Abstract
Introduction
Respiratory syncytial virus (RSV) is an important cause of lower respiratory tract disease in older adults, resulting in substantial morbidity and mortality.
Methods
This study estimates the public health impact of vaccination with the adjuvanted RSVPreF3 vaccine among adults aged ≥ 60 years in the United States (US). A static, multi-cohort Markov model was used to estimate RSV-related outcomes over a 3-year time horizon for scenarios with and without one-time RSV vaccination. The base-case analysis assumed the same vaccination coverage as for influenza vaccines, with key epidemiology and vaccine inputs obtained from the published literature and phase 3 clinical trial results for the adjuvanted RSVPreF3 vaccine. Model outcomes included the clinical burden of RSV (symptomatic RSV acute respiratory illness [RSV-ARI] cases [classified as upper or lower respiratory tract disease], pneumonia complications, and mortality) and RSV-related healthcare resource use (hospitalizations, emergency department visits, outpatient visits, and antibiotic prescriptions).
Results
In the base-case analysis, approximately 56.7 million adults aged ≥ 60 years received the vaccine, resulting in 2,954,465 fewer symptomatic RSV-ARI cases over 3 years compared with no vaccination, including 321,019 fewer X-ray confirmed pneumonia cases and 16,660 fewer RSV-related deaths. Vaccination also prevented a substantial number of RSV-related hospitalizations (203,891), emergency department visits (164,060), outpatient visits (1,577,586), and antibiotic prescriptions (1,343,915) over the 3-year period. A considerable public health impact was observed across a range of sensitivity analyses.
Conclusions
These findings highlight the potential of the adjuvanted RSVPreF3 vaccine to substantially reduce RSV disease burden among US older adults aged ≥ 60 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Respiratory syncytial virus (RSV) results in a substantial number of cases, complications, healthcare resource use, and deaths in older adults. |
Two vaccines are now available in the United States for the prevention of RSV in adults aged 60 years and older. This study modeled the public health impact of vaccinating older adults in the United States with the adjuvanted RSVPreF3 vaccine. Vaccination was given once at the start of the analysis and the impact was assessed over 3 years. |
What was learned from the study? |
Assuming the same vaccination coverage as for influenza vaccines, adjuvanted RSVPreF3 vaccination was estimated to prevent nearly 3 million symptomatic RSV cases over 3 years, including over 1.5 million outpatient visits, over 200,000 hospitalizations, and nearly 17,000 deaths compared with no vaccination. |
Vaccinating adults aged 60 years and older with the adjuvanted RSVPreF3 vaccine provides substantial public health benefits in the United States. |
Introduction
Respiratory syncytial virus (RSV) is highly contagious, with infection resulting in acute respiratory illness (ARI) in individuals of all ages [1]. Although RSV has long been recognized as a common cause of respiratory infections in young infants and children, it has been increasingly acknowledged as an important cause of severe respiratory illness in older adults and adults with certain chronic conditions, leading to substantial morbidity and mortality [2,3,4,5]. In a landmark prospective cohort study conducted over four consecutive winters (1999–2003), Falsey et al. found that RSV infection occurs in 3–7% of healthy older adults each year and in 4–10% of adults considered at increased risk of severe RSV outcomes, resulting in an estimated 177,525 RSV-related hospitalizations and 14,000 RSV-related deaths among adults aged ≥ 65 years in the United States (US) each year [2]. After adjusting for RSV testing sensitivity, a recent systematic literature review and meta-analysis estimated rates of medically attended RSV that ranged from 1722.2 to 2278.2 per 100,000 and rates of RSV-related hospitalizations that ranged from 66.9 to 266.7 per 100,000 for older adults aged 50–64 and ≥ 65 years, respectively [6]. Further, Tseng et al. reported that among adults aged ≥ 60 years who were hospitalized for RSV, mortality was 5.6% in-hospital, 8.6% within 1 month, and 25.8% within 12 months of admission [7].
Typically, RSV infections in adults result in mild, cold-like symptoms [8]. However, in older adults and adults with certain chronic conditions or weakened immune systems, RSV can lead to more serious outcomes, such as pneumonia, and exacerbation of underlying medical conditions (e.g., asthma or chronic obstructive pulmonary disease [COPD]) [9]. Symptomatic RSV-ARI cases can be classified as either lower respiratory tract disease (RSV-LRTD) or upper respiratory tract disease (RSV-URTD), with RSV-URTD cases generally being milder because they do not have lower respiratory tract involvement [10]. The study by Ackerson et al. compared RSV and influenza cases in hospitalized older adults (aged ≥ 60 years) and found that RSV cases were associated with more severe outcomes (e.g., prolonged hospital stays, higher rate of intensive care unit admission, higher rate of developing pneumonia, and higher probability of death within 1 year of admission) [11]. Similar findings were reported more recently by Surie et al., where older adults hospitalized with RSV had more severe outcomes than older adults hospitalized with influenza or SARS-CoV2 infection [12]. Some older adults with RSV (e.g., those who are hospitalized) may experience functional decline that persists beyond the acute RSV infection [13].
In 2023, two vaccines were approved by the US Food and Drug Administration (FDA) for the prevention of LRTD caused by RSV in individuals 60 years of age and older: the adjuvanted RSVPreF3 vaccine (Arexvy, GSK) [14] and the RSVpreF vaccine (Abrysvo, Pfizer) [15]. Both vaccines target the RSV viral F glycoprotein which facilitates RSV cell entry in its functional prefusion conformation, with demonstrated efficacy against RSV infection [16, 17]. The adjuvanted RSVPreF3 vaccine utilizes a recombinant F protein antigen (RSVPreF3) engineered to preferentially maintain its prefusion form, along with the long-established AS01E adjuvant which promotes induction/boosting of antibody and cellular responses to help overcome age-related decline in immunity [18], making it highly suited for use in older adults. In June 2023, the Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP) recommended that “adults aged ≥ 60 years may receive a single dose of an RSV vaccine, using shared clinical decision-making” [19]. Because of the relatively recent introduction of RSV vaccines in the US, few studies have evaluated their potential public health benefit. Estimating the impact of vaccination in terms of reductions in RSV cases, complications, and RSV-related mortality, as well as the broader impact on healthcare resource use (HCRU) can help inform healthcare professionals and patients of the potential benefits of RSV vaccination. Comparing such outcomes with RSV disease and HCRU burden in the absence of vaccination can provide a valuable benchmark for estimating the impact of recently introduced RSV vaccines.
The objective of this study was to estimate the public health impact of RSV vaccination with the adjuvanted RSVPreF3 vaccine compared with no vaccination in US adults aged ≥ 60 years. This included an evaluation of the clinical burden of RSV at the population level in the absence of vaccination (in terms of symptomatic RSV-ARI cases, complications, and RSV-related mortality) and estimated reductions in these outcomes with RSV vaccination. The impact of RSV on HCRU, in terms of RSV-related outpatient visits, emergency department (ED) visits, hospitalizations, and antibiotic use was also evaluated in both scenarios (without and with adjuvanted RSVPreF3 vaccination).
Methods
Model Overview and Modeling Approach
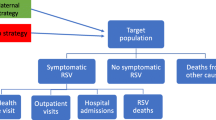
A static, multi-cohort Markov model was developed to evaluate the impact of the adjuvanted RSVPreF3 vaccine on symptomatic RSV-ARI cases and related morbidity and mortality compared with no vaccination in US adults aged ≥ 60 years. The modeled population was aligned to the current indication for the adjuvanted RSVPreF3 vaccine [14] and was stratified into seven age-specific cohorts (60–64, 65–69, 70–74, 75–79, 80–84, 85–89, and ≥ 90 years) to account for differential risks of key outcomes (e.g., RSV-related hospitalizations or deaths). The youngest age in a given cohort was used for the full cohort at the start of the simulation. The model was developed in Microsoft Excel (Microsoft Corporation; Redmond, WA, USA) and included a 3-year time horizon with adjuvanted RSVPreF3 vaccination occurring at the start of the simulation. The modeled time horizon was selected based on results from the adjuvanted RSVPreF3 vaccine’s phase 3 clinical trial. Specifically, data through two full RSV seasons suggest that while adjuvanted RSVPreF3 vaccine efficacy against RSV is greatest within the first year, some degree of protection is projected to persist for up to 3 years, accounting for waning [16, 20]. An overview of the model’s underlying Markov framework, as applied in the present analysis, is presented in Fig. 1.
Markov model structure. The multi-cohort Markov model follows US adults aged ≥ 60 years over 3 years, with a 1-month cycle length. The model includes seven age-specific cohorts and evaluates outcomes for scenarios with and without adjuvanted RSVPreF3 vaccination. The underlying model structure for RSV reinfections is the same as for initial RSV infections. ARI acute respiratory illness; LRTD lower respiratory tract disease; RSV respiratory syncytial virus; URTD upper respiratory tract disease; US United States
The Markov model and its inputs account for US population characteristics (size, age distribution, and all-cause mortality), RSV epidemiological data, HCRU, vaccine efficacy (VE) and waning, and vaccination coverage in older adults. The model follows older adults over 3 years, with a 1-month cycle length to account for seasonal variation in RSV epidemiology and for waning of VE over time. Use of the underlying Markov structure allows individuals to start in the “No RSV” health state and transition between different health states and events over the modeled time horizon (e.g., from the “No RSV” health state to the disease event “Symptomatic RSV-ARI” [classified as either “RSV-URTD” or “RSV-LRTD”] and then to the recovery health state [“Post-RSV”], also allowing for individuals to be reinfected). Additional details are provided in Table S1 in the Supplementary Material. These health states each represent important model outcomes for estimating the burden of RSV at an individual and population level. Symptomatic RSV-ARI cases provide a measure of the core disease burden of RSV infection within the older adult population, with those considered as RSV-LRTD more likely to have more severe RSV outcomes (e.g., ED visits, hospitalizations, X-ray confirmed pneumonia, RSV-related mortality). The model also accounts for all-cause mortality and allows for RSV reinfection.
Model Input Parameters
Model inputs were based on data from publicly available US sources, the published literature, and the pivotal Adult Respiratory Syncytial Virus (AReSVi-006) phase 3 clinical study (including data through two full RSV seasons) [16, 20] (Table 1). The model structure, data inputs, and underlying assumptions were informed by a targeted review of the literature and validated throughout the model development process by a number of RSV expert clinicians, epidemiologists, and health economists. For symptomatic RSV-ARI, core incidence data were based on data gathered over four consecutive seasons from Falsey et al. [2] (see below). The percentage of cases classified as RSV-LRTD was drawn from data across two seasons from the AReSVi-006 phase 3 study, with vaccine efficacy also based upon these longer-term data [16, 20] (and data on file). A more detailed description of these parameters and specific values are presented below.
Population Characteristics
In line with the indication for the adjuvanted RSVPreF3 vaccine, the modeled population included all US adults aged ≥ 60 years (n = 82,862,258), based on US Census Bureau population projections for 2023 (Table 1) [21]. All-cause mortality was derived from age-specific US 2020 annualized values for probability of death [22], converted to monthly probabilities (Table S2 in Supplementary Material).
Epidemiological Input Parameters
Epidemiological model input parameters are presented in Table 1. Incidence of symptomatic RSV-ARI was derived from data reported by Falsey et al. [2], calculated as the mean weighted average incidence among non-high-risk older adults (aged ≥ 65 years) and high-risk adults (aged ≥ 21 years with congestive heart failure or chronic lung conditions) across four winter seasons, after removing asymptomatic RSV cases. The derivation used weighting based on the proportions of non-high-risk and high-risk adults enrolled in a large influenza vaccine trial conducted in the US by DiazGranados et al. [23]. As Falsey et al. used multiple RSV testing methodologies [2], no further adjustment for under ascertainment was made. The estimated incidence of symptomatic RSV-ARI per person-year was 0.0465 (0.0272–0.0627); lower and upper bounds used in sensitivity analyses were calculated as the minimum and maximum incidence of symptomatic RSV-ARI across the four consecutive winter seasons evaluated by Falsey et al. [2].
RSV infection rates show substantial seasonality [24]. In our analysis, monthly RSV incidence estimates were adjusted for seasonality using 2018–2019 RSV surveillance data from the National Respiratory and Enteric Virus Surveillance System (NREVSS) [25]. These NREVSS data were prior to the Coronavirus Disease 2019 (COVID-19) pandemic, providing information on RSV seasonality in a typical year. The seasonality adjustment factors were derived from the NREVSS data by dividing the total number of polymerase chain reaction (PCR)-confirmed RSV cases in each month by the average monthly number of PCR-confirmed RSV cases (Table S3 in Supplementary Material). RSV reinfection rates were assumed to be the same as for initial infection. The percentages of symptomatic RSV-ARI cases characterized as RSV-URTD and RSV-LRTD were based on data from the AReSVi-006 phase 3 study; over two full seasons, 47.6% of symptomatic RSV-ARI cases in the placebo arm were categorized as RSV-LRTD [20] (and data on file).
Healthcare Resource Use and Complications
RSV-related HCRU inputs (Table 1) were primarily based on the age-specific rates of medically attended RSV reported from a systematic literature review and meta-analysis by McLaughlin et al. [6]. In the base-case analysis, rates of RSV-related outpatient visits, ED visits, and hospitalizations were adjusted for RSV underdetection (Table 1). A calibration process was used within the model to determine the input values needed in order for the model to estimate the adjusted rates reported by McLaughlin et al. [6]. Specific inputs included within this calibration process included the percentage of symptomatic RSV-ARI cases that are medically attended (where medically attended symptomatic RSV-ARI cases were assumed to have one outpatient visit each), the percentage of RSV-LRTD cases that experience ED visits, and the percentage of RSV-LRTD cases that experience hospitalizations. The model assumed that RSV-LRTD cases are approximately twice as likely to be medically attended compared with RSV-URTD cases based on the previous decision analytic model from Herring et al. [26]. All ED visits and hospitalizations were assumed to only occur among RSV-LRTD cases, and RSV-related deaths were assumed to occur only among hospitalized RSV-LRTD cases.
Model inputs for antibiotic use and X-ray confirmed pneumonia were obtained from Belongia et al. [27]. We assumed that pneumonia could develop only in those individuals with RSV-LRTD. Belongia et al. [27] provides the number of x-ray-confirmed pneumonia cases out of the total number of medically attended moderate-to-severe LRTD (msLRTD) cases. Because these data only include outcomes for medically attended RSV cases, we adjusted this percentage by the percentage of RSV-LRTD cases that are medically attended (estimated from McLaughlin et al. and Herring et al. [6, 26]). Mortality rates were estimated based on mortality following RSV-LRTD hospitalization from the study by Tseng et al. [7]. Although 30-day mortality data were only reported for the overall population aged ≥ 60 years (8.6%), Tseng et al. also reported an overall in-hospital mortality rate of 5.6%, with age-specific rates of 4.6% in adults aged 60–74 years and 6.1% in adults aged ≥ 75 years [7]. By applying the same age distribution to the reported 30-day mortality estimate among adults aged ≥ 60 years, we derived age-specific 30-day mortality inputs for use in the model (60–74 years: 7.1%; ≥ 75 years: 9.4%). These rates were then applied to the age-specific percentages of RSV-LRTD cases resulting in hospitalization to generate overall RSV-LRTD mortality estimates.
Vaccine Characteristics and Vaccine-Specific Parameters
VE inputs for the adjuvanted RSVPreF3 vaccine were based on results from the AReSVi-006 phase 3 clinical study with a median follow-up time of 18 months [16, 20] (and data on file). The model accounted for waning of VE against RSV-ARI and against RSV-LRTD across the 3-year study horizon. Specifically, weighted linear regression models were fitted on the trial data to estimate the monthly VE for RSV-ARI and RSV-LRTD during and beyond the clinical trial follow-up period (Fig. 2).
Adjuvanted RSVPreF3 vaccine efficacy against RSV-ARI and RSV-LRTD over modeled 3-year time horizon. In the month of first vaccination (i.e., 1st cycle of the model), 50% of peak VE is considered. Waning of the peak VE starts in the second month following vaccination. A linear decrease is applied to VE against RSV-ARI and against RSV-LRTD. The waning rates are applied each month as an absolute percentage point decrease in VE. Peak VE and waning rates were estimated based on the AReSVi-006 phase 3 clinical trial [16, 20] (and data on file). Solid lines represent data from the AReSVi-006 phase 3 clinical trial [16, 20] (and data on file), while dashed lines are extrapolations based on the weighted linear regression analysis. VE point estimates are shown over a 4-year period, with the model limited to a 3-year time horizon. ARI acute respiratory illness, LB lower bound, LRTD lower respiratory tract disease, RSV respiratory syncytial virus, UB upper bound, VE vaccine efficacy
Using this approach, peak VE against RSV-ARI and RSV-LRTD were estimated to be 74.2 and 88.0%, respectively. For the first month following vaccination, it was assumed that VE would be 50% of peak values to allow for an immune build-up period. Waning of the peak VE was assumed to start in the second month following vaccination, and to continue linearly in each subsequent month. Waning rates were applied each month as an absolute percentage point decrease in VE (monthly waning of 2.3% for VE against RSV-ARI and 2.1% for VE against RSV-LRTD). Further details about the VE calculations are described in the Supplementary Material, including Table S4 and Supplementary Fig. 1. It was conservatively assumed that RSV vaccination has no impact on the risk of reinfection following a breakthrough RSV case.
The model assumed vaccination in October to align with typical timing of influenza vaccinations. Vaccination coverage was based on coverage for influenza vaccines among US older adults during the 2021–2022 season (the most recent data that were available at the time of the analysis) [28]. This assumption has been used previously in models evaluating potential benefits of RSV vaccination [26, 29], in part because influenza and RSV follow similar seasonal patterns and influenza vaccination rates represent a potentially achievable target of older adults who are willing to be vaccinated against seasonal respiratory infections. However, we evaluated a wide range of coverage estimates in scenario analyses. As such, the base-case analysis assumes that 52.4% of adults aged 60–64 years and 73.9% of adults aged ≥ 65 years receive the adjuvanted RSVPreF3 vaccine once, at the start of the modeled 3-year time horizon [28].
Outcomes
Key health outcomes of interest focused on the number of symptomatic RSV-ARI cases (overall and by RSV-URTD and RSV-LRTD cases), and their associated HCRU (number of hospitalizations, ED visits, outpatient visits, and antibiotic prescriptions), complications (number of cases of X-ray confirmed pneumonia), and deaths. Outcomes over the 3-year time horizon were calculated for each strategy (vaccination with the adjuvanted RSVPreF3 vaccine and no vaccination), with the model also calculating the incremental differences.
Numbers of individuals needed to vaccinate (NNV) to avoid specific outcomes were also calculated based on the modeled results. NNV estimates were calculated by dividing the number of older adults who were vaccinated by the number of each outcome avoided as a result of vaccination.
Sensitivity and Scenario Analyses
Sensitivity and scenario analyses were conducted to assess the robustness of the results to uncertainties around key input parameters. Because of the wide range of RSV burden reported in the literature, we examined the impact of different input values for symptomatic RSV-ARI incidence (based on the minimum and maximum seasonal incidence from Falsey et al. [2, 23]), the percentage of symptomatic RSV-ARI cases that are RSV-LRTD (based on 95% confidence intervals), RSV-related hospitalization rates (based on data reported by Branche et al. [30] and Herring et al. [26]), and RSV-related mortality (based on 95% confidence intervals). Analyses were also conducted assuming alternate values for VE inputs and vaccination coverage (considering 25, 50, 75, and 125% of influenza vaccination coverage estimates, as well as considering 100% RSV vaccination coverage).
Statement of Ethics Compliance
This analysis is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Ethical approval for the development of the model was not required.
Results
Base-Case Analysis
In the absence of vaccination, RSV is estimated to result in over 11 million symptomatic RSV-ARI cases among older adults over a 3-year period, including nearly 5.8 million RSV-URTD cases and nearly 5.3 million RSV-LRTD cases. Of these modeled RSV cases, an estimated 515,200 cases are hospitalized, 425,566 cases result in ED visits, over 5.1 million cases result in outpatient visits, and nearly 4.3 million cases are prescribed antibiotics (Fig. 3). In the absence of vaccination, the model also estimates a total of 845,308 cases of X-ray confirmed pneumonia and 41,926 RSV-related deaths in older adults over a 3-year period.
In the base-case analysis, a total of 56,654,023 older adults received one dose of the adjuvanted RSVPreF3 vaccine at the start of the modeled time horizon. Compared to no vaccination, this adjuvanted RSVPreF3 vaccination is estimated to prevent nearly 3 million symptomatic RSV-ARI cases over the 3-year time horizon, including nearly 1 million RSV-URTD cases and nearly 2 million RSV-LRTD cases (Fig. 3). In addition, vaccination would result in 203,891 avoided RSV-related hospitalizations, 164,060 avoided ED visits, 1,577,586 avoided outpatient visits, 1,343,915 avoided antibiotic prescriptions, 321,019 avoided pneumonia cases, and 16,660 avoided deaths. Most of this averted RSV burden is achieved during the first year following vaccination (e.g., with nearly 1.7 million symptomatic RSV-ARI cases, 102,143 RSV-related hospitalizations, and 8364 RSV-related deaths avoided in year 1). RSV burden and the public health impact of adjuvanted RSVPreF3 vaccination during the first year of the modeled time horizon are further summarized in the Supplementary Material (Supplementary Fig. 2).
Over the 3-year time horizon, the NNV to prevent one symptomatic RSV-ARI case was 19, the NNV to avoid one RSV-LRTD case was 29, and the NNV to avoid one RSV-related outpatient visit was 36. In addition, the NNV to avoid one RSV-related hospitalization was 278, with an NNV of 3401 to avoid one RSV-related death (Supplementary Fig. 3). These results indicate that vaccinating 1 million US adults aged ≥ 60 years with the adjuvanted RSVPreF3 vaccine could prevent approximately 52,149 symptomatic RSV-ARI cases, 27,846 outpatient visits, 3599 hospitalizations, and 294 deaths over a 3-year time horizon.
Sensitivity and Scenario Analyses
Results from the sensitivity and scenario analyses are shown in Table 2. Assuming low and high values for the incidence of symptomatic RSV-ARI (based on the minimum and maximum incidence observed across four winter seasons by Falsey et al. [2]), the public health impact of the adjuvanted RSVPreF3 vaccine over 3 years resulted in a range of approximately 1.7–4.0 million fewer symptomatic RSV-ARI cases, 1.2–2.6 million fewer RSV-LRTD cases, 120,624–272,671 fewer RSV-related hospitalizations, and 9856–22,280 fewer RSV-related deaths. With regards to other key RSV epidemiology inputs, incremental hospitalization and death outcomes were less sensitive to the input for the percentage of symptomatic RSV-ARI cases that are RSV-LRTD (Table 2) and were more sensitive to the input for the percentage of RSV-LRTD cases resulting in hospitalizations. Assuming alternate input values for RSV hospitalization rates from the published literature, including those reported by Branche et al. [30] and Herring et al. [26], resulted in avoided hospitalizations ranging from 105,444 to 316,539 and avoided deaths ranging from 8761 to 25,864 among older adults over 3 years with adjuvanted RSVPreF3 vaccination (compared to no vaccination). A narrower range was observed for avoided RSV-related deaths when the model’s input for probability of death given RSV-LRTD was varied (ranging from 10,472 to 22,846 deaths avoided over 3 years compared to no vaccination).
Simultaneously assuming the lower and upper range of peak VE against both RSV-ARI and RSV-LRTD resulted in approximately 1.8–4.4 million fewer symptomatic RSV-ARI cases over the 3-year time horizon versus no vaccination, including 1.2–2.4 million fewer RSV-LRTD cases, 123,416–246,494 fewer RSV-related hospitalizations, and 10,091–20,138 fewer RSV-related deaths. When simultaneously assuming the lower and upper range of VE waning against both RSV-ARI and RSV-LRTD, the model estimated approximately 5.1–1.7 million fewer symptomatic RSV-ARI cases, 3.0–1.1 million fewer RSV-LRTD cases, 311,826–119,519 fewer RSV-related hospitalizations, and 25,455–9,780 fewer RSV-related deaths over the 3-year time horizon.
Assuming different RSV vaccination coverage rates (ranging from a low of 13.1% in adults aged 60–64 years and 18.5% in adults aged ≥ 65 years to a high of 100% coverage in all older adults), the model estimated approximately 0.7–3.7 million fewer symptomatic RSV-ARI cases resulting from vaccination, including approximately 0.5–2.5 million fewer RSV-LRTD cases, 50,973–254,863 fewer RSV-related hospitalizations, and 4165–20,825 fewer RSV-related deaths over 3 years.
Discussion
This analysis estimates substantial public health benefits associated with the adjuvanted RSVPreF3 vaccine for the prevention of RSV cases and RSV-related morbidity and mortality in US adults aged ≥ 60 years. Assuming the same vaccination coverage as for influenza vaccines, the adjuvanted RSVPreF3 vaccine has the potential to avert nearly 3 million symptomatic RSV-ARI cases over a 3-year period, consequently avoiding over 1.5 million outpatient visits, 164,000 ED visits, 200,000 hospitalizations, and nearly 17,000 RSV-related deaths. Although the current analysis was specifically designed to address public health benefits, the economic impact in terms of reduced healthcare spending and broader indirect societal costs realized with RSV vaccination may be considerable. A related economic evaluation of the impact of adjuvanted RSVPreF3 vaccination utilizing the same underlying model will be reported in a separate publication.
The availability of RSV vaccines for use in older adults marks a significant public health achievement that will help to improve and save the lives of many. In terms of humanistic burden, a previous study of adults aged ≥ 50 years found that RSV infection may adversely impact quality of life (e.g., with study participants reporting declines in physical function and engagement in leisure activities) [31]. Adults aged ≥ 60 years who are hospitalized with an RSV infection also may require discharges to higher levels of care or may experience acute functional decline that persists [13]. RSV vaccination among older adults is expected to not only promote healthy aging by avoiding RSV cases, complications, and deaths, but also free up healthcare resources for other uses. This is particularly important given the seasonality of RSV, where avoided outpatient visits, ED visits, and hospitalizations during the respiratory season could help to considerably reduce stress within healthcare systems and strengthen the quality of care provided to other patients.
Vaccination can also make substantial contributions to reductions in antibiotic use, helping to potentially mitigate downstream antimicrobial resistance [32]. One recent study estimated that, globally, a total of 4.95 million antimicrobial resistance-associated deaths occurred in 2019 [33]. Over a 3-year period, vaccination with the adjuvanted RSVPreF3 vaccine is estimated to result in over 1.3 million fewer antibiotic prescriptions among older adults in the US, which can assist efforts in reducing antimicrobial resistance.
Our model’s base-case results are consistent with other decision analytic models that have estimated substantial public health benefits of RSV vaccination among older adults. For example, Herring et al. found that vaccinating 65.3% of US adults aged ≥ 60 years with a hypothetical RSV vaccine with a VE of 50% against overall RSV and 65% against msLRTD would result in approximately 1.2 million fewer RSV infections overall, 323,000–396,000 fewer medically attended RSV cases, 44,000–82,000 fewer hospitalizations, and 8000–15,000 fewer deaths each year [26]. More recently, Moghadas et al. also assessed the potential impact of the adjuvanted RSVPreF3 vaccine, as well as the RSVpreF vaccine (Abrysvo, Pfizer) and a combination of both vaccines [34]. The study found that compared with no vaccination, vaccinating 66% of adults aged ≥ 60 years with the adjuvanted RSVPreF3 vaccine would result in 53.6% fewer outpatient visits, 60.5% fewer hospitalizations, and 60.4% fewer deaths (vs. reductions of 41.4, 57.6, and 58.6%, respectively, for vaccination with RSVpreF) [34]. Additional comparisons between our modeled annual RSV burden and results from previous studies are provided in the Supplementary Material.
The current model’s results were robust to a wide range of uncertainties in key parameters used to estimate the clinical burden of symptomatic RSV-ARI cases and HCRU avoided with the adjuvanted RSVPreF3 vaccine. Across all sensitivity and scenario analyses that were conducted, the adjuvanted RSVPreF3 vaccine was estimated to result in at least 738,616 fewer symptomatic RSV-ARI cases, 50,973 fewer RSV-related hospitalizations, and 4165 fewer RSV-related deaths among older adults over a 3-year period. The maximum public health impact estimated across all sensitivity and scenario analyses included approximately 5.1 million fewer symptomatic RSV-ARI cases, 311,826 fewer RSV-related hospitalizations, and 25,455 fewer RSV-related deaths among older adults over a 3-year period. Analyses can be updated further as additional data on RSV burden become available (e.g., if RSV testing, detection, and diagnosis becomes more widespread in clinical practice, allowing for the generation of additional RSV epidemiologic data that can help inform model inputs). Similarly, our analyses can also be updated as additional data on the uptake and duration of protection of the adjuvanted RSVPreF3 vaccine become available (following real-world analyses of vaccination uptake in the 2023–2024 season and the availability of longer-term follow-up data from the AReSVi-006 phase 3 clinical trial).
The present study includes several assumptions and limitations that should be noted. The current model’s structure and input values were informed by clinical trial data, the scientific literature, and external expert opinion where possible. When assumptions were needed, they were made with preference to conservative assumptions. First, although RSV vaccination is recommended by the ACIP for use in adults aged ≥ 60 years with shared clinical decision-making [19], the analysis presented here is based on a general population of older adults (i.e., without prioritizing those at highest risk of severe RSV outcomes). Uniform vaccination of older adults may not be representative of real-world uptake patterns, in which a preference may exist for RSV vaccination of certain individuals with risk factors for severe RSV. The adjuvanted RSVPreF3 vaccine has shown robust VE against RSV-LRTD in older adults with underlying cardiorespiratory and/or endocrine/metabolic conditions of interest [35], indicating that the public health impact of vaccination would be considerable in these populations. Second, the analysis excludes VE specific to severe RSV-LRTD, despite phase 3 clinical trial results demonstrating 94.1% VE against severe RSV-LRTD in the first season following vaccination and 78.8% VE through the full second season following vaccination [16, 20]. Severe RSV-LRTD cases may be more likely to seek medical care in real-world settings, potentially requiring higher levels of care (e.g., ED visits, hospitalizations) and/or resulting in death. The model excludes any disease attenuation resulting from the adjuvanted RSVPreF3 vaccine, although phase 3 clinical trial results indicate less severe symptoms (as measured by the Flu-PRO maximum Chest/Respiratory score endpoint) and reduced HCRU among breakthrough vaccinated RSV cases versus placebo cases [36]. For HCRU, the model focused on reduced outpatient visits, ED visits, and hospitalizations as a result of RSV vaccination and did not evaluate any further impacts to healthcare systems (e.g., through reduced use of intensive care units). Additionally, the model assumed that RSV-related deaths only occur following RSV-LRTD hospitalizations, potentially underestimating the total number of RSV-related deaths (i.e., excluding deaths among ED visit, outpatient visit, and non-medically attended RSV cases).
While the model’s vaccine efficacy inputs were derived from the pivotal AReSVi-006 phase 3 clinical study and accommodates efficacy waning over 3 years, such efficacy estimates may differ from those seen in real-world use. Furthermore, although model inputs for RSV epidemiology and HCRU were based on US data from the published literature, such estimates do vary, and there remains a need to better characterize RSV disease burden and its impact on healthcare resource use. Our input for the percentage of RSV-ARI cases that are RSV-LRTD was obtained from the placebo arm of the AReSVi-006 clinical study, and again this may differ from values seen in real-world settings. While we have tried to account for these considerations in our sensitivity analyses, our model can be updated as additional data become available.
Conclusions
Assuming the same vaccination coverage as for influenza vaccines, the public health benefit of the adjuvanted RSVPreF3 vaccine in older adults is expected to be substantial. This vaccine provides the opportunity to avoid nearly 3 million symptomatic RSV-ARI cases in US older adults over a 3-year period, which would have a considerable impact on associated morbidity and mortality. To achieve this public health impact, efforts will be needed to support RSV vaccination, particularly focusing on vaccination of older adults at highest risk for severe RSV outcomes.
Data Availability
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website.
References
Coultas JA, Smyth R, Openshaw PJ. Respiratory syncytial virus (RSV): a scourge from infancy to old age. Thorax. 2019;74(10):986–93. https://doi.org/10.1136/thoraxjnl-2018-212212.
Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–59. https://doi.org/10.1056/NEJMoa043951.
Villanueva DH, Arcega V, Rao M. Review of respiratory syncytial virus infection among older adults and transplant recipients. Ther Adv Infect Dis. 2022;9:20499361221091412. https://doi.org/10.1177/20499361221091413.
Nam HH, Ison MG. Respiratory syncytial virus infection in adults. BMJ. 2019;366: l5021. https://doi.org/10.1136/bmj.l5021.
Hansen CL, Chaves SS, Demont C, Viboud C. Mortality associated with influenza and respiratory syncytial virus in the US, 1999–2018. JAMA Netw Open. 2022;5(2): e220527. https://doi.org/10.1001/jamanetworkopen.2022.0527.
McLaughlin JM, Khan F, Begier E, Swerdlow DL, Jodar L, Falsey AR. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis. 2022;9(7):ofac300. https://doi.org/10.1093/ofid/ofac300.
Tseng HF, Sy LS, Ackerson B, et al. Severe morbidity and short- and mid- to long-term mortality in older adults hospitalized with respiratory syncytial virus infection. J Infect Dis. 2020;222(8):1298–310. https://doi.org/10.1093/infdis/jiaa361.
Centers for Disease Control and Prevention (CDC). Respiratory syncytial virus infection (RSV). https://www.cdc.gov/rsv/index.html. Accessed 24 Oct 2022.
Centers for Disease Control and Prevention (CDC). RSV in older adults and adults with chronic medical conditions. https://www.cdc.gov/rsv/high-risk/older-adults.html. Accessed 6 Jan 2022.
Meissner HC. Viral bronchiolitis in children. N Engl J Med. 2016;374(1):62–72. https://doi.org/10.1056/NEJMra1413456.
Ackerson B, Tseng HF, Sy LS, et al. Severe morbidity and mortality associated with respiratory syncytial virus versus influenza infection in hospitalized older adults. Clin Infect Dis. 2019;69(2):197–203. https://doi.org/10.1093/cid/ciy991.
Surie D, Yuengling KA, DeCuir J, et al. Disease severity of respiratory syncytial virus compared with COVID-19 and influenza among hospitalized adults aged ≥ 60 years - IVY network, 20 US States, February 2022–May 2023. MMWR Morb Mortal Wkly Rep. 2023;72(40):1083–8. https://doi.org/10.15585/mmwr.mm7240a2.
Branche AR, Saiman L, Walsh EE, et al. Change in functional status associated with respiratory syncytial virus infection in hospitalized older adults. Influenza Other Respir Viruses. 2022;16(6):1151–60. https://doi.org/10.1111/irv.13043.
US Food & Drug Administration (FDA). AREXVY (Respiratory Syncytial Virus Vaccine, Adjuvanted). https://www.fda.gov/media/167805/download. Accessed 29 Sept 2023.
US Food & Drug Administration (FDA). ABRYSVO (Respiratory Syncytial Virus Vaccine). https://www.fda.gov/media/168889/download. Accessed 29 Sept 2023.
Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med. 2023;388(7):595–608. https://doi.org/10.1056/NEJMoa2209604.
Walsh EE, Perez Marc G, Zareba AM, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465–77. https://doi.org/10.1056/NEJMoa2213836.
Didierlaurent AM, Laupeze B, Di Pasquale A, Hergli N, Collignon C, Garcon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16(1):55–63. https://doi.org/10.1080/14760584.2016.1213632.
Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the advisory committee on immunization practices - United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793–801. https://doi.org/10.15585/mmwr.mm7229a4.
Ison MG, Papi A, Athan E, et al. Efficacy and safety of respiratory syncytial virus prefusion F protein vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons. Clin Infect Dis. 2024. https://doi.org/10.1093/cid/ciae010.
US Census Bureau. Projected Population by Single Year of Age, Sex, Race, and Hispanic Origin for the United States: 2016 to 2060. 2018. https://www.census.gov/data/datasets/2017/demo/popproj/2017-popproj.html. Accessed 10 Mar 2022.
Arias E, Xu J, Tejada-Vera B, Murphy SL, Bastian B. US State life tables, 2020. Natl Vital Stat Rep. 2022;71(2):1–18.
DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–45. https://doi.org/10.1056/NEJMoa1315727.
Colosia AD, Yang J, Hillson E, et al. The epidemiology of medically attended respiratory syncytial virus in older adults in the United States: a systematic review. PLoS ONE. 2017;12(8): e0182321. https://doi.org/10.1371/journal.pone.0182321.
Centers for Disease Control and Prevention (CDC). National respiratory and enteric virus surveillance system (NREVSS). https://www.cdc.gov/surveillance/nrevss/. Accessed 22 Oct 2022.
Herring WL, Zhang Y, Shinde V, Stoddard J, Talbird SE, Rosen B. Clinical and economic outcomes associated with respiratory syncytial virus vaccination in older adults in the United States. Vaccine. 2022;40(3):483–93. https://doi.org/10.1016/j.vaccine.2021.12.002.
Belongia EA, King JP, Kieke BA, et al. Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults ≥ 60 years old. Open Forum Infect Dis. 2018;5(12):ofy316. https://doi.org/10.1093/ofid/ofy316.
Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage for persons 6 months and older. 2022. https://www.cdc.gov/flu/fluvaxview/interactive-general-population.htm. Accessed 12 Oct 2022.
Van Effelterre T, Hens N, White LJ, et al. Modeling respiratory syncytial virus adult vaccination in the United States with a dynamic transmission model. Clin Infect Dis. 2023;77(3):480–9. https://doi.org/10.1093/cid/ciad161.
Branche AR, Saiman L, Walsh EE, et al. Incidence of respiratory syncytial virus infection among hospitalized adults, 2017–2020. Clin Infect Dis. 2022;74(6):1004–11. https://doi.org/10.1093/cid/ciab595.
Curran D, Cabrera ES, Bracke B, et al. Impact of respiratory syncytial virus disease on quality of life in adults aged ≥ 50 years: a qualitative patient experience cross-sectional study. Influenza Other Respir Viruses. 2022;16(3):462–73. https://doi.org/10.1111/irv.12929.
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–55. https://doi.org/10.1016/S0140-6736(21)02724-0.
Kim C, Holm M, Frost I, Hasso-Agopsowicz M, Abbas K. Global and regional burden of attributable and associated bacterial antimicrobial resistance avertable by vaccination: modelling study. BMJ Glob Health. 2023;8(7): e011341. https://doi.org/10.1136/bmjgh-2022-011341.
Moghadas SM, Shoukat A, Bawden CE, et al. Cost-effectiveness of prefusion F protein-based vaccines against respiratory syncytial virus disease for older adults in the United States. Clin Infect Dis. 2023. https://doi.org/10.1093/cid/ciad658.
Feldman RG, Antonelli-Incalzi R, Steenackers K, et al. Respiratory syncytial virus prefusion F protein vaccine is efficacious in older adults with underlying medical conditions. Clin Infect Dis. 2023. https://doi.org/10.1093/cid/ciad471.
Curran C, Matthews S, Sabater E, et al. The respiratory syncytial virus prefusion F protein candidate vaccine (RSVPreF3 OA) attenuates the severity of RSV in breakthrough infections in adults ≥ 60 years of age. Presented at the 7th ReSViNET Conference (RSVVW'23); 22–24, 2023. Lisbon, Portugal.
Medical Writing, Editorial, and Other Assistance
The authors would like to thank Jessica Pickett, Marie-Pierre David, Sean Matthews, Ilse Van Vlaenderen, Will Herring, and Zinan Yi for their contribution to model development. The authors thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Iain O’Neill (freelance, on behalf of GSK) provided medical writing support.
Funding
GlaxoSmithKline Biologicals SA funded this study (GSK study identifier: VEO-000319) and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of this manuscript, including the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
All authors attest they meet the ICMJE criteria for authorship. Daniel Molnar, Elizabeth M La, Frederik Verelst, Sara Poston, Jonathan Graham, Laure-Anne Van Bellinghen, and Desmond Curran were involved in study conception and design. Daniel Molnar, Elizabeth M La, Frederik Verelst, Sara Poston, Jonathan Graham, and Desmond Curran were involved in data analysis and interpretation. All authors contributed to the initial manuscript drafts or critical revision for intellectual content and gave final approval for publication of the article.
Corresponding author
Ethics declarations
Conflict of Interest
Daniel Molnar, Elizabeth M La, Frederik Verelst, Sara Poston, and Desmond Curran are employees of GSK and hold shares in GSK. Jonathan Graham is an employee of RTI Health Solutions, which received consultancy fees from GSK to complete the work disclosed here and for other projects unrelated to the submitted work. Laure-Anne Van Bellinghen is an employee of CHESS in Health, which received funding from GSK to complete the work disclosed here and for additional projects unrelated to the present study.
Ethical Approval
This analysis is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Ethical approval for the development of the model was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior presentation: Preliminary public health impact results from the model were presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR-US) 2023 congress, 7–10 May, 2023, Boston, United States.
AREXVY is a trademark owned by or licensed to GSK. Abrysvo is a trademark of Pfizer.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Molnar, D., La, E.M., Verelst, F. et al. Public Health Impact of the Adjuvanted RSVPreF3 Vaccine for Respiratory Syncytial Virus Prevention Among Older Adults in the United States. Infect Dis Ther 13, 827–844 (2024). https://doi.org/10.1007/s40121-024-00939-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00939-w