Abstract
Pertussis, caused by Bordetella pertussis, remains one of the most widespread, contagious, and vaccine-preventable diseases. It results in notable morbidity and mortality as well as severe medical, social, and economic burden. Despite high global vaccine coverage, pertussis continues to be a significant epidemiologic problem, with outbreak episodes every few years just as in the pre-vaccination era. In Türkiye, there is a lack of comprehensive data on the current burden of pertussis in different age and risk groups, leading to underdiagnosis and underreporting of the disease, especially in adults who are often not considered at risk. Available data from Türkiye also reveal inadequate levels of protective antibodies in preterm newborns, emphasizing the need for additional preventive measures. Authors stated that improving physician awareness of pertussis symptoms in patients with prolonged cough, increasing access to routine pertussis tests, and conducting surveillance studies would aid in accurate diagnosis and reporting in Türkiye. As the Turkish Ministry of Health Antenatal Care Management Guide suggests routine second and third pregnancy check-up visits at weeks 18–24 and 28–32 correspondingly, this period can be considered the ideal vaccination time for Türkiye. Introducing a booster dose of Tdap at around 10 years of age or during national military service would reduce transmission and protect susceptible individuals. Identifying individuals at high risk of severe pertussis and prioritizing them for a booster dose is also crucial in Türkiye. Enhancing surveillance systems, increasing healthcare professionals’ awareness through training, and organizing catch-up visits for missed vaccinations during the COVID-19 pandemic are mentioned as additional strategies to improve pertussis prevention in Türkiye. This review focuses on the global and regional burden of pertussis and obstacles to effective prevention and evaluates existing strategies to achieve lifelong pertussis prevention. Literature and current strategies were also discussed from a Turkish national standpoint.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pertussis, a respiratory disease, poses a significant threat to newborns and vulnerable populations despite the availability of vaccines, particularly in low- and middle-income countries where underreporting is common. |
While both wP and aP vaccines are safe and effective, they do not confer lifelong immunity, leading to a shift in pertussis cases to older age groups and emphasizing the ongoing public health importance of the disease. |
The World Health Organization (WHO) and the Global Pertussis Initiative recommend implementing additional strategies like maternal immunization, cocooning, and booster doses in specific groups to enhance pertussis prevention and reduce outbreaks. |
Despite vaccination efforts, pertussis continues to cause mortality and morbidity in countries like Türkiye, necessitating increased awareness and further initiatives. Vaccinating pregnant women is considered a cost-effective strategy to prevent disease in infants who are too young to be immunized. |
To achieve lifelong pertussis prevention in Türkiye, it is recommended to incorporate maternal immunization into the national immunization program and consider administering booster doses. |
Introduction
Pertussis (whooping cough) is a severe respiratory infection caused by a highly contagious bacterium, Bordetella pertussis. Due to its high prevalence and severity, pertussis has been considered a serious epidemiologic problem for decades. The whole-cell pertussis (wP) vaccine was developed in the 1910s, and it became widely used in the 1930s. In the late 1940s, wP was combined with tetanus and diphtheria toxoids to develop the Diphtheria-Tetanus-Pertussis [DTP) vaccine [1]. Pertussis vaccination then became widely available with the launch of the Global Expanded Program on Immunization (EPI), significantly reducing pertussis-related childhood mortality [2]. However, due to the possibility of adverse events, particularly neurologic problems thought to be caused by the pertussis component of the vaccine, many countries suspended pertussis vaccination in the 1980s, and these concerns led to the development of the acellular pertussis vaccine [1]. Recent concerns include waning immunity following wP vaccination and an increase in adult-to-infant transmission despite a decrease in child-to-child transmission. These concerns emphasized the importance of booster vaccine doses for older children and adults, which were not possible with the wP vaccine because of its reactogenicity [2]. To avoid reactogenicity and side effects, the acellular pertussis (aP) vaccine was introduced, and DTP vaccines containing an acellular pertussis component (DTaP) were licensed for infant use in the early 1990s [1]. DTaP-containing combination vaccines are widely used in almost all developed countries, while wP vaccines are still used in low-income countries [3].
Despite high global vaccination coverage, pertussis epidemics occur approximately every 3–4 years, with varying patterns across countries [4]. Therefore, it is critical to keep infants, children, adolescents, pregnant women, and adults safe through optimal and prompt use of accessible vaccines to reduce the severe disease and mortality, especially in the early infantile period during which nearly all pertussis deaths occur.
This review article primarily aimed to investigate the global burden of pertussis, highlight obstacles in pertussis prevention, and evaluate existing strategies to achieve lifelong pertussis prevention. The secondary aim was to provide a regional perspective on Türkiye in each section as well as to interpret the current literature and strategies from a national standpoint.
Methods
An Expert Group Advisory Committee Meeting, involving eight experts in related fields (public health [2], pediatrics [1], pediatric infectious diseases [3], infectious diseases [1], obstetrics and gynecology [1]) from Türkiye was convened to identify the scope of the literature search and to evaluate the resources. Each expert was either a member of an academic association or had contributed to development of guidelines on the subject or published articles on pertussis prevention. Prior to that meeting, a literature review was performed to highlight the epidemiology of pertussis, burden of the disease, and obstacles to prevention and recommendations for effective pertussis prevention from a global and regional Turkish perspective. To identify relevant articles, we searched the MEDLINE® (via the PubMed interface), Web of Science, Google Scholar, and EMBASE databases. An electronic search of the literature published from 1 January 2000 to 28 February 2023 was conducted in these databases by using MeSH (Medical Subject Heading, Medline) and EMBASE terms, as well as free text words. The search included the terms “pertussis epidemiology,” “burden of pertussis,” “pertussis immunization,” and “pertussis and Türkiye.” The inclusion criteria were: (1) peer-reviewed articles and scientific reports; (2) original articles, review articles, and conference papers, including information about gene therapy for pertussis; (3) publication between 2000 and 2021. The exclusion criteria were: (1) articles not published in either English or Turkish and (2) case report(s). The reference lists of all manuscripts were manually reviewed for additional eligible articles. Most recent and up to date publications were chosen. Relying upon this literature review, experts created a national perspective and developed brief recommendations for a future immunization landscape in Türkiye to achieve lifelong pertussis prevention. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Epidemiology of the Disease
A global modeling study estimated that there were 24.1 million pertussis cases and 160,700 deaths from pertussis in children < 5 years in 2014, with the largest proportion in Africa. Global and regional epidemiologic data for pertussis have been summarized in Table 1. Globally, 21% of the estimated cases and 53% of the estimated deaths were in infants < 1 year old [5]. Between 2008 and 2016, substantial pertussis outbreaks were reported in many countries including the USA, Canada, Australia, Japan, UK, Sweden, Poland, Malaysia, Argentina, Brazil, Colombia, and Mexico [6,7,8,9]. Although no extensive outbreaks have been reported since 2016, several smaller local outbreaks have been reported. In 2019, a pertussis outbreak was announced in an early-years school in England among 7–11-year-old students who had their last booster dose at the age of 40 months. Vaccination coverage was found to be lower than the national average in this school and considered to be the main reason for the high transmission [10].
Since December 2019, a pandemic caused by novel coronavirus SARS-CoV-2 disrupted routine childhood immunization by reducing the use of healthcare facilities and increasing the risk of vaccine-preventable diseases including pertussis [11]. In 2021, 25.0 million children had not completed the three-dose DTP series worldwide, of whom 18.2 million (73%) had received no doses, and 6.8 million (27%) were incompletely vaccinated with DTP. This is almost 50% higher compared to the numbers in 2019. According to the World Health Organization (WHO) and UNICEF reports, global DTP1 coverage reduced from 90% in 2019 to 87% in 2020 and 86% in 2021, which is the lowest rate since 2005. The highest fall in first (DTP1) and third dose DTP (DTP3) coverage between 2019 and 2021 was in the Southeast Asia Region (from 94 to 86% for DTP1 and from 91 to 82% for DTP3) [12].
US and Canada
While approximately 4000 pertussis cases were reported annually in the US in the 1980s, reported cases increased to 25,827, 27,500, and 48,277 cases in 2004, 2010, and 2012, respectively [13]. From 2000 to 2016, 339,420 pertussis cases were reported in the USA. Throughout all age groups, almost 10% were hospitalized, and the mortality rate was 0.1%. Infants had the highest incidence of mortality (75.3/100,000 population), accounting for almost 90% of deaths. Incidence gradually rose, and this increase were observed for all groups except the age groups 0–12 months and 19–64 years [14]. Another study from the USA revealed that 11,378 pertussis cases were identified from 2006 to 2015. Adjusted pertussis incidence was 15.55 cases per 100,000 person-years. Although infants and children were the most affected population, around 60% of total cases were adolescents or adults [15].
A total of 33,481 pertussis cases were reported in Canada between 2005 and 2019 with an average annual incidence rate of 6.4 cases per 100,000 population. The highest average age-specific incidence rate was among infants < 1 year of age (n = 68.7 cases per 100,000 population). Hospitalization rates were almost eightfold higher in infants under the age of 3 months compared to those between 4 to 11 months of age [16]. However, there was a significant decrease in the incidence of pertussis during the pandemic. In British Columbia, one of the largest provinces of Canada, annual incidence of pertussis was found to be the lowest since 1990 in 2020 and 2021 (3/100,000 in 2020 and < 1/100,000 in 2021) [17].
European Countries
Surveillance data from Europe have shown that 35,627 cases of pertussis were reported in 30 European Union/European Economic Area (EU/EEA) countries in 2018; 72% of total European cases were reported from five countries including Germany, The Netherlands, Norway, Spain, and UK. Consistent with the previous years, 8.2 cases were reported per 100,000 population in 2018 whereas 62% of total cases were ≥ 15 years of age. Infants younger than 12 months were the most affected age group, with the highest rate 44.4 per 100,000 population (and three deaths reported), followed by the children between 10 to 14 years old [18]. A study funded by European Centre for Disease Prevention and Control evaluated the pertussis seroprevalence among adults of reproductive age (20–39 years) in 14 European countries. There was a noteworthy diversity in the ratio of samples with anti-PT IgG ≥ 100 IU/ml, illustrating a recent infection varying from 0.2% in Hungary to 5.7% in Portugal. However, the countries with the top reported incidences did not have the greatest degree of seroprevalence. In Portugal, the seroprevalence rate was 5.7%, whereas the incidence was only 0.1–2.2 per 100,000 people. Thus, many cases may have been underdiagnosed [19]. The pertussis incidence and hospitalization rate have been increasing in Spain with the highest rate in children under 12 months of age. However, vaccination has remarkably decreased hospital admissions; following the vaccination of pregnant women hospitalization rates showed a 20% reduction [20].
The number of pertussis cases remarkably reduced during the COVID-19 pandemic; a national Swedish cohort study showed that the average number of infant cases declined from 21 to 1 per quarter of a year during the pandemic [21]. Similarly, pertussis incidence decreased in England in all age groups, especially in infants younger than 12 months of age during the pandemic (0.50/100,000 during July 2020 to June 2021 compared to 24.49/100,000 from July 2014 to June 2019) [22].
Asia-Pacific Region
The WHO estimated that among children younger than 60 months of age, pertussis was the fourth most common vaccine-preventable disease after measles, diphtheria, and mumps, and the most prevalent one in the Asia-Pacific region [7]. The highest number of pertussis cases in the past 5 years in the Asia-Pacific region was reported in 2019 (63,483), whereas the lowest number of reported cases occurred in 2017 (27,624) [7]. Although surveillance systems are poor in some Asian countries, it has been reported that the burden of pertussis in children remains high in many Asian countries [25]. A recent surveillance study in Asian children and teenagers aged 10–18 years from China, India, Japan, South Korea, Sri Lanka, Taiwan, and Thailand (N = 1802) from July 2013 to June 2016 revealed that 4.8% had anti-PT IgG levels ≥ 62.5 IU/ml, which was interpreted as B. pertussis infection within the previous 12 months. Among the seropositive ones, 83.9% had minimum three doses of DTP vaccination before the age of 6 years [9, 23].
Middle East
While the surveillance data are insufficient in the Middle East, there was an alteration in reported pertussis cases in 2019 including nine in the United Arab Emirates, 78 in Lebanon, 242 in Iran, and 302 in Syria [24]. Data from the Middle East region suggest that pertussis is an extensive concern and that it might be affecting older age groups [24]. A few countries in the Middle East region administer a Tdap booster for adolescents. Israel was the only country with population health data that delivers Tdap, and the results showed that the utilization of the adolescent booster dose led to a remarkable drop in pertussis among children between 5 and 14 years of age [25].
Türkiye
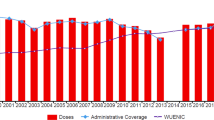
In Türkiye, diphtheria and pertussis immunization was initiated in 1937; routine childhood pertussis immunization with wP (DTP) was started in 1968, and aP (DTaP-IPV/Hib) has been given since 2008 [26]. The pertussis vaccine has been administered in the 2nd, 4th, and 6th months of age, and in combination with a booster dose administered in the 18th month, in accordance with the childhood vaccination schedule. Since 2010, a single-dose diphtheria, tetanus toxoid, acellular pertussis, and inactivated polio vaccine (DTaP-IPV) has also been applied to children at 7 years of age (Supplementary Fig. 1) [27]. After 2020, the DTaP-IPV dose was moved to the age of 48 months (Supplementary Table 1). In 2005, pertussis incidence decreased (0.38 per 100,000) compared to 1986 (2.03 per 100,000). However, the proportion of the patients ≥ 15 years of age increased from 6.5 to 16.9% within that period [28]. According to the data from national authorities, pertussis incidence decreased 99.5% with the vaccination from 1980 to 2014 [29]. In 2010, the number of pertussis cases was 48, the incidence of pertussis was 0.07 per 100,000 population, and no deaths due to pertussis were reported in Türkiye [30]. In a study conducted by Oksuz et al. in Istanbul, the largest city in Türkiye, in a sample of 410 nasopharyngeal aspirates taken from children who exhibited symptoms of whooping cough from 2010 to 2014, the Bordetella polymerase chain reaction (PCR) positivity rates were 36% in 2010 and gradually decreased as 29% in 2011, 15% in both 2012 and 2013. Since the initiation of DTaP-IPV vaccination at age 7 in 2010, pertussis has not been detected in the 5–9-year-old age group [31]. In another study from Türkiye, 214 adolescents and adults who had a cough lasting > 2 weeks were investigated for the presence of B. pertussis. Three (1.4%) patients were B. pertussis culture-positive; 15 (7%) were B. pertussis PCR-positive (including the culture-positive patients), and 11 (5.1%) were Bordetella spp. PCR-positive [32]. In another study, nasopharyngeal specimens were collected from 101 children between the age of 7–18 years with prolonged cough, and 19.8% were PCR positive for B. pertussis. Children who had their last vaccine dose > 5 years ago had a 6.13-fold higher risk of PCR-confirmed pertussis than those who were vaccinated within 5 years. Paroxysmal cough, whooping, and post-tussive vomiting were observed in approximately 30%, 15%, and 25% of the PCR-positive children, respectively [33]. Although pertussis is known to present generally with prolonged cough, prevalence of pertussis was found to be 3.5% among 115 adult patients with acute cough in a recent study from Türkiye [34].
In a seroprevalence study from Türkiye, high levels of anti-pertussis IgG (anti-PT IgG) (≥ 100 EU/ml) were detected in 9.7% (52/538) of the patients between 18 and 87 years who had a prolonged cough. Among cases with high antibody levels, age, gender, education level, vaccination, and smoking history or average daily cigarette consumption did not significantly differ [35]. Among a convenience sample of 228 adults who were admitted to the emergency departments without any respiratory problems, 40% were anti-PT IgG positive, 60% were negative. Anti-PT IgG positivity was increasing by age, as 26% at both 19–35 years and 36–50 years and 48% in the 51–65-year group, which may be illustrating a recent history for a typical or atypical pertussis infection [36].
In 2019, both DTP1 and DTP3 coverages were significantly high with a 99% rate in Türkiye [37]. A cross-sectional online survey study from Türkiye revealed that vaccination rates in Ankara, the capital city, fell 2–5% during the pandemic, with the highest drop in the vaccines administered after 18 months of age [38]. The pandemic has led to concern about other global health areas being neglected, including the delivery of vaccination and immunization programs particularly in low-income countries and among vulnerable populations.
Burden of Disease
Infants under 12 months of age, particularly those between 0 and 6 months, have the highest burden of pertussis disease [39]. According to Swiss National Surveillance Data, hospitalization rates were significantly higher (38.8 per 100,000 population) than the rate in patients < 16 years (2.6 per 100,000) [40]. During the 2010 outbreak in the US, although pertussis was detected in all age groups, the greatest incidence and hospitalization were seen in infants < 6 months old [41]. From 2010 to 2017, pertussis was reported in 27,370 infants aged < 12 months; 9199 cases (33.6%) occurred among infants aged < 2 months. Among the 7731 infant pertussis hospitalizations within that 8-year period, a total of 3928 (50.8%) were among infants aged < 2 months. Meanwhile, 69% of pertussis deaths were recorded in infants < 2 months old [42]. Similarly, during the pertussis resurgence in the UK in 2012, the highest incidence of pertussis was seen in infants < 3 months old whereas a remarkable rise was also reported in the age cohort of > 15 years [43]. In Spain, infants < 3 months of age accounted for almost 60% of hospitalizations due to pertussis, and > 90% of hospitalized patients were infants < 1 year of age [44]. Mortality rates in infants < 3 months old were the highest in all these epidemics [45]. In addition, the most recent data show that pertussis remains an important cause for deaths in infants and young children [20, 44]. Thus, when implementing elimination plans for pertussis, it is essential to develop routes that stop pertussis dissemination among both newborns and young infants.
Main symptoms of pertussis are cough with or without paroxysms, cyanosis, apnea, tachypnea, and difficulty in breathing. Pertussis can also cause important complications, particularly in infants who are too young to be vaccinated. In a study from Türkiye, pertussis was found in 44 (25.6%) of the 172 infants diagnosed with acute bronchiolitis and as a co-infection with respiratory viruses in 27 (61.4%) infants. Of those 44 pertussis-positive infants, only 17 (38.6%) had a paroxysmal cough, 13 (29.5%) experienced whooping, and 15 (34.1%) had post-tussive vomiting [46].
Pertussis can cause especially severe disease in newborns and unvaccinated infants [44]. A study conducted in Türkiye reviewed 18 patients in a pediatric intensive care unit with a diagnosis of pertussis, all of whom were unvaccinated. The median age was 40 (38–47.5) days. All patients had respiratory distress, 14 patients had cough (77.7%), 4 patients had fever (22.2%), and 3 patients had seizures (16.6%). Seven patients required mechanical ventilation. Three patients died because of multi-organ failure and cardiogenic shock despite extracorporeal life support [47]. A retrospective study from Singapore between 2007 and 2016 revealed that key risk factors for intensive care unit (ICU) admission included age < 3 months, positive contact history, underlying comorbidity, prematurity, and being unvaccinated [48].
Although pertussis is often considered a childhood disease, approximately 32% of all notified pertussis cases in England in 2019 were in people aged ≥ 45 years [49]. While adolescent and adult infections remain underrecognized and undiagnosed, they can be a source of transmission to higher risk groups, including as neonates and the patients with chronic diseases [24]. Although 1.4–7.5% of individuals 10–19 years of age and 3.5–5.7% of individuals ≥ 20 years of age required hospitalization, older individuals may require longer hospitalization. While death from pertussis occurs in ∼ 0.1% of cases among patients > 10 years old, complications of pertussis are not rare in adults and adolescents [50]. As the general population ages, the disease burden is therefore predicted to rise [51, 52].
Patients with respiratory and other systemic comorbidities—including chronic obstructive pulmonary disease (COPD) and asthma—may have an elevated chance of experiencing severe pertussis infections [53, 54]. Both human immunodeficiency virus (HIV) infection and exposure were also associated with greater pertussis incidences and rates of hospitalization and mortality [55].
In a study from the Philippines, the most frequent complications from pertussis disease were pneumonia requiring intubation (64%), acute respiratory distress syndrome (ARDS) (28%), seizures (21%), nosocomial pneumonia (11%), and myocarditis (11%) [56]. While the most common are pulmonary complications including both interstitial and alveolar pneumonia and, in serious cases, respiratory failure, neurologic and nutritional complications have also been observed. Cough paroxysms and related hypoxia may cause acute encephalopathy and/or cerebral hemorrhage, which may result in epilepsy or permanent brain damage [57]. Other pertussis-related complications include urinary incontinence, headache, sleep disturbances, rib fractures, fainting, sinusitis, and otitis media [24].
Obstacles in Pertussis Prevention
The primary goal of pertussis vaccination is to decrease the possibility of serious disease in infants and young children. Despite the success of widespread mass vaccination schemes, pertussis causes a noteworthy health burden in various countries. Furthermore, actual pertussis incidence is estimated to be considerably higher than the reported incidence [3]. The resurgence of pertussis reporting in the recent years may be partially attributable to improved recognition, reporting, and diagnosis of pertussis. Although the culture has 100% specificity, the sensitivity is very poor, and it is difficult because of the fastidious nature of the organism [58]. As the nasopharyngeal cultures should be collected in the first 15 days, diagnosis of pertussis may be missed because of its non-specific symptoms. Moreover, isolation of the bacterium can be affected by the antibiotic usage. Molecular techniques, including real time-polymerase chain reaction (rt-PCR), are currently extensively accessible allowing rapid diagnosis of pertussis with a higher sensitivity (70–99%) in the early stages of infection [59, 60]. Moreover, to verify the infection after this stage, serologic methods detecting anti-PT IgG in serum and saliva have been established [3]. Both PCR and serologic test applications may cause a relative increase in pertussis recognition. In Canada, clinical application of a sensitive PCR assay was related to an accompanying fivefold rise in the detection of pertussis-positive cases [61]. A comparison of diagnostic tests for pertussis is shown in Table 2.
Waning immunity is an important concern regarding pertussis prevention. Neither natural infection nor immunization with wP and aP vaccines gives life-long immunity [52]. A 10-year study of pertussis immunity in the UK showed that the protection afforded by vaccination remained effective in 85% of children during the first 4 years post-vaccination but declined to 50% within 3 years. A meta-analysis of 11 studies showed that during each year following administration of DTaP, the risk of pertussis rose 1.33-fold; consequently, 8.5 years after their last aP dose, only 10% of children were still immune against pertussis [62]. A comparison study of adolescent booster doses revealed that upon completion the absolute effectiveness of the full six-dose aPV series was estimated to be 85% but declined by 11.7% each year thereafter. At 18 years of age, protection was reduced to 28.2% of immunized patients [63]. This rapidly declining effectiveness indicates that additional booster doses are necessary to ensure continuing protection.
After the introduction of wP and aP vaccines, genetic mutations have been widely observed in the circulating strains of B. pertussis. The deletion of pertactin (PRN), over-expression of PT, as well as, in a few cases, deletion of PT or filamentous hemagglutinin have been documented [64]. PRN-deficient strains have become prevalent in Australia, Israel, and the USA [65,66,67]. From 2008 to 2012, during an important outbreak of pertussis in Australia, 30% (96/320) of B. pertussis isolates did not have PRN [65]. The pertussis flare-up in Israel was also related to the increased prevalence of PRN-negative strains [66]. Such mutant strains may evade a vaccine-induced immune response but not necessarily cause more disease or reduced vaccine efficacy.
Regional differences in the vaccination coverage, due to the varying national immunization schedules and vaccine hesitancy, may also foster pertussis resurgence. Although mean global DTP3 coverage was 81% in 2021, regional coverage levels vary extensively, ranging from 71 to 94%, depending on the country [12].
Strategies for Effective Pertussis Prevention
As pertussis remains an important global concern, to address the potential barriers for pertussis control and provide better prevention, several immunization strategies have been developed. These are maternal immunization during pregnancy, immunization of family and close contacts of newborns (cocooning), universal immunization of adolescents and adults, and immunization of healthcare workers. These should be carried out together with the application of a fourth or fifth booster dose for all pre-school children (4–6 years of age) and advancement of current infant and children immunization plans.
Maternal Immunization
Pertussis is a remarkable cause of morbidity and mortality in children, particularly in infants < 6 months of age via transmission from a relative in close contact [20, 44]. Generally, the initial pertussis vaccine is given at the age of 6 or 8 weeks but can be delayed up to 3 months in several countries [1]. Although the initial dose provides partial protection against severe disease, higher rates of immunity (80–90%) can be produced after the third dose [1, 3, 52]. The approaches to further decrease infant mortality from pertussis have therefore focused on supportive action plans such as maternal immunization during pregnancy and cocooning.
In the UK, pertussis-including vaccines have been delivered in infancy since 1957, at 2, 3, and 4 months of age since 1990, a preschool booster at 40 months of age, and aP vaccine since 2004. Although good disease control was achieved, a national rise in pertussis was recorded in late 2011, first limited to adolescents and adults, and then spread through younger children in 2012 [43]. As a response to this, in 2012 a five-component acellular-pertussis-containing vaccine was offered to all women between 28 and 38 weeks of pregnancy. There was a 78% fall in the number of confirmed cases and 68% drop in hospital admissions in infants < 3 months old. Vaccine effectiveness was 90% when the analysis was narrowed to cases < 2 months of age [43]. Pertussis vaccination in pregnancy was available in all regions of Spain in early 2016. While the national mean of death from pertussis was 5.1 per year between 2007 and 2015, annual mortality rate fell to 2.5 per year between 2016 and 2019 [44].
Since the introduction of maternal immunization in the US in 2012, the incidence of pertussis in infants < 6 months of age reduced from 169.0 per 100,000 population in 2014 to 57.2 per 100,000 in 2018. The number of deaths in infants < 12 months was 16 in 2012 and decreased to 4 in 2018 [13]. An ecologic study using the largest all-payer pediatric inpatient database in the US revealed that pertussis hospitalizations decreased from 5.06 to 2.15 per 100,000 infants with maternal Tdap vaccination [68]. A study from Brazil also showed that maternal pertussis vaccination reduced disease incidence in infants < 12 months of age, predominantly in those who are in their first 2 months of life [69]. Costa Rica implemented a dose of postpartum Tdap in 2007 and replaced it with intrapartum Tdap administration in 2011, which led to a significant decrease in both infant hospitalizations and deaths due to pertussis. Since 2011, there have been only four infant deaths associated with pertussis in Costa Rica [70]. Tdap vaccination has been recommended for pregnant women since November 2017 in Singapore. Based on a study performed in 2018–2019, maternal pertussis vaccination was 97.62% and 98.17% effective in protecting unvaccinated and partially vaccinated infants from pertussis retrospectively. Maternal Tdap vaccination was also found to be > 70% effective in preventing pertussis-related intensive care unit (ICU) admission in both unvaccinated and partially vaccinated infants [71].
Regarding the safety of Tdap immunization during pregnancy, there is no evidence of an elevated risk of serious adverse events or stillbirth [72,73,74]. A retrospective cohort study from Canada also showed that prenatal Tdap exposure was not related to any adverse health problems in early childhood while it was associated with a decreasing trend in upper respiratory infections, gastrointestinal infections, and medical care requirement [75].
Babies of vaccinated women were found to have higher transplacental antibodies against pertussis in the first 2 months of life [74]. A study comparing the immune responses against maternal immunization between preterm and term infants revealed that equal cord geometric mean concentrations (GMCs) of Ig G levels were observed in both groups. Preterm infants also profit from the maternal vaccination, whereas a longer period between maternal vaccination and delivery provided higher cord blood immunoglobulin levels in preterm [76].
In a seroprevalence study from Türkiye, antibodies against PT and FHA in maternal and cord blood samples were analyzed in 100 preterm infants and mothers and in 100 term infants and mothers. Anti-pertussis antibody levels were insufficient to protect infants until their vaccination schemes had been completed. Transplacental anti-pertussis antibody transmission and antibody levels were lower in the cord blood of preterm infants, predominantly the ones < 32 weeks of gestational age. Placental transfer ratios for anti-PT and anti-FHA in preterms were 68% and 72% and were 107% and 120% in term infants, respectively [77]. In another study, anti-pertussis antibody levels were found to be low in pregnant women in Türkiye, and only 34.6% of infants had sufficient anti-pertussis antibody titers (≥ 10 EU/ml) at birth [78]. As pertussis vaccination in pregnancy is currently not in practice in Türkiye, these findings support the need for maternal Tdap immunization during pregnancy.
Timing of the maternal vaccination is important to achieve optimal protection. Although surveillance data from England showed that vaccine effectiveness did not differ in infants with mothers vaccinated at different periods of pregnancy except in those with mothers vaccinated between 7 days before and 41 days after delivery, most of the studies showed that early third trimester Tdap vaccination provides the highest newborn immunity against pertussis [79,80,81,82,83]. A prospective study from Australia revealed that newborns had statistically significantly higher concentrations of high and very high avidity anti-PT IgG when their mothers were vaccinated 5–12 weeks before delivery compared to the ones whose mothers received vaccines within 4 weeks before delivery [84]. In another study from the USA, GMCs of PT antibodies were found to be highest when pregnant women had Tdap vaccine between the 27th and 30th weeks of pregnancy. Peak levels were obtained with vaccination at week 30 (57.3 IU/ml [95% CI 44.0–74.6]), and antibody levels decreased when mothers were vaccinated after 30 weeks [85].
Interference between maternal immunization and infants’ immune responses to their vaccines is another concern. Several meta-analysis studies revealed that lower IgG levels were recorded against both pertussis and diphtheria after their immunization in infants whose mothers received Tdap vaccine during pregnancy compared with infants of unimmunized mothers [86, 87]. Timing of maternal vaccination was not found effective against this interference [86]. While there are no available surveillance data to show the clinical impact of this interaction, further burden of disease data are needed to accurately assess the true clinical significance of this interaction as the cohort of infants born to pertussis-vaccinated women increases [87].
Vaccination of pregnant women is important as the most cost-effective supplementary approach to stop disease in infants too young to be vaccinated and seems to be more successful and beneficial than cocooning. Based on the evidence, The Global Pertussis Initiative (GPI) advices maternal vaccination during pregnancy as the primary approach to prevent pertussis disease [45, 88]. The Advisory Committee on Immunization Practices (ACIP) also suggests that women have a dose of Tdap during each pregnancy, ideally in the early stages of gestation as 27–36 weeks [89].
Cocooning
Cocooning is based on vaccinating the ones close to infants to provide secondary protection. Cocooning is immunologically feasible, as the antibody-mediated immunity after the vaccination is fast, and 88–94% of vaccinated individuals develop high levels of antibody in the following 2 weeks. However, there will still be a 2-week period during which infants can be at risk of pertussis infection; therefore, vaccination should be performed before the last month of pregnancy [90]. A study assessing the impact of maternal vaccination during the postpartum period on infant pertussis infection revealed that vaccinating only postpartum mothers did not decrease infection in infants ≤ 6 months of age [91]. Another study from Australia analyzed the association between parental vaccination status and incidence of pertussis in infants < 4 months old. Both the mothers and fathers of infants with pertussis were less likely to have been immunized at 22% vs. 32% and 20% vs. 31%, respectively. Parental cocooning was shown to reduce the risk of pertussis by only 51% in the infants [92]. Several simulation studies also showed that the cocoon strategy is not efficient and would be difficult to implement and expensive as many individuals would need to be vaccinated [45]. A mathematical modeling study predicted that maternal immunization during pregnancy is more cost-effective than cocooning and also decreases the number of the cases by 33% (vs. 20% for cocooning), hospitalizations by 38% (vs. 19% for cocooning), and deaths by 49% (vs. 16% for cocooning) [93]. Moreover, the number needed to vaccinate (NNV) is very high to achieve effective cocooning. According to a study evaluating all pertussis reports (confirmed and clinical/probable) in Québec and British Columbia from Canada between 1990 and 2010, the NNV for parental immunization was calculated as not less than 1 million to prevent one infant death, approximately 100,000 to prevent intensive care admission, and at least 10,000 to prevent hospitalization [94].
Adolescent/Adult Booster Doses
Several countries have included pertussis vaccination of adolescents in their national immunization programs. The Consensus on Pertussis Booster Vaccine in Europe (COPE group) suggested that adolescents between 10 and 17 years of age should have a combined Tdap vaccine instead of a Td vaccine, without considering the previous vaccination status [43]. A retrospective analysis of pertussis cases reported in the US between 1990 and 2009 illustrated that the application of Tdap for adolescents in 2005 led a significant reduction in the number of cases in those 11 to 18 years of age [95]. In Western Australia, Tdap was applied to all high school students in the 2008–2009 outbreak; a decrease was observed in not only adolescent but also infant pertussis cases [96]. It was also reported that adolescent boosters are cost-effective; immunization of adolescents in the US would prevent 0.7–1.8 million pertussis cases and save $0.6–$1.6 billion over a decade [97].
While adolescent boosters do not provide a life-long immunity, adults may also be susceptible to pertussis and become the source of infection. In a study performed in Türkiye, 90% of adult patients had no immunity against pertussis in an unrelated medical outpatient cohort [98]. Therefore, it is recommended that further doses of the vaccine should be administered after adolescence. In a prospective study, 95% of 15–65 years individuals who received Tdap were shown to have a sufficient immunity against pertussis [99]. However, this immunity wanes gradually, and renewing these boosters is recommended. Although kinetic antibody studies reveal that a booster interval of 10 years seems to be suitable, clinical protection may be more transient [100].
Geriatric Population
Pertussis is increasingly infecting the elderly, and a significant percentage of older people affected with pertussis may have remarkable morbidity and mortality. Therefore, the ACIP advises a single dose of Tdap for adults, including unvaccinated older adults [101].
Healthcare Workers
The COPE group and GPI recommend a single dose of Tdap instead of a Td, especially for healthcare workers who can be the source of disease for non-immune individuals under a high risk of severe pertussis [102]. Tdap is suggested for health care professionals without considering the administration time of the last Td containing vaccine in the USA [103].
Interpretation of Literature and Strategies from a Turkish National Perspective
While a nation-wide reporting system is in place and a few cross-sectional epidemiologic data are available in Türkiye, the current active disease and disease burden of pertussis in different age and risk groups are not well known [31, 47]. Physicians may miss the diagnosis of pertussis especially in the adult patient population because of the misperception that pertussis is a childhood disease. Increasing physicians’ awareness of pertussis symptoms in patients with prolonged cough and improving the availability of routine pertussis tests (PCR for newborns and serology for infants and older children) may contribute to adequate levels of pertussis diagnosis and reporting. Additionally, surveillance studies may provide a faster data generation opportunity in Türkiye regarding the challenges in executing seroprevalence studies [77].
The WHO states that the primary goal of pertussis immunization is to protect newborns and infants from severe complications of this disease [2]. Data from Türkiye showed that anti-pertussis antibody levels of preterm newborns were not sufficient to protect infants, and most infants did not have protective anti-pertussis antibody levels at birth [74]. Additionally, pertussis immunization during pregnancy is the most successful approach to protect infants < 6 months old who have not completed their primary course of vaccinations. The Infectious Diseases and Clinical Microbiology Specialty Society of Türkiye 2019 guideline recommends Tdap only as a suitable option to Td [104]. Although many countries recommend Tdap vaccination in each pregnancy preferably during 27–36 weeks of pregnancy, some countries recommend earlier than the 27th week, in different time frames and as early as 16 weeks. Health authority in UK recommends during 16–32 weeks, Ireland during 16–36 weeks, and Australia 20–32 weeks [81, 82, 105, 106].
As the estimated preterm birth rate is around 10% in Türkiye, to allow enough time for antibody production and to ensure the protection of these babies, pregnant women should have a dose of Tdap at the 2nd or 3rd routine prengancy check-up visit before the 32nd week. As the Turkish Ministry of Health Antenatal Care Management Guide suggests a routine 2nd and 3rd antenatal care visit in weeks 18–24 and 28–32 correspondingly, this period can be considered the ideal vaccination time in Türkiye [107]. The second antenatal care visit should be preferred, especially in high-risk pregnancies, to cover premature births as much as possible. During the third antenatal care visit, Tdap vaccination status should be checked, and if the vaccination is missed at the second visit, it should be completed. This recommendation for Turkey will be also compatible with the statement of WHO advising increasing routine immunization coverage by reducing missed opportunities for vaccination [108].
The last dose of DTaP-IPV in Türkiye’s vaccination scheme has been administered at 48 months of age since 2020. Considering available data, a booster dose of Tdap just after 10 years of age would decrease the transmission to unprotected subjects at high risk of severe pertussis [26, 32, 98]. Alternatively, a booster Tdap at the age of national military service duty would also be a practical way to immunize the young male population but would not provide immunization of females. Identification of individuals with high risk of severe pertussis is also vital to prioritize them for a booster Tdap in Turei.
In addition to all these suggested strategies, functioning of the surveillance systems should be enhanced, awareness of health care professionals should be increased by trainings, and catch-up visits should be organized for those who missed the opportunity to be vaccinated during the COVID-19 pandemic.
Conclusion
Pertussis is a respiratory disease with serious consequences for newborns and vulnerable populations such as pregnant women and the elderly. Even after the introduction of vaccines, pertussis remains a major cause of death and morbidity, particularly in low- and middle-income countries. As there is a lack of active surveillance in many countries, the true figures may be higher than reported [51].
Although both wP and aP vaccines have a high level of safety, efficacy, and effectiveness, neither provides lifelong immunity. After the introduction of childhood vaccination programs, pertussis disease has shifted to older age groups involving adolescents and adults, and pertussis has remained an important public health issue [52].
Additional strategies, such as maternal immunization, cocooning, and booster doses for adolescents, adults, and different risk groups, should be implemented to improve pertussis prevention and reduce outbreaks, as recommended by the WHO and the Global Pertussis Initiative [2, 45].
Despite widespread vaccination, pertussis continues to cause mortality and morbidity in Türkiye, as it does in many other developing countries; therefore, additional efforts to raise awareness are required. Vaccination of pregnant women is probably the most cost-effective additional strategy for preventing disease in infants too young to be vaccinated; thus, maternal immunization should be incorporated into the national immunization program, and booster dose administration should be considered to achieve lifelong pertussis prevention in Türkiye.
Limitations and Implications for Further Studies
The present study has all the limitations arising from the nature of a narrative review. General trends in pertussis incidence are also difficult to ascertain because of the heterogeneity of epidemiologic data. However, we believe this comprehensive review provided a broad perspective on this important topic and sufficiently synthesized the available literature to evaluate the current strategies to achieve lifelong pertussis prevention.
Data availability
Not applicable.
References
Guiso N, Meade BD, Wirsing-von-König CH. Pertussis vaccines: The first hundred years. Vaccine. 2020;38(5):1271–6.
Pertussis vaccines: WHO position paper, August 2015—recommendations. Vaccine. 2016;34(12):1423–5.
Esposito S, Stefanelli P, Fry NK, Fedele G, He Q, Paterson P, et al. Pertussis prevention: reasons for resurgence, and differences in the current acellular pertussis vaccines. Front Immunol. 2019;10:01344.
Domenech-de-Cellès M, Magpantay FM, King AA, Rohani P. The pertussis enigma: reconciling epidemiology, immunology and evolution. Proc Biol Sci. 2016;283(1822):20152309.
Yeung KHT, Duclos P, Nelson EAS, Hutubessy RCW. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis. 2017;17(9):974–80.
Amirthalingam G, Gupta S, Campbell H. Pertussis immunisation and control in England and Wales, 1957 to 2012: a historical review. Euro Surveill. 2013;18(38):20587.
Macina D, Evans KE. Bordetella pertussis in school-age children, adolescents, and adults: a systematic review of epidemiology, burden, and mortality in Asia. Infect Dis Ther. 2021;10:1115–40.
Smith T, Rotondo J, Desai S, Deehan H. Pertussis surveillance in Canada: trends to 2012. Can Commun Dis Rep. 2014;40(3):21–30.
Jog P, Memon IA, Thisyakorn U, Hozbor D, Heininger U, von König CHW, et al. Pertussis in Asia: recent country-specific data and recommendations. Vaccine. 2022;40(8):1170–9.
Tessier E, Campbell H, Ribeiro S, Andrews N, Stowe J, Nicholls M, et al. Investigation of a pertussis outbreak and comparison of two acellular booster pertussis vaccines in a junior school in South East England, 2019. Eurosurveillance. 2021;26(12):2000244.
Dinleyici EC, Borrow R, Safadi MAP, van Damme P, Munoz FM. Vaccines and routine immunization strategies during the COVID-19 pandemic. Hum Vaccin Immunother. 2021;17(2):400–7.
Rachlin A, Danovaro-Holliday MC, Murphy P, Sodha SV, Wallace AS. Routine vaccination coverage—worldwide, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(44):1396–400.
Centers for Disease Control and Prevention. Pertussis (Whooping Cough)—Surveillance and Reporting 2017 www.cdc.gov/pertussis/surv-reporting.html.
Skoff TH, Hadler S, Hariri S. The epidemiology of nationally reported pertussis in the United States, 2000–2016. Clin Infect Dis. 2018;68(10):1634–40.
Tong J, Buikema A, Horstman T. Epidemiology and disease burden of pertussis in the United States among individuals aged 0–64 over a 10-year period (2006–2015). Curr Med Res Opin. 2020;36(1):127–37.
Bhagat D, Saboui M, Huang G, Domingo FR, Squires SG, Salvadori MI, et al. Pertussis epidemiology in Canada, 2005–2019. Can Commun Dis Rep. 2023;49(1):21–8.
Reicherz F, Golding L, Lavoie PM, Abu-Raya B. Decay of anti-Bordetella pertussis antibodies in women of childbearing age following COVID-19 non-pharmaceutical measures. Vaccine. 2022;40(27):3746–51.
ECDC Report 2018.
Wehlin L, Ljungman M, Kühlmann-Berenzon S, Galanis I, Huygen K, Pierard D, et al. Pertussis seroprevalence among adults of reproductive age (20–39 years) in fourteen European countries. APMIS. 2021;129(9):556–65.
González-López JJ, Álvarez Aldeán J, Álvarez García FJ, Campins M, Garcés-Sánchez M, Gil-Prieto R, et al. Epidemiology, prevention and control of pertussis in Spain: new vaccination strategies for lifelong protection. Enfermedades Infecciosas y Microbiologia Clinica (English Ed). 2022;40(4):195–203.
Falkenstein-Hagander K, Appelqvist E, Cavefors AF, Källberg H, Nilsson LJ, Silfverdal SA, et al. Waning infant pertussis during COVID-19 pandemic. Arch Dis Child. 2022;107(3): e19.
Tessier E, Campbell H, Ribeiro S, Rai Y, Burton S, Roy P, et al. Impact of the COVID-19 pandemic on Bordetella pertussis infections in England. BMC Public Health. 2022;22(1):405.
Son S, Thamlikitkul V, Chokephaibulkit K, Perera J, Jayatilleke K, Hsueh PR, et al. Prospective multinational serosurveillance study of Bordetella pertussis infection among 10- to 18-year-old Asian children and adolescents. Clin Microbiol Infect. 2019;25(2):250.e1-e7.
Macina D, Evans KE. Bordetella pertussis in school-age children, adolescents, and adults: a systematic review of epidemiology, burden, and mortality in the Middle East. Infect Dis Ther. 2021;10(2):719–38.
Stein-Zamir C, Shoob H, Abramson N, Zentner G. The impact of additional pertussis vaccine doses on disease incidence in children and infants. Vaccine. 2010;29(2):207–11.
Kurugol Z. Pertussis epidemiology in Turkey: are booster doses necessary?/Turkiye’de bogmaca epidemiyolojisi pekistirme asi dozlari gerekli mi? J Pediatr Infect. 2009;14.
Türkiye Sağlıkta Dönüşüm Programı Değerlendirme Raporu [press release].
Dilli D, Bostanci I, Dallar Y, Buzgan T, Irmak H, Torunoğlu MA. Recent findings on pertussis epidemiology in Turkey. Eur J Clin Microbiol Infect Dis. 2008;27(5):335–41.
Asi Portali [Available from: https://asi.saglik.gov.tr/genel-bilgiler/27-a%C5%9F%C4%B1n%C4%B1n-yararlar%C4%B1.html.
Sağlık Bakanlığı İstatistik Yıllığı.
Öksüz L, Hançerli S, Somer A, Salman N, Gürler N. Pertussis in children in the İstanbul Faculty of Medicine: results for four years. Turk J Pediatr. 2014;56(6):632–7.
Karagul A, Ogunc D, Midilli K, Ongut G, Ozhak Baysan B, Donmez L, et al. Epidemiology of pertussis in adolescents and adults in Turkey. Epidemiol Infect. 2015;143(12):2613–8.
Aslan A, Kurugöl Z, Aydemir Ş, Gürsel D, Koturoğlu G. High frequency of pertussis in older children and adolescents with prolonged cough in Turkey. Turk J Pediatr. 2016;58(1):41–6.
İlbay A, Tanrıöver MD, Zarakol P, Güzelce E, Bölek H, Ünal S. Pertussis prevalence among adult patients with acute cough. Turk J Med Sci. 2022;52(3):580–6.
Sönmez C, Çöplü N, Gözalan A, Yılmaz Ü, Bilekli S, Demirci NY, et al. Serological evaluation of Bordetella pertussis infection in adults with prolonged cough. Mikrobiyol Bul. 2016;50(3):361–70.
Öksüz L, Gürler N, Ağaçfidan A. Investigation of seropositivity of Bordetella pertussis in adults in a university hospital. Mikrobiyol Bul. 2017;51(1):62–72.
Immunization Regional Snapshot 2019 Europe and Central Asia [press release].
Kara A, İlbay S, Topaç O, Arabulan EA, Tezer H, Tavukçu N, et al. Alteration in vaccination rates and an evaluation of physicians’ perceptions of the possible impact of the SARS-CoV-2 pandemic on childhood vaccinations in Ankara Turkey. Hum Vaccin Immunother. 2021;17(10):3457–62.
Masseria C, Martin CK, Krishnarajah G, Becker LK, Buikema A, Tan TQ. Incidence and burden of pertussis among infants less than 1 year of age. Pediatr Infect Dis J. 2017;36(3):e54–61.
Heininger U, Weibel D, Richard JL. Prospective nationwide surveillance of hospitalizations due to pertussis in children, 2006–2010. Pediatr Infect Dis J. 2014;33(2):147–51.
Mahmud AS, Lipsitch M, Goldstein E. On the role of different age groups during pertussis epidemics in California, 2010 and 2014. Epidemiol Infect. 2019;147: e184.
Lindley MC, Kahn KE, Bardenheier BH, D’Angelo DV, Dawood FS, Fink RV, et al. Vital signs: burden and prevention of influenza and pertussis among pregnant women and infants—United States. MMWR Morb Mortal Wkly Rep. 2019;68(40):885–92.
Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384(9953):1521–8.
Godoy P, Masa-Calles J. The effect of maternal pertussis vaccination on the epidemiology of pertussis in Spain. Enferm Infecc Microbiol Clin (Engl Ed). 2022;40(9):467–9.
Forsyth K, Plotkin S, Tan T, Wirsing-von-König CH. Strategies to decrease pertussis transmission to infants. Pediatrics. 2015;135(6):e1475–82.
Gökçe Ş, Kurugöl Z, Şöhret Aydemir S, Çiçek C, Aslan A, Koturoğlu G. Bordetella pertussis infection in hospitalized infants with acute bronchiolitis. Indian J Pediatr. 2018;85(3):189–93.
Şık G, Demirbuğa A, Annayev A, Çıtak A. The clinical characteristics and prognosis of pertussis among unvaccinated infants in the pediatric intensive care unit. Turk Pediatri Ars. 2020;55(1):54–9.
Chong CY, Yung CF, Tan NW, Acharyya S, Thoon KC. Risk factors of ICU or high dependency requirements amongst hospitalized pediatric pertussis cases: a 10 year retrospective series, Singapore. Vaccine. 2017;35(47):6422–8.
Laboratory confirmed cases of pertussis in England: annual report for 2019 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/881380/hpr0820_PRTSSS_annual.pdf.
Rothstein E, Edwards K. Health burden of pertussis in adolescents and adults. Pediatr Infect Dis J. 2005;24(5):S44–7.
Kandeil W, Atanasov P, Avramioti D, Fu J, Demarteau N, Li X. The burden of pertussis in older adults: what is the role of vaccination? A systematic literature review. Expert Rev Vaccines. 2019;18(5):439–55.
Esposito S, Principi N. Prevention of pertussis: an unresolved problem. Hum Vaccines Immunother. 2018;14(10):2452–9.
Aris E, Akpo EI, Bhavsar A, Harrington L, Merinopoulou E, Sawalhi-Leckenby N, et al. Late Breaking Abstract—The burden of pertussis in adults with asthma: a retrospective database study in England. Eur Respir J. 2020;56(suppl 64):4926.
Aris E, Harrington L, Bhavsar A, Simeone JC, Ramond A, Papi A, et al. Burden of pertussis in COPD: a retrospective database study in England. COPD J Chron Obstr Pulmon Dis. 2021;18(2):157–69.
Muloiwa R, Kagina BM, Engel ME, Hussey GD. The burden of laboratory-confirmed pertussis in low- and middle-income countries since the inception of the Expanded Programme on Immunisation (EPI) in 1974: a systematic review and meta-analysis. BMC Med. 2020;18(1):233.
Bonus RF, Delos-Reyes CA, Dy CAME, Alma RR. Clinical profile of pertussis among pediatric patients admitted at the Philippine General Hospital. Pediatr Infect Dis Soc Philipp J. 2015;1:21–7.
Decker MD, Edwards KM. Pertussis (whooping cough). J Infect Dis. 2021;224(Supplement 4):S310–20.
Wendelboe AM, Van Rie A. Diagnosis of pertussis: a historical review and recent developments. Expert Rev Mol Diagn. 2006;6(6):857–64.
van der Zee A, Schellekens JF, Mooi FR. Laboratory Diagnosis of pertussis. Clin Microbiol Rev. 2015;28(4):1005–26.
Abu Raya B, Bamberger E, Gershtein R, Peterman M, Srugo I. The laboratory diagnosis of Bordetella pertussis infection: a comparison of semi-nested PCR and real-time PCR with culture. Eur J Clin Microbiol Infect Dis. 2012;31(4):619–22.
Fisman DN, Tang P, Hauck T, Richardson S, Drews SJ, Low DE, et al. Pertussis resurgence in Toronto, Canada: a population-based study including test-incidence feedback modeling. BMC Public Health. 2011;11:694.
McGirr A, Fisman DN. Duration of pertussis immunity after DTaP immunization: a meta-analysis. Pediatrics. 2015;135(2):331–43.
Chit A, Zivaripiran H, Shin T, Lee JKH, Tomovici A, Macina D, et al. Acellular pertussis vaccines effectiveness over time: a systematic review, meta-analysis and modeling study. PLoS ONE. 2018;13(6): e0197970.
Lapidot R, Gill CJ. The pertussis resurgence: putting together the pieces of the puzzle. Trop Dis Travel Med Vaccines. 2016;2:26.
Lam C, Octavia S, Ricafort L, Sintchenko V, Gilbert GL, Wood N, et al. Rapid increase in pertactin-deficient Bordetella pertussis isolates Australia. Emerg Infect Dis. 2014;20(4):626–33.
Bamberger E, Abu Raya B, Cohen L, Golan-Shany O, Davidson S, Geffen Y, et al. Pertussis resurgence associated with pertactin-deficient and genetically divergent Bordetella pertussis isolates in Israel. Pediatr Infect Dis J. 2015;34(8):898–900.
Martin SW, Pawloski L, Williams M, Weening K, DeBolt C, Qin X, et al. Pertactin-negative Bordetella pertussis strains: evidence for a possible selective advantage. Clin Infect Dis. 2015;60(2):223–7.
Kim G, Berry JG, Janes JL, Perez A, Hall M. Association of maternal Tdap recommendations with pertussis hospitalizations of young infants. Hosp Pediatr. 2022;12(3):e106–9.
Santana CP, Luhm KR, Shimakura SE. Impact of Tdap vaccine during pregnancy on the incidence of pertussis in children under one year in Brazil—a time series analysis. Vaccine. 2021;39(6):976–83.
Avila-Agüero ML, Camacho-Badilla K, Ulloa-Gutierrez R, Espinal-Tejada C, Morice-Trejos A, Cherry JD. Epidemiology of pertussis in Costa Rica and the impact of vaccination: a 58-year experience (1961–2018). Vaccine. 2022;40(2):223–8.
Chong C-Y, Tan NW-H, Yung C-F, Li J, Kam K-Q, Nadua K, et al. Effectiveness of maternal pertussis vaccination in Singapore: a test-negative case-control study. Vaccine. 2022;40(46):6570–4.
Petousis-Harris H, Walls T, Watson D, Paynter J, Graham P, Turner N. Safety of Tdap vaccine in pregnant women: an observational study. BMJ Open. 2016;6(4): e010911.
Donegan K, King B, Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ. 2014;349: g4219.
Simayi A, Zhu L, Jin H. Safety and immunogenicity of pertussis vaccine immunization during pregnancy: a meta-analysis of randomized clinical trials. J Trop Med. 2022;2022:4857872.
Laverty M, Crowcroft N, Bolotin S, Hawken S, Wilson K, Amirthalingam G, et al. Health outcomes in young children following pertussis vaccination during pregnancy. Pediatrics. 2021;147(5):e2020042507.
Maertens K, Orije MRP, Herzog SA, Mahieu LM, Hens N, Van Damme P, et al. Pertussis immunization during pregnancy: assessment of the role of maternal antibodies on immune responses in term and preterm born infants. Clin Infect Dis. 2021;74:189–98.
Ercan TE, Sonmez C, Vural M, Erginoz E, Torunoğlu MA, Perk Y. Seroprevalance of pertussis antibodies in maternal and cord blood of preterm and term infants. Vaccine. 2013;31(38):4172–6.
Türkoğlu E, Sönmez C, Özer E, Çöplü N, Kurugöl Z. Low pertussis antibody levels in maternal and umbilical cord blood samples in Turkey. Turk J Pediatr. 2016;58(6):573–8.
Naidu MA, Muljadi R, Davies-Tuck ML, Wallace EM, Giles ML. The optimal gestation for pertussis vaccination during pregnancy: a prospective cohort study. Am J Obstet Gynecol. 2016;215(2):237.e1-6.
Abu Raya B, Bamberger E, Almog M, Peri R, Srugo I, Kessel A. Immunization of pregnant women against pertussis: the effect of timing on antibody avidity. Vaccine. 2015;33(16):1948–52.
Calvert A, Amirthalingam G, Andrews N, Basude S, Coleman M, Cuthbertson H, et al. Optimising the timing of whooping cough immunisation in mums (OpTIMUM) through investigating pertussis vaccination in pregnancy: an open-label, equivalence, randomised controlled trial. Lancet Microbe. 2023;4(5):e300–8.
Amirthalingam G, Campbell H, Ribeiro S, Stowe J, Tessier E, Litt D, et al. Optimization of timing of maternal pertussis immunization from 6 years of postimplementation surveillance data in England. Clin Infect Dis. 2023;76(3):e1129–39.
Abu Raya B, Srugo I, Kessel A, Peterman M, Bader D, Gonen R, et al. The effect of timing of maternal tetanus, diphtheria, and acellular pertussis (Tdap) immunization during pregnancy on newborn pertussis antibody levels—a prospective study. Vaccine. 2014;32(44):5787–93.
Abu-Raya B, Giles ML, Kollmann TR, Sadarangani M. The effect of timing of tetanus-diphtheria-acellular pertussis vaccine administration in pregnancy on the avidity of pertussis antibodies. Front Immunol. 2019;10:2423.
Healy CM, Rench MA, Swaim LS, Smith EO, Sangi-Haghpeykar H, Mathis MH, et al. Association between third-trimester Tdap immunization and neonatal pertussis antibody concentration. JAMA. 2018;320(14):1464–70.
Abu-Raya B, Maertens K, Munoz FM, Zimmermann P, Curtis N, Halperin SA, et al. Factors affecting antibody responses to immunizations in infants born to women immunized against pertussis in pregnancy and unimmunized women: individual-participant data meta-analysis. Vaccine. 2021;39(44):6545–52.
Abu-Raya B, Maertens K, Munoz FM, Zimmermann P, Curtis N, Halperin SA, et al. The effect of tetanus-diphtheria-acellular-pertussis immunization during pregnancy on infant antibody responses: individual-participant data meta-analysis. Front Immunol. 2021;12: 689394.
Abu-Raya B, Forsyth K, Halperin SA, Maertens K, Jones CE, Heininger U, et al. Vaccination in pregnancy against pertussis: a consensus statement on behalf of the global pertussis initiative. Vaccines (Basel). 2022;10(12):1990.
Liang JL, Tiwari T, Moro P, Messonnier NE, Reingold A, Sawyer M, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(2):1–44.
Healy CM, Rench MA, Baker CJ. Implementation of cocooning against pertussis in a high-risk population. Clin Infect Dis. 2011;52(2):157–62.
Castagnini LA, Healy CM, Rench MA, Wootton SH, Munoz FM, Baker CJ. Impact of maternal postpartum tetanus and diphtheria toxoids and acellular pertussis immunization on infant pertussis infection. Clin Infect Dis. 2012;54(1):78–84.
Quinn HE, Snelling TL, Habig A, Chiu C, Spokes PJ, McIntyre PB. Parental Tdap boosters and infant pertussis: a case-control study. Pediatrics. 2014;134(4):713–20.
Terranella A, Asay GR, Messonnier ML, Clark TA, Liang JL. Pregnancy dose Tdap and postpartum cocooning to prevent infant pertussis: a decision analysis. Pediatrics. 2013;131(6):e1748–56.
Skowronski DM, Janjua NZ, Sonfack Tsafack EP, Ouakki M, Hoang L, De Serres G. The number needed to vaccinate to prevent infant pertussis hospitalization and death through parent cocoon immunization. Clin Infect Dis. 2011;54(3):318–27.
Skoff TH, Cohn AC, Clark TA, Messonnier NE, Martin SW. Early Impact of the US Tdap vaccination program on pertussis trends. Arch Pediatr Adolesc Med. 2012;166(4):344–9.
Quinn HE, McIntyre PB. The impact of adolescent pertussis immunization, 2004–2009: lessons from Australia. Bull World Health Organ. 2011;89(9):666–74.
Purdy KW, Hay JW, Botteman MF, Ward JI. Evaluation of strategies for use of acellular pertussis vaccine in adolescents and adults: a cost-benefit analysis. Clin Infect Dis. 2004;39(1):20–8.
Tanriover MD, Soyler C, Ascioglu S, Cankurtaran M, Unal S. Low seroprevalence of diphtheria, tetanus and pertussis in ambulatory adult patients: the need for lifelong vaccination. Eur J Intern Med. 2014;25(6):528–32.
Ward JI, Cherry JD, Chang SJ, Partridge S, Keitel W, Edwards K, et al. Bordetella pertussis infections in vaccinated and unvaccinated adolescents and adults, as assessed in a national prospective randomized acellular pertussis vaccine trial (APERT). Clin Infect Dis. 2006;43(2):151–7.
Baxter R, Bartlett J, Rowhani-Rahbar A, Fireman B, Klein NP. Effectiveness of pertussis vaccines for adolescents and adults: case-control study. BMJ Brit Med J. 2013;347: f4249.
Kim DK, Hunter P. Recommended adult immunization schedule, United States, 2019. Ann Intern Med. 2019;170(3):182–92.
Heininger U. Vaccination of health care workers against pertussis: meeting the need for safety within hospitals. Vaccine. 2014;32(38):4840–3.
Immunization of Health-Care Personnel. recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(Rr-7):1–45.
EKMUD Erişkin Bağışıklama Rehberi 2019 [press release].
The Australian Immunisation Handbook.
Doğum Öncesi Bakım Yönetim Rehberi https://hsgm.saglik.gov.tr/depo/Yayinlarimiz/Rehberler/dogum_oncesi_bakim_08-01-2019_1.pdf.
Organisation WH. https://www.who.int/teams/immunization-vaccines-and-biologicals/essential-programme-on-immunization/implementation/reducing-missed-opportunities-for-vaccination-(mov).
Acknowledgements
Medical Writing/Editorial Assistance
Authors’ coordination, editorial assistance, and journal submission assistance were provided by Dr. Ferda Kiziltas at Remedium Consulting Group and funded by Sanofi.
Funding
Authors’ coordination and journal submission (including the rapid service fee) assistance were provided by Dr. Ferda Kiziltas at Remedium Consulting Group funded by Sanofi. Sanofi employees did not have any involvement in the manuscript content or the decision for journal submission.
Author information
Authors and Affiliations
Contributions
Tamer Pehlivan, Ener Cagri Dinleyici, Ates Kara, Zafer Kurugol, Hasan Tezer, Nur Baran Aksakal, Aydan Biri, and Alpay Azap contributed to literature review, data analysis, and manuscript development. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of Interest
Ener Cagri Dinleyici performs contract work funded by GSK, Sanofi Pasteur, and Pfizer. Tamer Pehlivan, Ates Kara, Zafer Kurugol, Hasan Tezer, Nur Baran Aksakal, Aydan Biri, and Alpay Azap declare no conflict of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pehlivan, T., Dinleyici, E.C., Kara, A. et al. The Present and Future Aspects of Life-Long Pertussis Prevention: Narrative Review with Regional Perspectives for Türkiye. Infect Dis Ther 12, 2495–2512 (2023). https://doi.org/10.1007/s40121-023-00876-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00876-0