Abstract
Introduction
Molnupiravir is an orally available prodrug of N-hydroxycytidine that received special approval for emergency treatment of coronavirus disease 2019 (COVID-19) in Japan in December 2021 and full approval in April 2023. To assess the real-world safety and effectiveness of molnupiravir in Japanese patients with COVID-19, we conducted nationwide post-marketing surveillance to collect data at registered institutions in Japan.
Methods
The surveillance data were collected from December 27, 2021, to May 2, 2023. All reported adverse events were collected for safety analysis. Adverse drug reactions (ADRs) were assessed by the treating physicians. Effectiveness was assessed by the composite of hospitalization or all-cause death in outpatients and the composite of oxygen/mechanical ventilation initiation or all-cause death in inpatients. The observation period was from molnupiravir initiation through day 29.
Results
Of 3214 patients enrolled in the survey, 3179 were analyzed for safety. At baseline, 52.31% (1663/3179) of patients were male, the median (range) age was 69.0 (18–107) years, 82.38% (2619/3179) received COVID-19 vaccines, and 95.72% (3043/3179) had risk factors for severe COVID-19 illness. COVID-19 severity at baseline was mild in 86.44% (2748/3179) and moderate I in 10.22% (325/3179). A total of 205 ADRs occurred in 5.50% (175/3179) of patients; ADRs that occurred in > 0.5% of patients were diarrhea (1.86% [59/3179]) and rash (0.69% [22/3179]). Seven serious ADRs were reported in seven patients. In the effectiveness analysis population, the incidence of all-cause death through day 29 was 1.14% (34/2988), and the incidence of death through day 29 related to COVID-19 was 0.40% (12/2988). The cumulative incidence of the composite endpoint was 2.34% (47/2006) in outpatients and 4.60% (38/826) in inpatients.
Conclusions
This large-scale survey showed that molnupiravir was safe and effective in real-world settings in highly vaccinated Japanese patients with COVID-19, including older patients and those with comorbidities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Molnupiravir is an orally available prodrug of N-hydroxycytidine that received special approval for emergency treatment of COVID-19 in Japan in December 2021 and full approval in April 2023. |
This post-marketing surveillance was conducted to assess the real-world safety and effectiveness of molnupiravir after its marketing approval for COVID-19 treatment in Japan, targeting all patients treated with molnupiravir at registered institutions in Japan from December 27, 2021, to May 2, 2023, the period during which Omicron replaced Delta as the dominant variant. |
What was learned from the study? |
This was a large-scale survey of molnupiravir use in Japanese patients with COVID-19 in real-world settings. |
Diarrhea (1.86% [59/3179]) and rash (0.69% [22/3179]) were the most common ADRs, and the cumulative incidence of hospitalization or all-cause death in outpatients and oxygen/mechanical ventilation initiation or all-cause death in inpatients from molnupiravir initiation through day 29 was 2.34% (47/2,006) and 4.60% (38/826), respectively. |
During the Omicron wave, molnupiravir was found to be safe and effective in highly vaccinated Japanese patients with COVID-19 in real-world settings, including older patients and those with comorbidities. |
Introduction
Since the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) in January 2020 (1), multiple vaccines and antiviral drugs using different existing and new technologies have been developed to fight against the responsible virus (severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) and its variants. With the rise in number of patients treated in outpatient settings, there is a growing need for oral antiviral treatments that are effective against COVID-19 infections caused by new emerging variants.
Molnupiravir is a broad-spectrum antiviral, an orally available prodrug of N-hydroxycytidine developed by Drug Innovation Ventures at Emory, LLC (2,3,4,5,6,7). Molnupiravir received special approval for emergency treatment of COVID-19 in December 2021 in Japan (8) and full approval in April 2023 after the review of supporting documents (9). In a phase 3, double-blind, randomized, placebo-controlled component of the MOVe-OUT study, molnupiravir reduced the risk of hospitalization or death through day 29 (7.3 vs. 14.1%) in at-risk, unvaccinated outpatients with mild-to-moderate COVID-19, if started within 5 days of symptom onset (10). In a UK-based, open-label, randomized controlled study comparing molnupiravir plus usual care versus usual care alone (PANORAMIC), which targeted highly vaccinated outpatients with COVID-19 at increased risk of adverse outcomes, molnupiravir did not reduce the risk of hospitalizations or death (which was low at 1% in both groups) but was associated with reduced time to first reported recovery and alleviation of all symptoms (11). Molnupiravir also showed a favorable safety profile with no significant differences in the incidence of adverse events (AEs) or intervention-related AEs (10, 11).
The real-world evidence also supports the safety and effectiveness of molnupiravir. A cohort study of patients who received a diagnosis of COVID-19 at the Cleveland Clinic in the United States from April 2022 to February 2023 (during the Omicron wave: variants from BA.2 to XBB/XBB.1.5) showed that the adjusted hazard ratio of death for patients with molnupiravir compared to no treatment was 0.23 (95% confidence interval [CI] 0.16–0.34), and that of hospitalization or death was 0.59 (95% CI 0.53–0.66) (12). An observational study of 1,074,856 outpatients with COVID-19 in Hong Kong showed that molnupiravir use was associated with a lower risk of death than non-use with a hazard ratio of 0.76 (95% CI 0.61–0.95) and a similar risk of hospitalization with a hazard ratio of 0.98 (95% CI 0.89–1.06) (13). A retrospective cohort study of 920 patients with mild-to-moderate COVID-19 who were admitted to hospitals in Fukushima Prefecture in Japan showed that the clinical deterioration rate after admission was significantly lower in molnupiravir users than in non-users (3.90 vs. 8.40%, P = 0.034) (14). In a study using electronic health records to emulate a randomized target study, molnupiravir was associated with fewer hospital admissions or death at 30 days with a relative risk of 0.72 (95% CI 0.64–0.79) compared to no treatment in adults with SARS-CoV-2 infection in the community during the Omicron wave (15).
As of May 9, 2023, the cumulative number of confirmed COVID-19 cases was about 33.8 million in Japan, and the cumulative number of deaths due to COVID-19 was 74,694, mainly consisting of people older than 60 years (16). Considering that Japan has the world’s highest proportion of people aged 65 and older (28.9% in 2021) (17), comprehensive data collection is imperative to ascertain if molnupiravir is safe and effective in Japanese patients with COVID-19 in real-world settings, including older patients and those with multiple comorbidities such as chronic kidney disease (CKD).
To assess the real-world safety and effectiveness of molnupiravir in Japanese patients with COVID-19, we conducted nationwide post-marketing surveillance (PMS) to collect data on patients treated with molnupiravir at registered institutions in Japan. In the interim analysis, we reported the preliminary safety and effectiveness data in 1031 Japanese patients (18). Here we report the final results of the PMS of molnupiravir use in 3214 Japanese patients.
Methods
Survey Design and Patients
This PMS employed a single-arm observational study design. The primary aim of this PMS was to confirm the safety of molnupiravir in Japanese patients with infection caused by SARS-CoV-2 as early as possible after approval, and secondarily to confirm effectiveness. The final report includes data collected from December 27, 2021, to May 2, 2023, when Omicron replaced Delta as the dominant SARS-CoV-2 variant in Japan (19).
This survey was conducted in accordance with the Ministry of Health, Labour, and Welfare (MHLW) ordinance for Good Post-Marketing Study Practice (GPSP) and the Pharmaceutical and Medical Device Act. This survey complied with the Helsinki Declaration of 1964 and its later amendments, and all other applicable regulations. Institutional review board (IRB) approval was not mandatory for this survey, according to the GPSP.
Molnupiravir was started within 5 days of the onset of COVID-19 symptoms in patients with COVID-19 who are at risk for severe COVID-19 outcomes, as stated in the Japanese package insert (20). To reduce selection bias, we collected data using the continuous registration method from patients who received molnupiravir at registered medical institutions and from whom informed consent was obtained regardless of the timing of molnupiravir initiation. After explaining to the patients or their legal representatives the objective of the survey, the information to be collected, and how the survey results would be used, the treating physicians obtained informed consent from the patients or their legal representatives.
The following data were collected: baseline patient characteristics, administration status of molnupiravir and other medications, respiratory status, laboratory test results, outcome at the last observation day, and AEs. Patients were categorized as outpatients if they started molnupiravir in the outpatient settings, inpatients if they developed COVID-19 during hospitalization for other reasons or were hospitalized for COVID-19-related reasons, and others if they were nursing home residents or home-visit medical care recipients. The severity of COVID-19 in this survey was classified according to Japanese guidance from MHLW (21) as mild (patients had a peripheral oxygen saturation [SpO2] of ≥ 96%, absence of pneumonia), moderate I (patients had a SpO2 of < 96% and > 93%, presence of pneumonia), moderate II (patients had a SpO2 of ≤ 93% and required oxygen), and severe (patients required ICU admission or mechanical ventilation). Risk factors for severe COVID-19 illness in this survey consisted of older age (≥ 65 years); comorbidities, such as active malignancies, chronic obstructive pulmonary disease, CKD, type 2 diabetes mellitus, hypertension, dyslipidemia, and immunodeficiency after solid-organ transplantation; obesity with a body mass index of ≥ 30 kg/m2; smoking; and other risk factors reported by the treating physicians.
Safety Assessments
All AEs were collected throughout the observation period and coded to primary system organ classes and preferred terms in a Japanese version of the Medical Dictionary for Regulatory Activities (Version 26.0). Adverse drug reactions (ADRs) were AEs for which a causal relationship to molnupiravir was not ruled out by the treating physicians. AEs were classified into mild (AEs not interfering with activities of daily living), moderate (AEs interfering with activities of daily living and requiring therapeutic interventions), and severe (AEs seriously disrupting activities of daily living) by the treating physicians.
Effectiveness Assessments
We assessed the effectiveness of molnupiravir through day 29 from the initiation of treatment in the following patient cohorts: (i) outpatients and (ii) inpatients without oxygen or mechanical ventilation when starting molnupiravir. Outpatient effectiveness was assessed in relation to unscheduled hospitalization, death, and their composites. Inpatient effectiveness was assessed in relation to the initiation of oxygen, mechanical ventilation, death, and their composites.
Statistical Analysis
All statistical analyses were performed using SAS Release 9.4 (SAS Institute, Inc., Cary, NC, USA). Data were shown as n (%) or median (range). For the safety analysis, Fisher’s exact test and Wilcoxon rank-sum test were used to find factors associated with ADRs. Both tests were two-sided, and a P value less than 0.05 was considered statistically significant. For the effectiveness analysis, Kaplan–Meier curves were used to show the cumulative incidence of each event during the observation period. The crude incidence rates per 10,000 person-days with their 95% CIs were also calculated. Univariable logistic regression analyses were performed to obtain the odds ratios (ORs) and 95% CIs between baseline characteristics and outpatient hospitalization or death through day 29. A forest plot was used to represent the effect of each characteristic graphically.
Results
Patient Disposition and Characteristics at Baseline
Of 3214 patients enrolled in the survey, 3179 (outpatients, 67.44% [n = 2144]; inpatients, 30.67% [n = 975]; others, 1.89% [n = 60]) were analyzed for safety (Fig. 1 and Table 1). At baseline, 52.31% (1663/3179) were male, the median (range) age was 69.0 (18–107) years, 82.38% (2619/3179) were vaccinated against SARS-CoV-2 at least once and 77.32% (2458/3179) twice or more, and 95.72% (3043/3179) had risk factors for severe COVID-19 illness (Table 1). COVID-19 symptoms were experienced by 97.48% (3099/3179), and COVID-19 severity was mild in 86.44% (2748/3179). At baseline, 3.59% (114/3179) of patients needed oxygen or mechanical ventilation, and 8.52% (271/3179) had CKD (including 2.99% [95/3179] on hemodialysis).
Administration Status of Molnupiravir and Other Medications
In most patients, molnupiravir was initiated within 5 days of the onset of COVID-19 symptoms (Table 2), with a median of 2 days. Medications other than molnupiravir were used in 25.70% (817/3179) of patients and included symptomatic treatment medications (14.94% [475/3179]), neutralizing antibodies (9.00% [286/3179]), corticosteroids (2.80% [89/3179]), heparins (1.45% [46/3179]), and other antivirals (0.66% [21/3179]).
Safety of Molnupiravir
During the median observation period of 30.0 days, AEs and serious AEs occurred in 10.16% (323/3179) and 2.80% (89/3179) of patients, respectively (Supplementary Table 1). A total of 205 ADRs as assessed by the treating physicians occurred in 5.50% (175/3179) of patients (Table 3). The most common ADRs were diarrhea (1.86% [59/3179]; non-serious, n = 59; serious, n = 0) and rash (0.69% [22/3179]; non-serious, n = 21; serious, n = 1). The most common occurrence of diarrhea was the day1-2 of administration (27/59), and that of rash was the day 3–4 (7/22). Seven serious ADRs were reported in seven patients (COVID-19 [aggravated], n = 2; cardiac failure congestive [aggravated], n = 1; acute respiratory failure, n = 1; hypoxia, n = 1; hepatic function abnormal, n = 1; rash, n = 1) (Supplementary Table 2).
Treatment was completed by 95.88% (3048/3179) of patients and discontinued by 4.12% (131/3179) of patients (Table 4). Overall, 1.64% (52/3179) of ADRs resulted in treatment discontinuation; the most common ADRs that led to treatment discontinuation were diarrhea (n = 12) and rash (n = 11).
Although most patients recovered or were recovering from ADRs, during the observation period, seven patients did not recover from eight events (anemia, n = 2; neutrophil count decreased, n = 2; rash, n = 1; alanine aminotransferase increased, n = 1; blood uric acid increased, n = 1; vertigo, n = 1), and three patients died from three events (hypoxia, n = 1; cardiac failure congestive, n = 1; acute respiratory failure, n = 1) (Table 5). These three patients died at home, and the causal relationships between molnupiravir and their deaths were not fully investigated.
The occurrence of ADRs was not associated with sex, age group (18 to < 65, 65 to < 75, ≥ 75), COVID-19 severity and oxygen/mechanical ventilation use at molnupiravir initiation, comorbidities (including renal or hepatic impairment), risk factors for severe COVID-19 illness, or SARS-CoV-2 vaccination status (Supplementary Table 3).
Effectiveness of Molnupiravir
Of 3179 patients in the safety analysis set, 2988 (outpatients, 67.14% [n = 2006]; inpatients, 30.96% [n = 925]; others, 1.91% [n = 57]) were included in the effectiveness analysis set, and 191 were excluded because (i) they used a dosage or a route that had not been approved (n = 24) and/or (ii) they were not evaluated for effectiveness (n = 168, one patient met both exclusion criteria) (Fig. 1). The incidence of all-cause death through day 29 was 1.14% (34/2988) in the effectiveness analysis population, and the incidence of death through day 29 related to COVID-19 was 0.40% (12/2988) (Supplementary Table 4).
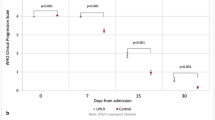
The cumulative incidence of hospitalization (for reasons other than isolation or assessment), all-cause death, and their composite endpoint from molnupiravir initiation through day 29 among outpatients was 2.29% (46/2006), 0.10% (2/2006), and 2.34% (47/2006), respectively. After excluding 99 inpatients (96 patients who used oxygen, two patients on non-intubated mechanical ventilation, and one patient who used unspecified respiratory support at baseline), the cumulative incidence of oxygen initiation, all-cause death, and their composite endpoint from molnupiravir initiation through day 29 among 826 inpatients was 3.75% (31/826), 1.57% (13/826), and 4.60% (38/826), respectively (Fig. 2, Table 6).
Cumulative incidence of events in outpatients and inpatients. Graphs show the cumulative incidence of outpatient hospitalization or all-cause death (a) and inpatient oxygen initiation or all-cause death (b) from molnupiravir initiation through day 29, with magnified insets. aInpatients who used oxygen (n = 96), mechanical ventilation (n = 3), and unspecified respiratory support (n = 1) at baseline are excluded from the analysis
Factors Associated with Outpatient and Inpatient Composite Outcome
Factors associated with outpatient hospitalization or all-cause death through day 29 were as follows: older age (≥ 75 vs. 65 to < 75, OR 5.77, 95% CI 2.21–15.06), more severe COVID-19 disease (Moderate I vs. Mild, OR 5.32, 95% CI 2.63–10.79; Moderate II vs. Mild, OR 6.71, 95% CI 1.49–30.35), more risk factors for severe COVID-19 illness (2 vs. 1, OR 3.84, 95% CI 1.41–10.47; 3 vs. 1, OR 3.63, 95% CI 1.25–10.53; ≥ 4 vs. 1, OR 8.18, 95% CI 2.91–22.95), other medications (Yes vs. No, OR 3.93, 95% CI 2.09–7.37), lower baseline SpO2 (94–95 vs. ≥ 96%, OR 3.25, 95% CI 1.19–8.84; ≤ 93 vs. ≥ 96%, OR 13.59, 95% CI 4.57–40.41), renal impairment (Yes vs. No, OR 9.22, 95% CI 4.68–18.17), and hemodialysis (Yes vs. No, OR 8.11, 95% CI 2.28–28.86) (Fig. 3, Supplementary Table 5).
Factors associated with outpatient hospitalization or all-cause death through day 29 of molnupiravir initiation. Older age, more severe COVID-19 disease, more risk factors for severe COVID-19 illness, other medications, lower baseline SpO2, renal impairment, and hemodialysis are associated with hospitalization or all-cause death of outpatients through day 29 of molnupiravir initiation. BMI body mass index, COVID-19 coronavirus disease 2019, MOV molnupiravir, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, SpO2 peripheral oxygen saturation
Factors associated with inpatient oxygen initiation or all-cause death through day 29 were as follows: baseline SpO2 (94–95 vs. ≥ 96%, OR 2.31, 95% CI 1.07–4.96) and hemodialysis (Yes vs. No, OR 4.14, 95% CI 1.87–9.17) (Supplementary Table 6).
Discussion
This is the largest survey conducted in routine patient care in Japan that provides safety and effectiveness data for 3179 and 2988 patients, respectively, exceeding the number of patients included in the previously published interim analysis (1031 and 884 patients, respectively) (18). The results obtained in this larger population of patients were consistent with those in the interim analysis in terms of the safety and effectiveness data (18). The frequency of ADRs as assessed by the treating physicians was 5.50% (175/3179). Most ADRs were non-serious, and 1.64% (52/3179) of ADRs resulted in treatment discontinuation. The cumulative incidence of outpatient hospitalization or all-cause death from molnupiravir initiation through day 29 was 2.34% (47/2006), and the cumulative incidence of inpatient oxygen initiation or all-cause death from molnupiravir initiation through day 29 was 4.60% (38/826). No new safety concerns were identified in this PMS.
Molnupiravir was first approved for the treatment of COVID-19 in the UK in November 2021 (22) and shortly after in Japan in December 2021. The safety and effectiveness of molnupiravir were evaluated in the international phase 3 (MOVe-OUT) study (10). To our knowledge, the present PMS is the first large scale survey to report the safety and effectiveness of molnupiravir in the real-world setting in Japan during the period when the predominant circulating SARS-CoV-2 variant was Omicron. Compared to the MOVe-OUT study where patients were largely unvaccinated, this survey population was highly vaccinated (82.38% of patients were vaccinated against SARS-CoV-2 at least once), which is comparable to the national COVID-19 vaccination rate in Japan of 78% (23). In addition, people at high risk of progression to severe disease were included: 58.79% of the patients were aged 65 and older, and 95.72% had risk factors for severe COVID-19 illness including being ≥ 65 years, hypertension, smoking history, type 2 diabetes mellitus, and dyslipidemia.
In this survey, molnupiravir was initiated within 5 days of the onset of COVID-19 symptoms in 94.81% of patients, which may reflect the easy accessibility of molnupiravir in Japan and the convenience of oral administration. Molnupiravir also showed good tolerability and adherence in this survey, with a treatment completion rate of 95.88%, which is higher than the reported treatment completion rate of anti-influenza drugs for seasonal influenza in France (82.73% for the inhaled route and 85.73% for the oral route, both of which were given twice daily for five days; which is the same frequency and duration as molnupiravir) (24).
No new safety concerns were identified in this survey. The AE and ADR profiles in this survey were generally consistent with those identified in previous studies (10, 11), the FDA Adverse Event Reporting Systems (25), and are specified in the approved product label. Despite 83.45% of patients in the safety analysis set in this survey having comorbidities, the incidence of ADRs was 5.50% (175/3179), which was lower than that reported in the MOVe-OUT study (8.0%, 57/710) (10). The most common ADR in this survey was diarrhea (1.86% [59/3179]), similar to the MOVe-OUT study (10) and the FDA Adverse Event Reporting Systems (25). In the MOVe-OUT study, diarrhea occurred in 1.7% (12/710) of patients in the molnupiravir group and 2.1% (15/701) of those in the placebo group (10). Notably, diarrhea was non-serious in all 59 patients in this survey, and all (except for one with an unknown outcome) recovered or were recovering with a median time of 5.5 days and 6.5 days, respectively. Rash was the second most common ADR occurring in 0.69% in this survey, which was comparable to the rate observed in the MOVe-OUT study (< 1%) (10), and most of the cases of rash were non-serious. Since COVID-19 is often considered a systemic viral disease that can cause extrapulmonary manifestations such as gastrointestinal tract (26, 27) and cutaneous symptoms (28, 29), some AEs that were deemed to be related to molnupiravir might have been COVID-19 symptoms.
Serious ADRs as assessed by the treating physicians occurred in 0.22% (7/3179) of patients in this survey (Supplementary Table 2). Of 39 fatal cases, 36 deaths were assessed as unrelated to molnupiravir by the treating physicians. Whether there was a causal relationship between molnupiravir and death in three patients who died at home (one from hypoxia, one from cardiac failure congestive, and one from acute respiratory failure) was not assessable due to lack of sufficient information. Considering that in this survey the median age was 69.0 years (35% of patients were ≥ 75 years), and that many patients had underlying diseases such as malignant tumors, CKD, and heart disease in this survey, the small number of fatal cases is particularly noteworthy.
In the MOVe-OUT study, molnupiravir reduced the risk of hospitalization or death through day 29 (7.3 vs. 14.1%; difference in percentage − 6.8, 95% CI − 11.3 to − 2.4) (10). In this survey, molnupiravir treatment resulted in a cumulative incidence of outpatient hospitalization or all-cause death of 2.34% (47/2006) through day 29, which was consistent with the figure of 2.61% (16/612) in the interim analysis (18). In addition, among inpatients treated with molnupiravir and who did not use oxygen or mechanical ventilation at baseline, oxygen initiation or all-cause death occurred in only 4.60% (38/826), and none required mechanical ventilation through day 29. According to the MHLW reports, the mortality rate due to COVID-19 regardless of treatment status was higher in older patients in Japan: 0.28% (573/204,031) in patients aged 60 to 79 years, and 2.41% (1828/75,956) in patients aged ≥ 80 years (January–April and July–September 2022) (16, 30). In the effectiveness analysis set of the present PMS, the all-cause mortality rate was 3.16% (24/759) in patients aged ≥ 80 years (Supplementary Table 4). It is noteworthy that the mortality rate was 0.57% (2/350) among outpatients aged ≥ 80 years and 0.00% (0/1633) among outpatients aged < 80 years. The mortality rate was 4.48% (16/357) in inpatients aged ≥ 80 years and 11.54% (6/52) in others aged ≥ 80 years. Furthermore, the mortality rate due to COVID-19 was 0.00% (0/2006) in outpatients and 1.08% (10/925) in inpatients. While a direct comparison is not possible owing to differences in patient backgrounds, such as inpatient to outpatient ratio, and proportion of patients with comorbidities, our findings are consistent with previous reports showing the effectiveness of molnupiravir in reducing severe COVID-19 outcomes in patients aged > 80 years (11, 31).
In this survey, the risk of being hospitalized or dying through day 29 was higher for outpatients with baseline characteristics including older age (≥ 75), severe COVID-19 disease at the start of treatment, multiple risk factors for progression to severe disease, other medications, lower baseline SpO2, renal impairment, and hemodialysis. At the same time, the occurrence of ADRs in this survey was unrelated to patient characteristics (e.g., age and comorbidities). Even though these patient characteristics generally have a significant impact on the incidence of ADRs (32), findings from this survey show that older patients and those with comorbidities, such as hepatic or renal impairment (including those on hemodialysis), can be treated safely with molnupiravir without dose adjustment in clinical practice. It should also be noted that about one in four patients were taking medications to treat or alleviate symptoms of COVID-19 other than molnupiravir. This is probably because molnupiravir has no known drug–drug interactions that require temporary discontinuation or dose adjustment of other medications. Other observational studies have also shown the safety and effectiveness of molnupiravir among patients on hemodialysis (33,34,35) or after a kidney transplant (33). Therefore, these findings suggested that molnupiravir can be used safely regardless of renal or hepatic impairment, concomitant medications, and comorbidities in clinical practice.
This survey has some limitations. First, because of the noninterventional, single-arm observational design of this survey, we could not statistically compare the safety and effectiveness of molnupiravir treatment with other medications or a placebo and had to exclude 168 patients whose effectiveness data were missing or unavailable. Second, the survey forms collected in this survey were from patients at 266 institutions, which might not reflect the general population in Japan. Third, this survey included data collected for 16 months, during which a series of COVID-19 waves were observed in Japan. Therefore, the effectiveness of molnupiravir might have been different among patients infected with different SARS-CoV-2 variants.
Conclusions
In conclusion, this large-scale survey showed that molnupiravir was safe and effective in real-world settings among highly vaccinated Japanese patients with COVID-19, including older patients and those with comorbidities. Considering the lack of existing safety and effectiveness data in older Japanese patients, this PMS will provide valuable information for routine clinical practice, serving as a vital supplement to the results obtained from clinical studies.
Data Availability
The datasets generated and/or analyzed during the survey are available from the corresponding author upon reasonable request.
References
World Health Organization. Press briefing on WHO Mission to China and novel coronavirus outbreak. https://www.who.int/director-general/speeches/detail/press-briefing-on-who-mission-to-china-and-novel-coronavirus-outbreak. Accessed 10 Jun 2023.
Yoon J-J, Toots M, Lee S, et al. Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses. Antimicrob Agents Chemother. 2018;62(8):e00766-e818.
Cox RM, Wolf JD, Plemper RK. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat Microbiol. 2021;6(1):11–8.
Sheahan TP, Sims AC, Zhou S, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12(541):eabb5883.
Wahl A, Gralinski LE, Johnson CE, et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591(7850):451–7.
Abdelnabi R, Foo CS, De Jonghe S, Maes P, Weynand B, Neyts J. Molnupiravir inhibits replication of the emerging SARS-CoV-2 variants of concern in a hamster infection model. J Infect Dis. 2021;224(5):749–53.
Agostini ML, Pruijssers AJ, Chappell JD, et al. Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J Virol. 2019;93(24):e01348-e1419.
MSD K.K. LAGEVRIO® Interview Form [in Japanese]. https://www.msdconnect.jp/wp-content/uploads/sites/5/2022/09/if_lagevrio_cap200.pdf.
Ministry of Health, Labour and Welfare. Changes in approval conditions of Molnupiravir (LAGEVRIO®) capsules [in Japanese]. https://www.mhlw.go.jp/content/001090926.pdf. Accessed 24 Jun 2023.
Jayk Bernal A, Gomes-Da-Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–20.
Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. The Lancet. 2023;401(10373):281–93.
Lin DY, Abi Fadel F, Huang S, et al. Nirmatrelvir or molnupiravir use and severe outcomes from omicron infections. JAMA Netw Open. 2023;6(9): e2335077.
Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022;400(10359):1213–22.
Suzuki Y, Shibata Y, Minemura H, et al. Real-world clinical outcomes of treatment with molnupiravir for patients with mild-to-moderate coronavirus disease 2019 during the Omicron variant pandemic. Clin Exp Med. 2023;23(6):2715–23.
Xie Y, Bowe B, Al-Aly Z. Molnupiravir and risk of hospital admission or death in adults with COVID-19: emulation of a randomized target trial using electronic health records. BMJ. 2023;380: e072705.
Ministry of Health, Labour and Welfare. Visualizing the data: information on COVID-19 infections. COVID-19 as of May 7, 2023. https://covid19.mhlw.go.jp/en/. Accessed 10 Jun 2023.
Organisation for Economic Co-operation and Development. OECD Data. Elderly population. https://data.oecd.org/pop/elderly-population.htm#indicator-chart. Accessed 10 Jun 2023.
Kimata M, Watanabe A, Yanagida Y, Kinoshita D, Maekawa S. Safety and effectiveness of molnupiravir (LAGEVRIO®) capsules in Japanese patients with COVID-19: interim report of post-marketing surveillance in Japan. Infect Dis Ther. 2023;12(4):1119–36.
National Institute of Infectious Diseases. The effectiveness of SARS-CoV-2 vaccines against severe COVID-19 disease: an interim analysis of a case-control study during the Delta and early Omicron wave [in Japanese]. https://www.niid.go.jp/niid/ja/2019-ncov/2484-idsc/12019-covid19-9999-2.html. Accessed 18 Jul 2023.
MSD K.K. LAGEVRIO Capsules 200mg package insert [in Japanese]. https://www.msdconnect.jp/wp-content/uploads/sites/5/2022/09/pi_lagevrio_cap200.pdf. Accessed 10 Jun 2023.
Ministry of Health, Labour and Welfare. Clinical management of patients with COVID-19 [in Japanese]. https:// www.mhlw.go.jp/content/000936655.pdf. Accessed 10 Jun 2023.
Syed YY. Molnupiravir: first approval. Drugs. 2022;82(4):455–60.
Digital Agency. The status of COVID-19 Vaccines in Japan [in Japanese]. https://info.vrs.digital.go.jp/dashboard. Accessed 10 Aug 2023.
Flicoteaux R, Protopopescu C, Tibi A, et al. Factors associated with non-persistence to oral and inhaled antiviral therapies for seasonal influenza: a secondary analysis of a double-blind, multicentre, randomised clinical trial. BMJ Open. 2017;7(7): e014546.
Santi Laurini G, Montanaro N, Motola D. Safety profile of molnupiravir in the treatment of COVID-19: a descriptive study based on FAERS data. J Clin Med. 2022;12(1):34.
Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001.
Patel KP, Patel PA, Vunnam RR, et al. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J Clin Virol. 2020;128: 104386.
Daneshgaran G, Dubin DP, Gould DJ. Cutaneous manifestations of COVID-19: an evidence-based review. Am J Clin Dermatol. 2020;21(5):627–39.
Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71–7.
COVID-19 Advisory Board. Summary of newly positive cases, hospitalized patients, severely ill patients, and deaths during the third, fifth, sixth, and seventh waves based on the COVID-19 registry [in Japanese]. https://www.mhlw.go.jp/content/10900000/001010896.pdf. Accessed 23 Aug 2023.
Ma BH-M, Yip TC-F, Lui GC-Y, et al. Clinical outcomes following treatment for COVID-19 with nirmatrelvir/ritonavir and molnupiravir among patients living in nursing homes. JAMA Netw Open. 2023;6(4):e2310887.
Alomar MJ. Factors affecting the development of adverse drug reactions (Review article). Saudi Pharm J. 2014;22(2):83–94.
Poznański P, Augustyniak-Bartosik H, Magiera-Żak A, et al. Molnupiravir when used alone seems to be safe and effective as outpatient COVID-19 therapy for hemodialyzed patients and kidney transplant recipients. Viruses. 2022;14(10):2224.
Chang YC, Chen YC, Huang CC, et al. Clinical effectiveness of molnupiravir in patients with COVID-19 undergoing haemodialysis. Int J Antimicrob Agents. 2023;62(1): 106834.
Kikuchi K, Nangaku M, Ryuzaki M, et al. Efficacy of molnupiravir and sotrovimab in Japanese dialysis patients with COVID-19 in clinical practice during the Omicron (BA.1 and BA.2) pandemic. Ther Apher Dial. 2023;27(6):1064–9.
Acknowledgements
The authors would like to thank all healthcare professionals and patients who participated in this survey and provided valuable data.
Medical Writing and Editorial Assistance
Statistical analysis and data management were provided by CMIC HOLDINGS CO., Ltd., Tokyo, Japan. Medical writing was provided by Yuka Kinoshita, MD, PhD (SunFlare Co., Ltd.), and English language editing was provided by SunFlare Co., Ltd. Both statistical and medical writing support was funded by MSD K.K
Funding
This survey was funded by MSD K.K., Tokyo, Japan. All authors are employed by MSD K.K. This journal’s Rapid Service Fee was provided by MSD K.K.
Author information
Authors and Affiliations
Contributions
Shohei Shinozaki, Asuka Watanabe, Masahiro Kimata, Makoto Miyazaki and Shinichiroh Maekawa made substantial contributions to all of the following: (i) the conception and design of the survey, or acquisition of data, or analysis and interpretation of data, (ii) drafting the article or revising it critically for important intellectual content, and (iii) final approval of the version to be submitted. All the authors meet the ICMJE authorship criteria.
Corresponding author
Ethics declarations
Conflict of Interest
Shohei Shinozaki, Asuka Watanabe, Masahiro Kimata, Makoto Miyazaki and Shinichiroh Maekawa are all employees of MSD K.K.
Ethical Approval
This survey was conducted in accordance with the MHLW ordinance for GPSP and Pharmaceutical and the Medical Device Act. This survey complied with the protocol, the Helsinki Declaration of 1964 and its later amendments, and all other applicable regulations. IRB approval was not mandatory for this survey, according to the GPSP.
After explaining to the patients or their legal representatives the objective of the survey, the information to be collected, and the uses of the survey results, the treating physicians obtained informed consent from the patients or their legal representatives.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior Publication: The interim report of the present study was published as follows: Kimata M, Watanabe A, Yanagida Y, Kinoshita D, Maekawa S. Safety and Effectiveness of Molnupiravir (LAGEVRIO®) Capsules in Japanese Patients with COVID-19: Interim Report of Post-marketing Surveillance in Japan. Infect Dis Ther. 2023;12(4):1119–36.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shinozaki, S., Watanabe, A., Kimata, M. et al. Safety and Effectiveness of Molnupiravir in Japanese Patients with COVID-19: Final Report of Post-marketing Surveillance in Japan. Infect Dis Ther 13, 189–205 (2024). https://doi.org/10.1007/s40121-023-00915-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00915-w