021. https://www.gov.uk/government/news/covid-19-vaccines-have-prevented-7-2-million-infections-and-27-000-deaths. Accessed 09 Nov 2023.
GOV.UK. Appendix 1: UKHSA report estimating the number needed to vaccinate to prevent COVID-19 hospitalisation for booster vaccination in autumn 2023 in England. 2023; Updated 05/10/2023. https://www.gov.uk/government/publications/covid-19-autumn-2023-vaccination-programme-jcvi-advice-26-may-2023/appendix-1-ukhsa-report-estimating-the-number-needed-to-vaccinate-to-prevent-covid-19-hospitalisation-for-booster-vaccination-in-autumn-2023-in-engla. Accessed 23 Nov 2023.
Office for National Statistics. Coronavirus (COVID-19) latest insights: Hospitals. 2023. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/hospitals#hospital-admissions-by-age. Accessed 08 Dec 2023.
Office for National Statistics. Single year of age and average age of death of people whose death was due to or involved coronavirus (COVID-19). 2023. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/singleyearofageandaverageageofdeathofpeoplewhosedeathwasduetoorinvolvedcovid19. Accessed 08 Dec 2023.
Kouhpayeh H, Ansari H. Adverse events following COVID-19 vaccination: a systematic review and meta-analysis. Int Immunopharmacol. 2022;109: 108906.
Medicines and Healthcare products regulatory agency. Coronavirus vaccine - summary of Yellow Card reporting. 2023. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting. Accessed 17 Aug 2023.
NHS. About COVID-19 vaccination. 2023; Updated 21/03/2023. https://www.nhs.uk/conditions/covid-19/covid-19-vaccination/about-covid-19-vaccination/#:~:text=Find%20out%20more%20about%20the,19%20vaccine%20on%20GOV.UK. Accessed 07 Dec 2023.
GOV.UK. Coronavirus vaccine - summary of Yellow Card reporting. 2023; Updated 08/03/2023. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting. Accessed 06 Dec 2023.
GOV.UK. JCVI statement on the COVID-19 booster vaccination programme for autumn 2022: update 3 September 2022. 2022. https://www.gov.uk/government/publications/covid-19-vaccines-for-autumn-2022-jcvi-advice-15-august-2022/jcvi-statement-on-the-covid-19-booster-vaccination-programme-for-autumn-2022-update-15-august-2022. Accessed 25 July 2023.
Medical Writing, Editorial and Other Assistance.
The authors wish to thank Professor Katharina Hauck and Dr Pablo Perez Guzman for their analytical assistance throughout model development. Medical writing was provided by Aisling Morrin, PhD and Emma Crouch-Baker, PhD of Health Economics and Outcomes Research Ltd and was funded by Pfizer.
Funding
This study was sponsored by Pfizer, alongside the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Diana Mendes, Sheeja Machira Krishnan, Esme O’Brien, Thomas Padgett, Cale Harrison, W David Strain, Andrea Manca, Andrew Ustianowski, Rebecca Butfield, Elizabeth Hamson, Charlie Reynard, and Jingyan Yang were involved in the conception and design of the study and contributed to the analysis and interpretation. All authors critically revised manuscript drafts, approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of Interest
Diana Mendes, Jingyan Yang, Cale Harrison, Rebecca Butfield, Elizabeth Hamson, and Charlie Reynard are employees of Pfizer and may own stocks. Sheeja Machira Krishnan, Thomas Padgett, and Esmé O’Brien are employees of Health Economics and Outcomes Research Ltd, which received funding from Pfizer in relation to the study and in connection with the development of this manuscript. Cale Harrison was an employee of Health Economics and Outcomes Research Ltd at the time of study, received fees for consultancy, and is now an employee of Pfizer. Andrea Manca, Andrew Ustianowski, and W David Strain were paid consultants to Pfizer in relation to the study and in connection with the development of this manuscript. Andrew Ustianowski also received advisory board and/or speaker fees from Gilead Sciences, Merck/MSD, Valneva, GSK/ViiV, Sanofi, unrelated to this study.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mendes, D., Machira Krishnan, S., O’Brien, E. et al. Modelling COVID-19 Vaccination in the UK: Impact of the Autumn 2022 and Spring 2023 Booster Campaigns. Infect Dis Ther 13, 1127–1146 (2024). https://doi.org/10.1007/s40121-024-00965-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00965-8