Abstract
Introduction
Rare myocarditis and pericarditis cases have occurred in coronavirus disease 2019 (COVID-19) messenger RNA (mRNA) vaccine recipients. Troponin levels, a potential marker of myocardial injury, were assessed in healthy participants before and after BNT162b2 vaccination.
Methods
Vaccine-experienced 12- to 30-year-olds in phase 3 crossover C4591031 Substudy B (NCT04955626) who had two or three prior BNT162b2 30-μg doses were randomized to receive BNT162b2 30 μg followed by placebo, or placebo followed by BNT162b2 30 µg, 1 month apart. A participant subset, previously unvaccinated against COVID-19, in the phase 3 C4591007 study (NCT04816643) received up to three vaccinations (BNT162b2 10 μg or placebo [5- to 11-year-olds]) or open-label BNT162b2 30 μg (12- to 15-year-olds). Blood samples collected pre-vaccination, 4 days post-vaccination, and 1-month post-vaccination (C4591031 Substudy B only) were analyzed. Frequencies of elevated troponin I levels (male, > 35 ng/l; female, > 17 ng/l) were assessed.
Results
Percentages of 12- to 30-year-olds (n = 1485) in C4591031 Substudy B with elevated troponin levels following BNT162b2 or placebo receipt were 0.5% and 0.8% before vaccination, 0.7% and 1.0% at day 4, and 0.7% and 0.5% at 1 month, respectively. In Study C4591007 (n = 1265), elevated troponin I levels were observed in 0.2, 0.4, and 0.2% of 5- to 11-year-old BNT162b2 recipients at baseline and 4 days post-dose 2 and 3, respectively; corresponding values in 12- to 15-year-olds were 0.4, 0.4, and 0.7%. No 5- to 11-year-old placebo recipients had elevated troponin levels. No myocarditis or pericarditis cases or deaths were reported.
Conclusions
Among 5- to < 30-year-olds in both studies, troponin levels were rarely elevated (≤ 1.0%) and similar before and post-vaccination; troponin levels were also similar between BNT162b2 and placebo in 12- to 30-year-old and 5- to 11-year-old recipients in the respective studies. No myocarditis or pericarditis cases were reported. These findings did not provide evidence that BNT162b2 causes troponin elevations. No utility of routine measurement of troponin levels in asymptomatic BNT162b2 recipients was identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Rare cases of myocarditis and pericarditis have occurred in coronavirus disease 2019 (COVID-19) messenger RNA (mRNA) vaccine recipients; however, these are not well-characterized phenomena, and the underlying pathogenesis and risk factors for myocarditis and pericarditis are not well elucidated. |
Serum troponin I levels, as a potential marker of myocardial injury, were assessed in healthy 5- to 30-year-old participants in two clinical trials before and after BNT162b2 vaccination. |
What has been learned from the study? |
Percentages of participants with elevated troponin I levels were similar before and after vaccination and between BNT162b2 and placebo recipients; across both clinical trials, percentages of 5- to 30-year-olds (46.6% of whom were males) with elevated troponin I levels before and after the investigated BNT162b2 doses were ≤ 1.0%. |
These findings did not provide evidence that BNT162b2 vaccination causes troponin elevations, a potential indicator of subclinical myocarditis or pericarditis; therefore, no utility of routine measurement of troponin levels in asymptomatic BNT162b2 recipients was identified. |
Introduction
Myocarditis and pericarditis are inflammatory diseases that typically present as a chest pain syndrome [1,2,3]. Although the extent of injury following myocarditis and pericarditis is highly variable, more severe injury to the myocardium or pericardial sac, albeit rare, can lead to heart failure, arrhythmias, pericardial tamponade, constrictive pericarditis, cardiac arrest, or sudden death [1,2,3].
While the prevalence of myocarditis and pericarditis has been reported to be 22 and 28 cases per 100,000, respectively, these are thought to underestimate the true burden of these conditions [2, 3]. Myocarditis and pericarditis have been caused by drug or vaccine hypersensitivity, autoimmunity, or are viral in origin [2, 3], and abnormal electrocardiogram (ECG), echocardiogram, or troponin findings consistent with myocarditis with no cardiac symptoms have been reported in association with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [4,5,6]. However, subclinical myocarditis (i.e., without cardiac symptoms) is not a well-characterized phenomenon, and a widely accepted definition is lacking.
Rare symptomatic myocarditis and pericarditis cases have been reported following immunization with messenger RNA (mRNA) coronavirus disease 2019 (COVID-19) vaccines (e.g., 17.7 cases per million second mRNA COVID-19 vaccine doses administered in US adolescents and 22.6 cases per million doses in a meta-analysis of data from > 290 million mRNA COVID-19 vaccine doses) [7,8,9,10]. Typically, these have occurred more often in young males, after the second dose, and within days after vaccination [7, 9,10,11,12]. These events are generally mild, and often self-limiting, but deaths have been reported [7, 11, 13].
Differential diagnosis of myocarditis or pericarditis can be challenging because of the range of manifestations [3]. Definitive myocarditis diagnosis has required histologic or immunohistologic confirmation of an endomyocardial biopsy or other heart specimen (e.g., from autopsy) [1]. Because of their invasiveness, biopsies are rarely obtained, and myocarditis diagnosis is usually based on a compatible clinical scenario in tandem with biomarker and imaging studies [1].
Serum biomarkers of cardiac injury, such as troponin I, which is part of a complex of regulatory proteins in striated muscle, are used clinically for suspected myocarditis cases [1, 14]. Troponin I elevations provide evidence of cell degradation, allowing identification of damaged cardiac tissue, such as myocardial necrosis associated with myocarditis [14, 15]. Notably, troponin levels may also be elevated in association with other cardiac and noncardiac processes and conditions, including stress, exercise, rhabdomyolysis, and autoimmunity [14,15,16].
To further characterize the safety profile after receipt of the COVID-19 mRNA BNT162b2 vaccine, including potential risk of myocarditis and pericarditis, here we report additional findings from two phase 3 clinical trials in 5- to 30-year-olds. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Methods
Study Designs and Participants
The first study (C4591031 Substudy B) was a crossover study in healthy 12- to 30-year-olds who had previously received two or three doses of BNT162b2 30 µg, with the last dose administered ≥ 4 months before randomization. Participants were randomized at a 1:1 ratio to receive either BNT162b2 followed by placebo 1 month later (i.e., Sequence 1 [BNT162b2 → placebo]) or placebo followed by BNT162b2 1 month later (i.e., Sequence 2 [placebo → BNT162b2]) (Fig. S1). Randomization was stratified by age (i.e., 12–17, 18–24, and 25–30 years).
The second study (Study C4591007) is a phase 1/2/3 clinical trial evaluating safety, tolerability, immunogenicity, and efficacy of BNT162b2 in healthy children (NCT04816643). Primary results and the design of the phase 2/3 portion of Study C4591007 in 5- to < 12-year-old participants who received up to three doses of BNT162b2 were reported previously [17, 18]. The current analysis reported here is from a group of participants from Study C4591007 who were specifically enrolled for the purpose of troponin I testing. Participants 5–11 years old were initially randomized 2:1 to receive two study vaccinations (BNT162b2 10 μg or placebo) with a third dose of open-label BNT162b2 10 μg later added. Participants 12–15 years old received three study vaccinations of open-label BNT162b2 30 μg. Open-label BNT162b2 30 μg was administered because the vaccine had previously received emergency use authorization (EUA) in this age group [19]. The BNT162b2 dose levels administered were based on age at time of administration of the specific vaccine dose.
Eligibility criteria for both studies are summarized in the Supplementary Material; notably, no specific cardiac inclusions or exclusions were included in either study.
Ethical Approval
Both studies were conducted in accordance with the protocols and consensus ethical principles derived from international guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use good clinical practice guidelines, and other applicable laws and regulations, including privacy laws. Participants or their legally authorized representatives provided informed consent, and participant assent if applicable, for enrollment in the studies. A listing of Independent Ethics Committees or Institutional Review Boards is provided in the Supplementary Material.
Interventions and Blinding
In C4591031 Substudy B, BNT162b2 30 µg and placebo (0.9% sodium chloride [NaCl] for injection) were administered intramuscularly. In Study C4591007, 5- to < 12-year-old participants received BNT162b2 10 µg or placebo (0.9% NaCl for injection) intramuscularly, and 12- to 15-year-old participants received open-label BNT162b2 30 µg intramuscularly.
In both studies, study staff receiving, storing, dispensing, preparing, and administering the study interventions were unblinded. All other study and site personnel (apart from those who received open-label BNT162b2 in Study C4591007), including the investigator, investigator staff, and participants, were blinded to study intervention assignments. Individuals who evaluated participant safety were blinded.
Assessments and Endpoints
In both studies, troponin I assessments were conducted to evaluate whether elevated troponin I levels (as assessed by frequency of elevated measurements), potentially indicative of subclinical myocarditis, occurred after BNT162b2 vaccination. Blood samples were collected for troponin testing. In Study C4591031 Substudy B, samples were obtained before each vaccination, 4 days after each vaccination, and 1 month after the second vaccination. Details on the timing for assessment of troponin I levels in Study C4591007 are further clarified in the Supplementary Material.
Samples were analyzed using the Abbott ARCHITECT STAT High Sensitivity Troponin-I assay. An elevated troponin I result was defined as > 35 ng/l in males and > 17 ng/l in females. These values were established as the 99th percentile cutoff in a reference range study conducted by the assay manufacturer in healthy 21- to 75-year-olds [20]. Troponin I levels were also assessed according to age group, sex, and troponin value range.
In both studies, confirmed myocarditis or pericarditis diagnoses after vaccination were defined as adverse events (AEs) of special interest (AESIs). Any participant reporting acute chest pain, shortness of breath, palpitations, or any other symptoms potentially indicative of myocarditis or pericarditis within 4 weeks after study vaccination was to be evaluated for possible myocarditis or pericarditis, preferably by a cardiologist. In addition to clinical evaluation, an ECG and troponin level measurement (besides protocol-specified measurements described previously) were to be performed, followed by cardiac imaging (echocardiogram and/or cardiac magnetic resonance imaging [MRI]) if myocarditis or pericarditis was suspected based on initial evaluation.
Safety evaluations in C4591031 Substudy B reported here also included frequency of local reactions and systemic events occurring up to 7 days after vaccination.
Statistics
C4591031 Substudy B (Participants 12–30 Years of Age)
Analyses were conducted in the safety population, which included all participants who received ≥ 1 dose of study intervention. Analysis of troponin I levels was limited to participants with valid and determinate troponin I results from the blood sample collected within the protocol-defined window at each visit after vaccination.
All objectives are descriptive. Statistics for categorical variables included percentages and 95% confidence intervals (CIs) where applicable. Exact 95% CIs for binary endpoints for each group were computed using the Clopper–Pearson method, and for between-group comparisons of binary endpoints, the 95% CI for the between-group difference in proportions was calculated using the Miettinen and Nurminen method. For within-group comparison of binary endpoints, the two-sided 95% CI for the difference in percentages was calculated using the adjusted Wald interval.
Study C4591007 (Participants 5–15 Years of Age)
All objectives are descriptive. Counts and percentages of participants with elevated troponin I levels at baseline and after each dose of vaccine with the associated Clopper–Pearson 95% CIs are provided.
Results
Participants
C4591031 Substudy B (Participants 12–30 Years Old)
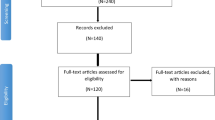
The study was conducted at sites in Germany, South Africa, and the United States from December 20, 2021, to November 29, 2022. Participant disposition is summarized in Fig. 1A; 1485 participants received study intervention and comprised the safety population (Sequence 1 [BNT162b2 → placebo], n = 753; Sequence 2 [placebo → BNT162b2], n = 732).
Disposition of participants in a C4591031 Substudy B (12–30 years of age), b in Study C4591007 (5– < 12 years of age), and c in Study C4591007 (12–15 years of age). aTwo participants (0.1%) were randomized to Sequence 2 (placebo → BNT162b2) in error and were withdrawn from the study before receiving the study vaccine. bOne participant randomized to Sequence 1 (BNT162b2 → placebo) received BNT162b2 instead of placebo for their second vaccination. cOne participant received BNT162b2 10 µg as dose 1 because of a dosing error, and BNT162b2 30 µg was received for dose 2 and dose 3. In C4591031 Substudy B, participants received BNT162b2 at the 30-µg dose level. In Study C4591007, the decrease in the number of participants who received placebo as dose 2 and with no participants receiving placebo as dose 3 is a result of the emergency use authorization. In Study C4591007, 149 participants were unblinded after the first vaccination, 110 were unblinded after the second vaccination, and one participant was not unblinded but lost to follow-up after the first vaccination
Demographic characteristics in the two vaccine sequence groups were balanced (Table 1). Overall, 40.7% of participants were male, 66.3% White, 21.1% Black, and 78.9% non-Hispanic/non-Latino. Median time between the last prior dose of BNT162b2 (received before the study) and the first study vaccination was 8.9 months; 22.4% of participants were obese (≥ 16 years old, 24.5%; 12–15 years old, 13.5%). Medical history included myocardial infarction in one participant, occurring before study enrollment; two participants with a history of obesity had a medical history of fatty liver disease.
Study C4591007 (Participants 5–15 Years Old)
This portion of the study was conducted at sites in Mexico, Spain, and the United States from October 2021 to December 2023. Disposition of 5- to < 12-year-old participants is summarized in Fig. 1B. The safety population of 5- to < 12-year-old participants included 778 (BNT162b2 10 µg, n = 518; placebo, n = 260) of the 784 randomized participants; six randomized participants did not receive study vaccination and were excluded from the safety population. In accordance with protocol allowance and because of the two-dose primary series EUA for BNT162b2 10 μg for 5- to < 12-year-olds, participants who originally received placebo were unblinded and offered the opportunity to receive BNT162b2. Therefore, percentages of participants who received placebo declined from the first to second dose (99.6–42.1%), with no participants receiving placebo as dose 3. Eleven participants in the BNT162b2 group were ≥ 12 years old at the time of dose 3 administration and therefore received the age-appropriate 30-µg dose level. Disposition of 12- to 15-year-old participants who received open-label BNT162b2 30 µg is summarized in Fig. 1C. The safety population of 12- to 15-year-old participants included 487 of the 507 assigned participants; one participant did not receive study vaccination and 19 participants’ parent/guardian did not provide compliant informed consent; these participants were therefore excluded from the safety population.
Demographic characteristics of 5- to < 12-year-old participants are shown in Table 1 and were balanced across the BNT162b2 and placebo groups. Overall, 52.2% of 5- to < 12-year-old participants were male, 82.8% White, 88.9% non-Hispanic/non-Latino, 14.7% SARS-CoV-2 positive at baseline, and 10.2% obese. Demographic characteristics of 12- to 15-year-old participants are also shown in Table 1. Overall, 55.6% of 12- to 15-year-old participants were male, 46.8% White, 86.7% Hispanic/Latino, and 21.4% obese. In contrast to 5- to < 12-year-old participants, most participants in this age group were SARS-CoV-2positive at baseline (88.7 vs. 14.7%), reflecting the longer duration of enrollment for 12- to 15-year-old participants encompassing the period when Omicron sublineages were predominant.
Troponin I Levels
C4591031 Substudy B (Participants 12–30 Years Old)
The data after BNT162b2 or placebo administration were summarized by combining data from participants in Sequence 1 (BNT162b2 → placebo) and Sequence 2 (placebo → BNT162b2), thereby providing the overall number of elevated troponin I results in each group (results by vaccine sequence are provided in Table S1). Before receipt of BNT162b2 or placebo, seven (0.5%) and 11 (0.8%) participants, respectively, had elevated troponin I results (Fig. 2A). At 4 days after receipt of BNT162b2 or placebo, nine (0.7%) and 14 (1.0%) participants, respectively, had elevated troponin I results, and the difference in the percentage of participants with elevated troponin I results between the BNT162b2 and placebo groups was −0.5% (95% CI −1.1%, 0.2%). One month after receipt of BNT162b2 or placebo, nine (0.7%) and seven (0.5%) participants, respectively, had elevated troponin I results; the difference in the percentage of participants with elevated troponin I results between the BNT162b2 and placebo groups was 0.2% (95% CI −0.3%, 0.7%).
Percentages of elevated troponin I results after receipt of BNT162b2 and after receipt of placebo were similar across age groups and by sex at all time points (Fig. 3). After receipt of BNT162b2 or placebo, percentages of elevated troponin I results were generally higher in younger compared with older age groups and in males compared with females. Additionally, there were similar percentages of participants with elevated troponin I results before and after receipt of BNT162b2 or placebo (Table S2). Results by troponin value range are summarized in Fig. S2.
Study C4591007 (Participants 5–15 Years Old)
Four 5- to < 12-year-old participants had elevated troponin I levels at any measured time point, all of whom received BNT162b2. One female participant (0.2%; 95% CI 0.0, 1.1) who received BNT162b2 10 µg had an elevated troponin value range of > 17–35 ng/ml at baseline (Fig. 2B). This participant did not have subsequent elevated troponin I levels after dose 2 or 3. At 4 days after dose 2 of BNT162b2 10 µg, two participants (0.4%; 95% CI 0.0, 1.4) had elevated levels (1 male, troponin value range of > 200 ng/ml; 1 female, troponin value range of > 17–35 ng/ml); neither had elevated levels at baseline nor reported symptoms of potential myocarditis or pericarditis, and additional troponin I levels are not available for these participants because neither received dose 3 (1 withdrew from study and 1 lost to follow-up). At 4 days after dose 3 of BNT162b2 10 µg, one male participant (0.2%; 95% CI 0.0, 1.3) had an elevated troponin I level (troponin value range > 50–100 ng/ml); this participant did not have elevated levels at the other time points. No participant in the placebo group had an elevated troponin I result.
Seven 12 to 15-year-old participants who received open-label BNT162b2 30 µg had elevated troponin I results and none reported symptoms of potential myocarditis or pericarditis. Two male participants (0.4%; 95% CI 0.0, 1.5) had an elevated troponin I value range of > 50–100 ng/ml at baseline, with no further elevated levels at the other time points (Fig. 2B). At 4 days after dose 2, two participants (0.4%; 95% CI 0.1, 1.5) had elevated levels (1 male, troponin value range > 35–50 ng/ml; 1 female, troponin value range > 17–35 ng/ml); neither had elevated levels at the other time points. At 4 days after dose 3, three participants (0.7%; 95% CI 0.1, 2.1) had elevated levels (1 male, troponin value range > 35–50 ng/ml; 1 male, troponin value range > 50–100 ng/ml; 1 female, troponin value range > 35–50 ng/ml); none had elevated levels at the other time points. Results by sex and troponin value range are provided in Table S3.
Adverse Events
C4591031 Substudy B (Participants 12–30 Years Old)
No clinical cases of myocarditis or pericarditis were reported. AESIs reported in participants after receiving BNT162b2 30 µg included COVID-19 (8 [0.6%]), dyspnea (2 [0.1%]), chest pain (2 [0.1%]), tachycardia (1 [< 0.1%]), and chest discomfort (1 [< 0.1%]; Table S4). AESIs reported in participants after receiving placebo included COVID-19 (2 [0.1%]) and chest discomfort (1 [< 0.1%]).
Seven participants (4 after receipt of BNT162b2; 3 after receipt of placebo) underwent evaluation for potential myocarditis or pericarditis based on protocol-defined symptoms (i.e., acute chest pain, shortness of breath, palpitations, or any other symptoms potentially indicative of myocarditis or pericarditis) in the 4 weeks after vaccination. Following investigations including troponin (I and T) levels, ECG, echocardiogram, and cardiac MRI, none were determined to be myocarditis or pericarditis. Of these seven participants, four (1 after receipt of BNT162b2 30 µg; 3 after receipt of placebo) had normal troponin results that were assessed by a local laboratory. Troponin results for the other three participants were not reported.
One participant experienced chest discomfort and dyspnea on the same day of BNT162b2 vaccination that were assessed by the investigator as related to study vaccination, with an abnormal ECG (heart rate 109 bpm; ST elevation) the next day. The echocardiogram and cardiac MRI were normal. Both events were reported as resolved after 2 days. The protocol-specified day 4 troponin level made available at the end of the study was 53.2 ng/l (prevaccination level was < 3.5 ng/l).
Frequencies of local reactions and systemic events were consistent with that reported in other clinical trials assessing additional BNT162b2 doses. No new safety or tolerability signals were identified.
Study C4591007 (Participants 5–15 Years Old)
No 5- to < 12-year-old or 12- to 15-year-old participants in the troponin group with elevated troponin I levels at baseline or after vaccination reported symptoms of potential myocarditis or pericarditis. Six participants (2 male; 4 female) reported symptoms within 4 weeks after a dose of vaccine that prompted evaluation for potential myocarditis or pericarditis. These participants reported chest pain (n = 5), shortness of breath (n = 3), and palpitations (n = 3); symptoms resolved in all participants. Three of the six participants had troponin evaluations and four of the six participants had ECG performed; all results were normal. One of the six participants had cardiac MRI performed with abnormal results (i.e., peribronchial parahilar linear interstitial opacities suggestive of reactive airway disease/viral infection) but no cardiac abnormalities reported. No confirmed myocarditis or pericarditis cases were observed in either age group.
Discussion
Randomized controlled trials and ongoing real-world surveillance support the favorable safety and tolerability profile of COVID-19 mRNA vaccines, including BNT162b2, among children, adolescents, and adults [9, 17,18,19, 21, 22]. However, rare cases of myocarditis and pericarditis have occurred following receipt of mRNA vaccines while an increased risk of myocarditis and pericarditis has not been confirmed to date with other COVID-19 vaccine platforms [7, 8]. In an analysis of US surveillance data reported to the Vaccine Adverse Event Reporting System (VAERS; a passive vaccine safety surveillance system) up to May 2022 among the > 32 million BNT162b2 vaccine doses administered to 12- to 17-year-olds, there were 20,240 AEs reported in this age group and 17.7 cases of myocarditis per million second doses administered, with 90% of cases occurring in males and 77% fully recovered at the time of reporting [9]. In another report from the US Centers for Disease Control and Prevention of VAERS data to October 2022 on booster doses with either the bivalent Omicron BA.4/BA.5 BNT162b2 or bivalent mRNA-1273 vaccine, at which time 22.6 million doses (bivalent BNT162b2, 14.4 million doses in ≥ 12-year-olds; bivalent mRNA-1273, 8.2 million doses in ≥ 18-year-olds) were administered, 5542 AEs (bivalent BNT162b2, n = 2928; bivalent mRNA-1273, n = 2615) were reported among those 12 years and older who received a bivalent booster, among which there were five reports of myocarditis (bivalent BNT162b2, n = 3; bivalent mRNA-1273, n = 2) and four of pericarditis (bivalent BNT162b2, n = 1; bivalent mRNA-1273, n = 3); three and four of these reports of myocarditis and pericarditis, respectively, were confirmed by medical review [23]. Additionally, in a meta-analysis comparing the incidence of myocarditis and pericarditis following COVID-19 vaccine receipt to that of receipt of non-COVID-19 vaccines, the risk in the general population after COVID-19 vaccination was low (18.2 cases [95% CI 10.9, 30.3] per million vaccine doses) and did not differ significantly to the risk in individuals who received vaccines against other diseases (56.0 cases [95% CI 10.7, 293.7] per million vaccine doses) [10]. These surveillance data and meta-analysis indicate that the occurrence of reported myocarditis and pericarditis cases following mRNA COVID-19 vaccination is extremely rare, which has implications for the ability to detect cases in typical clinical trial population sizes.
Reported cases of symptomatic myocarditis and pericarditis following COVID-19 mRNA vaccination have occurred more often in young males, after the second vaccine dose, and soon after vaccination [7, 10,11,12]. These events are generally mild and often self-limiting [7, 11]; however, serious outcomes, including fatalities, have occurred [13]. These are not well-characterized phenomena, and underlying pathogenesis and risk factors for myocarditis and pericarditis are not well elucidated. Therefore, further characterization of markers potentially associated with clinical or subclinical injury to better inform potential pathogenesis and risk factors for myocarditis and pericarditis following mRNA COVID-19 vaccination is critical.
Here we report safety findings from two clinical trials in 5- to 30-year-olds who had BNT162b2 vaccination. In the C4591031 Substudy B of 12- to 30-year-old participants who had received two or three previous doses of BNT162b2 30 µg, similar percentages of participants with elevated troponin I levels before and after receipt of a further dose of BNT162b2 or placebo were observed, and no myocarditis or pericarditis cases were reported. In this study, there was no evidence that observed elevations in troponin I were risks for development of myocarditis and pericarditis in younger males and females after receipt of a third or fourth BNT162b2 30-µg dose. Similarly, in Study C4591007 of 5- to 15-year-old participants who received up to three study vaccinations (BNT162b2 10 μg or placebo [5–11-year-olds]) or open-label BNT162b2 30 μg (12–15-year-olds), the percentage of participants with elevated troponin I levels at baseline and after receipt of BNT162b2 dose 2 and dose 3 at the age-appropriate dose level was < 1%, and there were no myocarditis or pericarditis cases.
Notably, the definition of elevated troponin I values in these clinical studies was based on values established in an adult population. In pediatric populations, evidence-based reference standards for troponin I testing are lacking, and few studies have evaluated performance of the assay used in our trials in healthy children and adolescents. The limited available data suggest that age-specific variation in the 99th percentile of troponin I exists, declining from 166.3 ng/l (cord blood) in term infants, to 30.9 ng/l (serum) in children 1–18 years old, and 41.3 ng/l (plasma) in adolescents 10–18 years old [24,25,26]. Additional data are needed to establish robust pediatric reference intervals and to ensure appropriate interpretation of assay-specific troponin I values in children and adolescents.
We found that some participants had elevations of troponin I levels at baseline. Limitations regarding use of troponin I elevations as a marker of subclinical myocarditis or pericarditis should be noted. For instance, other cardiac and noncardiac conditions may be accompanied by elevated troponin I, including stress, exercise, and autoimmunity [14,15,16]. Additionally, because troponin I has both cytosolic and structural distributions, troponin increases can be seen in true myocardial injury (i.e., myocyte necrosis) and may also result from transient leakage from the cytosolic pool [27]. Potential analytical errors or assay interference should also be considered when interpreting the clinical relevance of troponin I elevations [28,29,30].
Other limitations of these studies should be noted. Real-world surveillance data support the rarity of myocarditis and pericarditis cases following COVID-19 mRNA vaccination; therefore, it is not feasible to power clinical trials to detect these cases and serum troponin I elevations were used as a marker of potential cardiac injury, which is consistent with clinical recommendations [1]. It is also recommended that diagnosis of myocarditis is typically based on a compatible clinical scenario with biomarker and imaging studies [1]. In our studies, any participant reporting clinical symptoms potentially indicative of myocarditis or pericarditis within 4 weeks after study vaccination was to be clinically evaluated, and ECG and further troponin level measurement were to be performed, followed by cardiac imaging if myocarditis or pericarditis was suspected based on the initial clinical, ECG, and troponin evaluation. The timing of ECG and cardiac imaging assessments relative to symptom onset is important in ensuring an accurate assessment and may have varied.
In conclusion, in a clinical trial of 12- to 30-year-olds, no clinically significant differences in percentages of participants with elevated troponin I levels before and after BNT162b2 or placebo were observed. Across two clinical trials, the percentages of 5- to 30-year-old participants with elevated troponin I levels before and after the investigated doses of BNT162b2 were ≤ 1.0%, and there were no confirmed myocarditis or pericarditis cases. Additionally, across the full age spectrum under study, elevated troponin results were also observed in placebo recipients and at baseline for BNT162b2 and placebo recipients. These findings did not provide evidence that BNT162b2 vaccination causes troponin elevations, a potential indicator of subclinical myocarditis or pericarditis. The findings do not provide convincing support that routine troponin measurements help identify subclinical myocarditis or pericarditis cases in asymptomatic BNT162b2 recipients. It is anticipated that continued safety data surveillance following COVID-19 mRNA vaccine administration, including those targeting more contemporary circulating variants, will enable further characterization of postvaccination myocarditis and pericarditis events.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
Bozkurt B, Colvin M, Cook J, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016;134:e579–646.
Chiabrando JG, Bonaventura A, Vecchié A, et al. Management of acute and recurrent pericarditis: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:76–92.
Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40:1499–511.
Daniels CJ, Rajpal S, Greenshields JT, et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the big ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021;6:1078–87.
Long B, Brady WJ, Bridwell RE, et al. Electrocardiographic manifestations of COVID-19. Am J Emerg Med. 2021;41:96–103.
Piccioni A, Brigida M, Loria V, et al. Role of troponin in COVID-19 pandemic: a review of literature. Eur Rev Med Pharmacol Sci. 2020;24:10293–300.
Gao J, Feng L, Li Y, et al. A systematic review and meta-analysis of the association between SARS-CoV-2 vaccination and myocarditis or pericarditis. Am J Prev Med. 2023;64:275–84.
Guo BQ, Li HB, Yang LQ. Incidence of myopericarditis after mRNA COVID-19 vaccination: a meta-analysis with focus on adolescents aged 12–17 years. Vaccine. 2023;41:4067–80.
Hesse EM, Hause A, Myers T, et al. COVID-19 vaccine safety first year findings in adolescents. Pediatrics. 2023. https://doi.org/10.1542/peds.2022-060295.
Ling RR, Ramanathan K, Tan FL, et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med. 2022;10:679–88.
Pillay J, Gaudet L, Wingert A, et al. Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review. BMJ. 2022;378: e069445.
Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA Vaccine against COVID-19 in Israel. N Engl J Med. 2021;385:2140–9.
Verma AK, Lavine KJ, Lin CY. Myocarditis after COVID-19 mRNA vaccination. N Engl J Med. 2021;385:1332–4.
Wu AHB. Release of cardiac troponin from healthy and damaged myocardium. Front Lab Med. 2017;1:144–50.
Goldmann BU, Christenson RH, Hamm CW, Meinertz T, Ohman EM. Implications of troponin testing in clinical medicine. Curr Control Trials Cardiovasc Med. 2001;2:75–84.
McCarthy CP, Raber I, Chapman AR, et al. Myocardial injury in the era of high-sensitivity cardiac troponin assays: a practical approach for clinicians. JAMA Cardiol. 2019;4:1034–42.
Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 COVID-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386:35–46.
Simões EAF, Klein NP, Sabharwal C, et al. Immunogenicity and safety of a third COVID-19 BNT162b2 mRNA vaccine dose in 5- to 11-year olds. J Pediatr Infect Dis Soc. 2023;12:234–8.
Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N Engl J Med. 2021;385:239–50.
US Food and Drug Administration. 510(k) Substantial Equivalence Determination Decision Summary. Food and Drug Administration. https://www.accessdata.fda.gov/cdrh_docs/reviews/K191595.pdf. Accessed 26 Sept 2023.
Moreira ED Jr, Kitchin N, Xu X, et al. Safety and efficacy of a third dose of BNT162b2 COVID-19 vaccine. N Engl J Med. 2022;386:1910–21.
Santi Laurini G, Montanaro N, Broccoli M, Bonaldo G, Motola D. Real-life safety profile of mRNA vaccines for COVID-19: an analysis of VAERS database. Vaccine. 2023;41:2879–86.
Hause AM, Marquez P, Zhang B, et al. Safety monitoring of bivalent COVID-19 mRNA vaccine booster doses among persons aged ≥12 years—United States, August 31–October 23, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1401–6.
Bohn MK, Adeli K. Comprehensive pediatric reference limits for high-sensitivity cardiac troponin I and NT-proBNP in the CALIPER cohort. J Appl Lab Med. 2023;8:443–56.
Kavsak PA, Rezanpour A, Chen Y, Adeli K. Assessment of the 99th or 97.5th percentile for cardiac troponin I in a healthy pediatric cohort. Clin Chem. 2014;60:1574–6.
Caselli C, Cangemi G, Masotti S, et al. Plasma cardiac troponin I concentrations in healthy neonates, children and adolescents measured with a high sensitive immunoassay method: high sensitive troponin I in pediatric age. Clin Chim Acta. 2016;458:68–71.
Rahman A, Broadley SA. Review article: elevated troponin: diagnostic gold or fool’s gold? Emerg Med Australas. 2014;26:125–30.
Laguë M, Turgeon PY, Thériault S, Steinberg C. A false-positive troponin assay leading to the misdiagnosis of myopericarditis. CMAJ. 2022;194:E456–9.
Apple FS, Sandoval Y, Jaffe AS, Ordonez-Llanos J. Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem. 2017;63:73–81.
Januzzi JL Jr, McCarthy CP. Cardiac troponin and the true false positive. JACC Case Rep. 2020;2:461–3.
Acknowledgements
The authors thank the participants and their family members for their contributions to these studies. The authors also acknowledge the members of the Data Monitoring Committee (Dr. Jonathan Zenilman, Dr. Kathryn Edwards, Dr. Robert Belshe, Dr. Lawrence Stanberry, Dr. Steve Self, Dr. Robert Heine, and Dr. Heather Lipkind) and the members of the C4591031 Substudy B and Study C4591007 teams, including the site staff. The authors also acknowledge Dr. Efrén Alberto Sánchez Campos for his contributions to the study.
Medical Writing/Editorial Assistance.
The authors thank Tricia Newell, PhD, of ICON (Blue Bell, PA, USA), who wrote the first draft under direction from the authors, with funding from Pfizer Inc.
Funding
Sponsored by BioNTech, funded by Pfizer and BioNTech. Pfizer is funding the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Consortia
Contributions
Timothy E. Albertson, Caitlin Hansen, and Juleen Gayeed were involved in data collection, data analysis, drafting of the manuscript, and approved the final manuscript for submission. Smiti Bihari, Georgina Keep, Ye Feng, and Charu Sabharwal were involved in study conception/design, data collection, data analysis, drafting of the manuscript, and approved the final manuscript for submission. Xia Xu, Federico J. Mensa, Hua Ma, and Nicholas Kitchin were involved in study conception/design, data analysis, drafting of the manuscript, and approved the final manuscript for submission. J. Abraham Simón-Campos, Kenneth Koury, Susan Mather, Claudia Ana Ianos, Annaliesa S. Anderson, Özlem Türeci, Uğur Şahin, William C. Gruber, and Alejandra Gurtman were involved in data analysis, drafting of the manuscript, and approved the final manuscript for submission. Michael E. Dever, Jose F. Cardona, Essack Mitha, Jeffrey B. Baker, and Islamiat Oladipupo were involved in data collection, drafting of the manuscript, and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Conflict of Interest
Timothy E. Albertson: None. J. Abraham Simón-Campos: AstraZeneca/Other-personal fees; Pfizer/Other-personal fees; Roche/Other-personal fees. Michael E. Dever: None. Jose F. Cardona: None. Essack Mitha: None. Jeffrey B. Baker: Pfizer/BioNTech/Other-received payment as a principal investigator for Study C4591007. Federico J. Mensa: BioNTech/Other-employee/Stock. Özlem Türeci: BioNTech/Board membership and board member/Advisor/Consultant/Honoraria/Intellectual property/Patents/Other-Cofounder and Chief Medical Officer/Stock. Uğur Şahin: BioNTech/Other-employee and board member/Stock. Caitlin Hansen, Smiti Bihari, Juleen Gayed, Xia Xu, Georgina Keep, Islamiat Oladipupo, Ye Feng, Hua Ma, Kenneth Koury, Susan Mather, Claudia Ana Ianos, Annaliesa S. Anderson, William C. Gruber, Charu Sabharwal, and Nicholas Kitchin are current or former employees of Pfizer and may hold stock or stock options.
Ethical Approval
Both studies were conducted in accordance with the protocols and consensus ethical principles derived from international guidelines, including the Declaration of Helsinki and CIOMS International Ethical Guidelines, applicable ICH GCP Guidelines, and other applicable laws and regulations, including privacy laws. Participants or their legally authorized representatives provided informed consent, and participant assent if applicable, for enrollment in the studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Albertson, T.E., Hansen, C., Bihari, S. et al. Serum Troponin I Assessments in 5- to 30-Year-Olds After BNT162b2 Vaccination. Infect Dis Ther 13, 699–714 (2024). https://doi.org/10.1007/s40121-024-00927-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00927-0