Abstract
Introduction
Remdesivir is a registered treatment for hospitalised patients with COVID-19 that has moderate clinical effectiveness. Anecdotally, some patients’ respiratory insufficiency seemed to recover particularly rapidly after initiation of remdesivir. In this study, we investigated if this rapid improvement was caused by remdesivir, and which patient characteristics might predict a rapid clinical improvement in response to remdesivir.
Methods
This was a multicentre observational cohort study of hospitalised patients with COVID-19 who required supplemental oxygen and were treated with dexamethasone. Rapid clinical improvement in response to treatment was defined by a reduction of at least 1 L of supplemental oxygen per minute or discharge from the hospital within 72 h after admission. Inverse probability of treatment-weighted logistic regression modelling was used to assess the association between remdesivir and rapid clinical improvement. Secondary endpoints included in-hospital mortality, ICU admission rate and hospitalisation duration.
Results
Of 871 patients included, 445 were treated with remdesivir. There was no influence of remdesivir on the occurrence of rapid clinical improvement (62% vs 61% OR 1.05, 95% CI 0.79–1.40; p = 0.76). The in-hospital mortality was lower (14.7% vs 19.8% OR 0.70, 95% CI 0.48–1.02; p = 0.06) for the remdesivir-treated patients. Rapid clinical improvement occurred more often in patients with low C-reactive protein (≤ 75 mg/L) and short duration of symptoms prior to hospitalisation (< 7 days) (OR 2.84, 95% CI 1.07–7.56).
Conclusion
Remdesivir generally does not increase the incidence of rapid clinical improvement in hospitalised patients with COVID-19, but it might have an effect in patients with short duration of symptoms and limited signs of systemic inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
We investigated if rapid improvement in hospitalized patients with COVID-19 was caused by remdesivir, and which patient characteristics predict rapid clinical improvement in response to remdesivir. |
What was learned from the study? |
Remdesivir generally does not increase the incidence of rapid clinical improvement in hospitalised patients with COVID-19. |
Remdesivir might have an effect on rapid clinical improvement in patients with short duration of symptoms and limited signs of systemic inflammation. |
Remdesivir might have an effect on in-hospital mortality. |
Introduction
Remdesivir was granted conditional marketing authorization for coronavirus disease 2019 (COVID-19) in June 2020. In guidelines worldwide, it is currently recommended as treatment for hospitalised patients requiring supplemental oxygen [1,2,3]. Despite 2 years of use, there is still controversy about the clinical effectiveness and the optimal timing of remdesivir for treatment of hospitalised patients with COVID-19. Randomised controlled trials showed an effect on mortality and progression of disease, but these effects were moderate, and the number needed to treat remained large [4,5,6]. Despite the moderate overall effect of remdesivir in hospitalised patients with COVID-19, there might be subgroups of patients who benefit more than this average effect.
Remdesivir is an adenosine analogue prodrug and after extensive metabolism works by incorporation into viral RNA where it causes chain termination, resulting in the inhibition of viral replication [7]. The effect of remdesivir is therefore, from the pharmacological point of view, maximal during the viral replication phase of COVID-19 [7]. After viral replication an ongoing inflammatory response is likely to cause pulmonary damage and morbidity in affected patients [8]. When inflammation is the primary driver of disease, not antivirals but other drugs like corticosteroids, Jak inhibitors or interleukin-6 antagonists are likely more beneficial as has been shown in multiple studies [9,10,11]. Patient characteristics and biomarkers predicting active viral replication are therefore of importance in determining the window of opportunity for remdesivir as active antiviral therapy.
Worldwide, clinicians have reported on cases of individuals with an extraordinary quick clinical recovery during remdesivir therapy [12,13,14,15,16]. Studies explicitly aimed at exploring the phenomenon of such rapid response to remdesivir are unavailable, despite the antiviral working mechanism and pharmacological profile of remdesivir providing a rationale for an early response [7, 14]. Exploring the characteristics of the patients with rapid clinical improvement could aid in selecting the optimal population for remdesivir treatment in hospitalised patients.
In the Netherlands, the national COVID-19 guideline changed over time from weak recommendation to discommend the use of remdesivir in hospitalised patients with COVID-19. However, differences between Dutch hospital practices remained, ranging from no to standard remdesivir treatment. As prescription of remdesivir was dependent on the date of hospitalisation and the local hospital treatment policy, this created a unique opportunity to evaluate the benefit of remdesivir in daily clinical practice. We performed a multicentre observational cohort study investigating if remdesivir resulted in an increased incidence of rapid clinical improvement, and which patient characteristics are associated with rapid clinical improvement associated with remdesivir treatment.
Methods
Study Design and Setting
This was a multicentre retrospective observational cohort study performed in three teaching hospitals and one university hospital in the Leiden–Hague region, the Netherlands. The study was conducted in compliance with the Declaration of Helsinki and was approved by the COVID-19 Scientific and Ethics Committee for observational studies of the Leiden University Medical Centre and local scientific boards of the participating hospitals. Given the extraordinary nature of the COVID-19 pandemic, the ethics committee waived the need for informed consent for this study that used pseudonymised data based on routinely collected information.
Study Population
The study population consisted of patients who were diagnosed with severe COVID-19 and hospitalised within 7 days after registration of the diagnosis. Diagnosis was based on a positive reverse transcription polymerase chain reaction (RT-PCR) for SARS-CoV-2 or, in case no RT-PCR result was available, on a registered COVID-19 diagnosis code or notification of verified COVID-19 in the electronic patient file. To be included, patients needed to be hospitalised for at least 24 h between 1 March 2020 and 1 March 2021, to require at least 1 L/min of supplemental oxygen at any point during the 24 h after presentation to the hospital and to be treated with dexamethasone. Dexamethasone was chosen as an inclusion criterion because this is currently recommended as standard treatment for severe COVID-19 [1]. Patients were excluded if they were admitted from or transferred to another hospital within 72 h after presentation. Also, patients who were admitted to the ICU or died within 24 h after hospital admission were excluded.
Exposure
All patients receiving at least one dose of remdesivir within 24 h after admission to the hospital were included into the remdesivir group. The standard dosing regimen for remdesivir was a loading dose of 200 mg followed by 4 days of 100 mg daily. In some cases, this was extended to a total of 10 days. Whether a patient received remdesivir was mainly dependent on the location of treatment (due to changes between hospital treatment protocols), the period of presentation (local treatment protocols changed over time) and availability of remdesivir (e.g. scarcity).
Outcomes
The primary endpoint was rapid clinical improvement defined as clinical improvement between 24 and 72 h after hospitalisation. Clinical improvement was defined by a decrease in supplemental oxygen requirement of at least 1 L/min or discharge from the hospital alive excluding those who were discharged for hospice care which was considered as ‘no relief of symptoms’. Patients with an increase in oxygen requirement compared to baseline, admission to the ICU and in-hospital death by any cause were considered to have no rapid clinical improvement. Secondary endpoints were in-hospital mortality, ICU admissions and duration of hospitalisation.
Data Sources
All data were collected during routine practice and extracted from patients’ electronic hospital records using a natural language processing tool and Clinical Data Collector (CTcue B.V., Amsterdam, the Netherlands) [17]. The results collected with automatic text mining from unstructured text in the patient files were compared to manual review within a sample of the population.
Statistical Analysis
All analyses were performed using R (version 4.0.3). Descriptive statistics, continuous variables were presented as means and standard deviation or as medians with interquartile range, and categorical variables were presented as numbers and percentages. Missing data were imputed 30 times using multiple imputation by chained equations.
Because of the non-random assignment to treatment with remdesivir and thereby potential confounding by indication, the propensity score of receiving remdesivir was estimated using binary logistic regression analysis. Variables that were included in the propensity score (PS) model were chosen on the basis of clinical experience and were expected to either influence the probability of prescription of remdesivir or were expected to be associated with the primary outcome. The following variables were selected: age, duration of complaints prior to admission, admission quartile, registered limitation of treatment effort, baseline supplemental oxygen requirement, renal function tests (estimated glomerular filtration rate (eGFR) calculated using the CKD-EPI creatinine equation, creatinine, urea), laboratory tests (alanine aminotransferase (ALT), bilirubin, lymphocyte count, C-reactive protein (CRP), creatine kinase), renal transplantation and chronic renal disease. Inverse probability of treatment weighting (IPTW) using the PS was performed to correct for confounder imbalances and create a pseudo population for analysis, in which potential confounding variables were balanced between treatment groups. Weights were truncated at the 1st and 99th percentiles of the observed PS weighting distribution to minimize the impact of extreme weights. The treated and untreated group were considered balanced if the standardised mean difference (SMD) of any clinically relevant parameters was less than 10%.
Within the weighted pseudo population, logistic regression analysis without inclusion of covariates was performed to estimate the effect of remdesivir on rapid clinical improvement. Odds ratios for the secondary outcomes mortality and ICU admission were also estimated using logistic regression analysis. Time to discharge was analysed using Cox regression modelling and presented as hazards ratio. All analyses were performed separately within each imputed dataset and results were pooled using Rubin’s rules.
Subgroup Analysis
To explore patient characteristics that might influence the association between remdesivir and rapid clinical improvement, several post hoc subgroup analyses were performed. These were CRP (≤ 75 mg/L and > 75 mg/L), based on the cut-ff criterion used for systemic inflammation in the RECOVERY trial [9]. Days of complaints prior to admission (< 7 days and ≥ 7 days), the same interval as used for remdesivir in outpatients in the PINETREE study [18]. Baseline supplemental oxygen requirement (1–4 L/min, 4–12 L/min and ≥ 12 L/min), age (< 60 years, 60–75 years and ≥ 75 years), and a combination of CRP and days of complaints prior to admission (< 7 days and CRP ≤ 75 mg/L). The last one was used to explore the combination of two measurements that could predict the viral replication phase in COVID-19. Logistic regression analyses with interaction terms were used to explore effect modification and thereby the subgroup effect; all performed subgroup analyses were reported in the manuscript.
Sensitivity Analysis
Three sensitivity analyses were performed. The first, to establish the robustness of the confounder correction with IPTW analysis, a second analysis using logistic regression modelling with traditional correction for confounders was performed. The selected confounders were the same variables as included in the analysis with IPTW. Second, a sensitivity analysis also including patients who did not receive dexamethasone as COVID-19 treatment was performed. These patients were not included in the main analysis but added to the corresponding groups based on the prescription of remdesivir. Third, we performed the primary analysis, but only a reduction of more than 2 L/min in supplemental oxygen requirement was considered rapid clinical improvement.
Results
Population
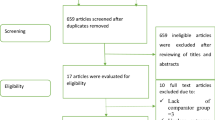
In total, 3365 patients were treated for COVID-19 during the study period; 871 of those met the study eligibility criteria, 445 of whom were treated with remdesivir and 426 were not. Inclusion and exclusion of the patients are presented in the flowchart in Fig. 1. Details on percentages missing data are visualised in supplementary Fig. 1.
The median age was 70 years; 531 (61%) were male. The most frequent comorbidities were hypertension (27%) and diabetes mellitus (22%). Median oxygen requirement at baseline was 4 L/min (IQR 2–10) and the median duration of complaints prior to admission was 8.9 days (IQR 6.1–11). The median duration of remdesivir therapy was 4 days (IQR 3–5). Patient characteristics before and after IPTW are described in Table 1. Following IPTW, all variables were balanced across treatment groups (i.e., SMD < 0.1), except for haemoglobin concentration and diastolic blood pressure (see Love plot in supplementary Fig. 2).
Outcomes
Rapid clinical improvement occurred in 540 patients (62%) in the unweighted population; 209 patients (24%) were discharged from the hospital within 72 h and 331 (38%) were still hospitalised but required less oxygen; 235 (27%) patients were still on the general ward but required more oxygen suppletion, 52 (6.0%) were admitted to the ICU and 47 (5.4%) died within 72 h. Differences in patient characteristics between patients with and without rapid clinical improvement are presented in supplementary Table 1. After adjustment with IPTW there was no association between remdesivir and rapid clinical improvement in the entire population (62% vs 61% OR 1.05, 95% CI 0.79–1.40; p = 0.76).
The in-hospital mortality was lower in the remdesivir-treated group (14.6% vs 19.8% OR 0.70, 95% CI 0.48–1.02; p = 0.06). ICU admission (11.3% vs 11.4% OR 0.99, 95% CI 0.64–1.52; p = 0.97) and hospitalisation duration (HR for discharge 1.13, 95% CI 0.97–1.31; p = 0.13) were similar.
Subgroup Analysis
The results from the subgroup analysis are visualised in Fig. 2. We did not find an association in the subgroups based on days of symptoms prior to admission or supplemental oxygen requirement at baseline or CRP concentration. An association was found in the subgroup with low CRP (≤ 75 mg/L) and short duration of symptoms (< 7 days) prior to admission. There were 66 patients treated with remdesivir (15% of all remdesivir-treated patients) and 48 non-treated patients (11% of all non-remdesivir-treated patients) with low CRP and short duration of symptoms prior to IPTW. There was an increase in rapid clinical improvement associated with remdesivir (OR 2.83, 95% CI 1.07–7.56) in this subgroup compared to the rest of the patients (OR 0.92, 95% CI 0.86–1.25; p for interaction 0.03).
Sensitivity Analysis
The direction and magnitude of the association seen in the sensitivity analysis with logistic regression modelling with confounder selection were like the association seen in the main analysis. The results are presented in supplementary Fig. 3. When patients without dexamethasone treatment were included, the results were similar. A total of 1334 patients were available for this analysis. No association between remdesivir and rapid clinical improvement in the entire population was found (OR 1.05, 95% CI 0.82–1.34; p = 0.69). In the subgroup with CRP ≤ 75 mg/L and days of symptoms < 7 days, remdesivir resulted in an increase in rapid clinical improvement (OR 2.35, 95% CI 1.08–5.09) (supplementary Fig. 4). Also, when > 2-L reduction of supplemental oxygen was considered rapid clinical improvement, the results were similar. There was no association in the general population (OR 1.06, 95% CI 0.80–1.40; p = 0.69) but there might be an association in the subgroup with CRP ≤ 75 mg/L and days of symptoms < 7 days (OR 2.49, 95% CI 0.98–6.30) (supplementary Fig. 5).
Discussion
In this multicentre propensity score weighted observational cohort study, we evaluated rapid clinical response to remdesivir treatment in a cohort of hospitalised patients with COVID-19 receiving oxygen and dexamethasone. We found that remdesivir overall did not lead to an increased incidence of rapid clinical improvement, except for patients with low inflammation (CRP ≤ 75 mg/L) and a short symptom duration (< 7 days) prior to admission. Thus, these data suggest that the efficacy of remdesivir treatment in hospitalised patients with COVID-19 might depend on the right timing and extent of inflammation.
The overall absence of rapid clinical improvement on remdesivir treatment in hospitalised patients with COVID-19 is not unexpected. The ACCT-1 trial and a large retrospective cohort study showed that the median time to improvement with remdesivir treatment was respectively 10 and 7 days, which is later than the interval used for rapid clinical improvement (24–72 h) [5, 19]. The in-hospital mortality in our population was comparable with the patients requiring supplemental oxygen in the SOLIDARITY trial (17% vs 15%) [4]. Remdesivir has been shown to reduce the mortality risk in the SOLIDARITY trial and other real-world studies [4, 19,20,21]. In our study the OR (0.70, 95% CI 0.48–1.02) for mortality also suggests a potential survival benefit for remdesivir, though not statistically significant, which is likely a result of the relatively smaller sample size of our study. It seems like remdesivir has an effect on mortality but not rapid response; an explanation for this could be an effect of remdesivir on deterioration later during admission. Based on the working mechanism of remdesivir it might mitigate the inflammatory response in patients by preventing prolonged viral replication.
We did not find an association between remdesivir and hospitalisation duration. This might be explained by the urge of clinicians to finish the 5 or 10 days of treatment before discharging recovered patients, as has also has been shown in the SOLIDARITY trial and real-world data [4, 22]. Whether the full 5 days of treatment are necessary for optimal effectiveness is currently unknown. In our study, an effect of remdesivir was already seen within 24–72 h after admission in the subgroup of patients with limited signs of systemic inflammation (CRP ≤ 75 mg/l). A shorter duration of treatment could therefore be considered in these patients, like the 3 days of therapy currently recommended and proven to be effective in outpatients [18, 23]. This might be beneficial in reducing the hospitalisation duration and thereby treatment costs. However, future studies on remdesivir treatment duration among hospitalised patients are needed.
In the subgroup analysis, an association between remdesivir treatment and rapid clinical improvement was found in patients with a duration of symptoms < 7 days and a CRP ≤ 75 mg/L. We did not find an association between duration of symptoms and rapid clinical improvement alone. Nevertheless, previous studies indicated that remdesivir works best in outpatients who started within 7 days and in hospitalised patients remdesivir is more effective in patients within 7 or 10 days after the start of symptoms [5, 18, 24]. Apart from the difference in primary endpoint, an explanation is that the exact symptom duration at admission is often uncertain. Especially in elderly patients with multiple comorbidities, the patients’ history is often not clear about the time COVID-19 symptoms started. The combination of this parameter with a laboratory-confirmed measurement of inflammation improved the identification of patients who benefit from remdesivir treatment.
This was also found in a previous study that explored the influence of CRP combined with a short duration of symptoms as predictor of remdesivir response. Padilla et al. found an effect on mortality in patients treated with remdesivir, tocilizumab and dexamethasone in combination with low CRP (< 38 mg/L), a high viral load (low cycle threshold value of SARS-CoV-2 PCR on nasopharyngeal swab) and duration of symptoms < 5 days [25]. Even though the population, the treatment regimen and the CRP cutoff differed from our study, the results indicate that remdesivir works best in the early stages from COVID-19 when the inflammatory response is still limited. A possible explanation is that during COVID-19, persistent viral replication can lead to a second inflammatory response that results in cytokine release and progression to more severe disease [26]. It has also been shown that persistent high SARS-CoV-2 viral load is associated with worse outcomes [27]. Remdesivir, through interaction with the SARS-CoV-2 polymerase and inhibition of RNA synthesis, could thus contribute to reducing viral replication and prevent the secondary inflammatory reaction shown in clinical practice as rapid clinical improvement.
To our knowledge this study is the first to specifically investigate rapid clinical improvement as a primary endpoint in hospitalised patients with COVID-19. Rapid clinical improvement can best be classified as a measurement of prevention of deterioration to worse outcomes/clinical status in hospitalised patients and therefore a plausible result of remdesivir therapy based on pharmacokinetics and working mechanism [7, 28]. It can be used to identify patients who benefit most from remdesivir treatment, but could also be used in future studies to identify patients who benefit temporarily from remdesivir and deteriorate later on. In the latter group, remdesivir therapy only postpones deterioration, which is also beneficial as it gives clinicians more time to start alternative supportive treatment or concomitant therapies.
This study has limitations, as this was a retrospective observational study. Although efforts were made to balance the differences between patients with and without remdesivir and correct for missing data using multiple imputation, we are unable to fully exclude the potential influence of residual confounding. Several conclusions result from subgroup analyses which were not corrected for multiple testing and therefore might be chance findings that need verification in future studies. However, as the combination of short duration of symptoms and low signs of inflammation are indicative of the viral replication phase, we consider the association we found between remdesivir treatment and rapid clinical improvement to be biologically sound.
Also, the study was performed with data collected primarily during the alpha and beta wave of the COVID-19 pandemic and almost exclusively from unvaccinated patients. Hospitalisation rates for COVID-19 and the severity of disease in hospitalised have changed over the course of the pandemic as a result of natural immunity and vaccinations. Therefore, the results of our study cannot be extrapolated to every patient admitted with COVID-19 in 2023. However, if patients are treated for COVID-19 as primary diagnosis and require supplemental oxygen, the effects of remdesivir are likely similar in current clinical practice, because the EC50 of remdesivir for SARS-CoV-2 has not significantly changed between the variants. Furthermore, we only selected patients treated with concomitant dexamethasone which is still considered standard treatment for patients with COVID-19 requiring supplemental oxygen [1, 29]. Nonetheless, verification of the study results in a population of vaccinated patients with COVID-19 infected with the current circulating SARS-CoV-2 strains would provide more insight into the added value of remdesivir in COVID-19 treatment.
Future studies are needed to verify the criteria for selection of patients who benefit most from remdesivir treatment. As a result of the unavailability of quantitative measures for the SARS-CoV-2 viral load and a relatively low percentage of immunosuppressed patients in our cohort, exploring the effects of remdesivir in subgroups stratified by these parameters was not feasible. Also, we only looked at baseline characteristics and treatments initiated within 24 h after hospitalisation. It would be interesting to see if low inflammation at later time points during hospitalisation is also associated with better effectiveness of remdesivir when initiated later during hospitalisation. Future studies should also investigate alternative remdesivir dosing regimens, duration of treatment and the combination of remdesivir treatment with other therapies like nirmatrelvir or anti-SARS-CoV-2 antibodies, especially in patients with initial rapid clinical improvement in response to remdesivir monotherapy, but deterioration later in the course of disease [30].
Conclusion
There was no general association between remdesivir and rapid clinical improvement in hospitalised patients with COVID-19. However, patients with a CRP ≤ 75 mg/L and < 7 days of symptoms prior to hospital admission might have an increased chance of rapid clinical improvement when treated with remdesivir. This indicates that patients with COVID-19 who present at the hospital with limited signs of systemic inflammation and short duration of symptoms benefit most from remdesivir treatment.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Infectious Diseases Society of America. ISDA guidelines on the treatment and management of patients with COVID-19. Infectious Diseases Society of America. 2022. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed 5 Jan 2023.
Lamontagne F, Agarwal A, Rochwerg B, et al. A living WHO guideline on drugs for COVID-19. BMJ. 2020;370:m3379.
Bartoletti M, Azap O, Barac A, et al. ESCMID COVID-19 living guidelines: drug treatment and clinical management. Clin Microbiol Infect. 2022;28:222–38.
WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet. 2022;399:1941–53.
Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19: final report. N Engl J Med. 2020;383:1813–26.
Ader F, Bouscambert-Duchamp M, Hites M, et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis. 2022;22:209–21.
Jorgensen SCJ, Kebriaei R, Dresser LD. Remdesivir: review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19. Pharmacotherapy. 2020;40:659–71.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4.
RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–45.
Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalised patients with COVID-19. N Engl J Med. 2021;384:693–704.
Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9:1407–18.
Buckland MS, Galloway JB, Fhogartaigh CN, et al. Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat Commun. 2020;11:6385.
NEJM Journal Watch. Does remdesivir actually work? 2020. https://blogs.jwatch.org/hiv-id-observations/index.php/does-remdesivir-actually-work/2020/10/18/. Accessed 18 Dec 2022.
Cubeddu LX, Cubeddu RJ. Early remdesivir treatment in COVID-19: why wait another day? J Med Virol. 2021;93:4078–80.
Kajova M, Kekäläinen E, Anttila VJ, Paajanen J. Successful treatment with a short course of remdesivir in a case of prolonged COVID-19 in a lymphoma patient. Infect Dis (Lond). 2022;54:455–9.
Palomba E, Carrabba M, Zuglian G, et al. Treatment of SARS-CoV-2 relapse with remdesivir and neutralizing antibodies cocktail in a patient with X-linked agammaglobulinaemia. Int J Infect Dis. 2021;110:338–40.
CTcue. 2023. https://ctcue.com/. Accessed 5 Jan 2023.
Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med. 2022;386:305–15.
Garibaldi BT, Wang K, Robinson ML, et al. Real-world effectiveness of remdesivir in adults hospitalised with coronavirus disease 2019 (COVID-19): a retrospective, multicenter comparative effectiveness study. Clin Infect Dis. 2022;75:e516–24.
Marrone A, Nevola R, Sellitto A, et al. Remdesivir plus dexamethasone versus dexamethasone alone for the treatment of coronavirus disease 2019 (COVID-19) patients requiring supplemental O2 therapy: a prospective controlled nonrandomized study. Clin Infect Dis. 2022;75:e403–9.
Mozaffari E, Chandak A, Zhang Z, et al. Remdesivir treatment in hospitalised patients with coronavirus disease 2019 (COVID-19): a comparative analysis of in-hospital all-cause mortality in a large multicenter observational cohort. Clin Infect Dis. 2022;75:e450–8.
Garibaldi BT, Wang K, Robinson ML, et al. Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalised patients with COVID-19. JAMA Netw Open. 2021;4:e213071.
Piccicacco N, Zeitler K, Ing A, et al. Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge. J Antimicrob Chemother. 2022;77:2693–700.
Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76:3296–302.
Padilla S, Polotskaya K, Fernández M, et al. Survival benefit of remdesivir in hospitalised COVID-19 patients with high SARS-CoV-2 viral loads and low-grade systemic inflammation. J Antimicrob Chemother. 2022;77:2257–64.
Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, et al. COVID-19 infection: an overview on cytokine storm and related interventions. Virology J. 2022;19:92.
Gao Y-D, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76:428–55.
Humeniuk R, Mathias A, Cao H, et al. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin Transl Sci. 2020;13:896–906.
Vangeel L, Chiu W, De Jonghe S, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 omicron and other variants of concern. Antivir Res. 2022;198: 105252.
Abdelnabi R, Maes P, de Jonghe S, Weynand B, Neyts J. Combination of the parent analogue of remdesivir (GS-441524) and molnupiravir results in a markedly potent antiviral effect in SARS-CoV-2 infected Syrian hamsters. Front Pharmacol. 2022;13:1072202.
Funding
The Apotheek Haagse Ziekenhuizen (EL & EW) received an unrestricted grant from Gilead Sciences, including the journal’s Rapid Service Fee. Gilead Sciences had no role in the design and conduct of this study; data collection; analysis and interpretation of the data; review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Emiel Leegwater, Erik B Wilms and Cees van Nieuwkoop conceived the idea and designed the study; Emiel Leegwater, Cees van Nieuwkoop, Menno Benard, Eveline E Roelofsen and Rachel Knevel collected the data. Emeil Leegwater, Cees van Nieuwkoop, Rolf HH Groenwold and Frits R Rosendaal designed the statistical analysis. Eemil Leegwater performed the statistical analysis and drafted the first version of the manuscript. Lisa Dol, Nathalie M Delfos, Machteld van der Feltz, Femke PN Mollema, Liesbeth BE Bosma, Loes E Visser, Thomas H Ottens, Nathalie D van Burgel, Sesmu M Arbous, Lahssan H El Bouazzaoui, Mark GJ de Boer and Leo G Visser provided critical feedback on the study protocol, discussed the results, and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Emiel Leegwater and Erik B Wilms of The Hague Hospital Pharmacy received an unrestricted research grant from Gilead Sciences Inc. T.H.O. participated in a COVID-19 Digital Advisory Board from Gilead Sciences Inc. Lisa Dol, Menno R Benard, Eveline E Roelofsen, Nathalie M Delfos, Machteld van der Feltz, Femke PN Mollema, Liesbeth BE Bosma, Loes E Visser, Thomas H Ottens, Nathalie D van Burgel, Sesmu M Arbous, Lahssan H El Bouazzaoui, Rachel Knevel, Rolf HH Groenwold, Mark GJ de Boer, Leo G Visser, Frits R Rosendaal, and Cees van Nieuwkoop have nothing to disclose.
Ethical Approval
The study was conducted in compliance with the Declaration of Helsinki and was approved by the COVID-19 Scientific and Ethics Committee for observational studies of the Leiden University Medical Centre and local scientific boards of the participating hospitals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Leegwater, E., Dol, L., Benard, M.R. et al. Rapid Response to Remdesivir in Hospitalised COVID-19 Patients: A Propensity Score Weighted Multicentre Cohort Study. Infect Dis Ther 12, 2471–2484 (2023). https://doi.org/10.1007/s40121-023-00874-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00874-2