Abstract
Recombinant vaccines against invasive meningococcal disease due to Neisseria meningitidis serogroup B (MenB) have shown substantial impact in reducing MenB disease in targeted populations. 4CMenB targets four key N. meningitidis protein antigens; human factor H binding protein (fHbp), Neisserial heparin binding antigen (NHBA), Neisseria adhesin A (NadA) and the porin A protein (PorA P1.4), with one or more of these expressed by most pathogenic MenB strains, while MenB-FHbp targets two distinct fHbp variants. While many countries recommend MenB immunisation in adults considered at high risk due to underlying medical conditions or immunosuppression, there are no recommendations for routine use in the general adult population. We reviewed the burden of MenB in adults, where, while incidence rates remain low (and far lower than in young children < 5 years of age at greatest risk), a substantial proportion of MenB cases (20% or more) is now observed in the adult population; evident in Europe, Australia, and in the United States. We also reviewed immunogenicity data in adults from clinical studies conducted during MenB vaccine development and subsequent post-licensure studies. A 2-dose schedule of 4CMenB generates hSBA titres ≥ 1:4 towards all four key vaccine target antigens in up to 98–100% of subjects. For MenB-FHbp, a ≥ fourfold rise in hSBA titres against the four primary representative test strains was observed in 70–95% of recipients following a 3-dose schedule. While this suggests potential benefits for MenB immunisation if used in adult populations, data are limited (especially for adults > 50 years) and key aspects relating to duration of protection remain unclear. Although a broader adult MenB immunisation policy could provide greater protection of the adult population, additional data are required to support policy decision-making.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Neisseria meningitidis serogroup B (MenB) is an important cause of invasive meningococcal disease in Europe, North America, Oceania and China. |
While incidence remains highest in young children and adolescents, up to 20% of MenB cases are now observed in the adult population. |
Effective MenB vaccines (4CMenB and MenB-FHbp) are now widely available, although recommended use in routine immunisation is focused towards use in infants and young children (in Europe) and adolescents and younger adults (in North America). Use in adults is limited to those with specific medical co-morbidities. Clinical studies have shown substantial impact in reducing MenB disease in targeted populations. |
Immunogenicity data indicate robust protective immune responses in adults. However, data are limited in those aged > 50 years, and persistence of response is unclear. |
While greater routine use of MenB vaccination in adult immunisation strategies can help reduce the case burden and adverse clinical outcomes observed in adults, additional data are required to inform such recommendations. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.23823081.
Introduction
Invasive meningococcal disease (IMD) due to Neisseria meningitidis remains an important global public health concern [1, 2]. Mortality is high, with reported case fatality rates (CFRs) ranging from 4% to 20% (and up to 30% in older adults) [3]. Serious sequelae, including a broad range of neurological complications (seizures, hearing loss, visual upset and language impairment), are reported in up to 20% of survivors [4,5,6], resulting in long-term physical disability, social impairment and reduced quality of life (QoL) [6, 7]. Such sequelae contribute to the reduced lifetime expectancy and premature death observed in those recovering from initial infection [6, 8].
Recognised risk factors for IMD span a range of individual and social elements. The greatest risk (as evident from greatest incidence rates) is seen in infants and young children, and then in adolescents and young adults [2, 9, 10]. IMD risk is higher in individuals with congenital or acquired immunosuppressive conditions (asplenia/splenic dysfunction, complement deficiency) or receiving specific immunosuppressive medications (e.g. complement component inhibitors) [11,12,13], and in specific groups such as men who have sex with men (MSM) [14]. In addition, social aspects such as overcrowding, including dormitory accommodation (e.g. in higher education facilities and for military personnel) and that associated with lower socioeconomic status are also associated with greater risk (and disease outbreaks) [1, 11, 15,16,17]. Mass gatherings and religious events such as the Hajj and Umrah pilgrimages are also well-recognized risk factors for meningococcal transmission and subsequent outbreaks [18, 19]. Risk is also greater in people experiencing homelessness [20], and in immigrant and refugee populations [21]. Tobacco smoking and passive exposure may also increase IMD risk [22].
Twelve N. meningitidis serogroups are recognised based on distinct capsular polysaccharide antigen expression. Of these, five serogroups are responsible for the great majority of global IMD cases; serogroups A, B, C, W and Y (MenA, MenB, MenC, MenW and MenY) [2, 10, 23, 24], with serogroup X also important in some settings, e.g. sub-Saharan Africa [25]. At present, MenB is the predominant causative IMD serogroup in many countries [26], evident across Europe [23, 27,28,29], the United States [30, 31], Canada [32], Australia [33,34,35,36], New Zealand [37] and elsewhere, including much of North Africa [38, 39], and also in China [40, 41] and Taiwan [42].
The development and introduction of effective MenB vaccines has transformed the meningococcal immunisation landscape [2, 43,44,45,46]. Two vaccines are widely available: the 4-component meningococcal serogroup B vaccine (4CMenB, Bexsero, GSK) and the bivalent factor H binding protein meningococcal serogroup B vaccine (MenB-FHbp, Trumenba, bivalent rLP2086; Pfizer) although licensing differences exist (Table 1). In Europe and Australia, 4CMenB is approved from the age of 2 months upwards (with no upper age restriction) [47,48,49]. MenB-FHbp is approved in individuals ≥ 10 years in these settings [50,51,52]. In the United States, approval for 4CMenB or MenB-FHbp is only for use in those aged 10–25 years [53, 54]. In Canada, 4CMenB is approved for use in individuals from 2 months through 25 years of age [55] and MenB-FHbp in those aged 10–25 years [56]. In general, approval in other countries for 4CMenB or MenB-FHbp aligns with those used in Europe (although in some, e.g., Brazil, an upper age limit of 50 years exists) [57].
As we detail later in this article, adoption and implementation of MenB vaccines in routine immunisation programmes continues to evolve, with the current focus in most countries towards younger children or in adolescents (and also younger adults in the United States); at present, there are no recommendations for routine use in the general adult population [57,58,59,60]. Evidence for vaccine impact derived from such routine use (or when used in focused vaccination campaigns) have shown substantial impact in reducing MenB disease in targeted populations [61]. However, in contrast to conjugated MenC and MenACWY vaccines, studies evaluating the impact of MenB vaccines on N. meningitidis carriage have shown no evidence for any significant effect [61,62,63,64,65], underlining the importance of MenB vaccination for direct protection of all those at risk of IMD due to MenB.
One impact of the introduction of MenB immunisation targeted towards children and adolescents (with subsequent substantial declines in IMD in these age groups) is that significant proportion of overall MenB cases in many countries (approximately 20%) now occur in adults and the elderly; a feature seen in Europe [28, 29, 66], the United States [30, 31], and Australia [33,34,35,36]. Despite approval for use in adult populations in most countries where MenB vaccines are available (except North America), the current focus on childhood or adolescent MenB immunisation is such that the increasing burden of MenB disease in adults is likely to remain unchanged.
In the present review, we consider the burden of MenB in adults and describe the development and implementation of MenB vaccines. While data are limited, we review the immunogenicity and safety data for 4CMenB and MenB-FHbp when given to adult populations. While these are supportive of broader use of MenB immunisation in adult populations, an approach in alignment with growing support for a lifetime immunisation approach to vaccination policy [67, 68], we also highlight the need for additional data to inform such decision-making.
Methods
To inform this review, we initially searched PubMed electronic databases using search terms relating to meningitis and meningococcal disease to identify relevant publications reporting IMD epidemiology, meningococcal immunization policy, and clinical studies on 4CMenB and MenB-FHbp. The search strategy included a range of free-text and MeSH search terms, e.g. “meningococcal infection”, “N. meningitidis”, “IMD”, “4CMenB”, “MenB-FHbp” “Bexsero”, “Trumenba” in various combinations. Identified articles were supplemented by snowball sampling of relevant citations from the consulted papers. In addition, we reviewed relevant immunisation policy documents from a broad range of countries and publicly available reports on 4CMenB and MenB-FHbp approval and licensed indications. While comprehensive, our approach was not formally systematic. Searches were focused primarily on English language publications, although with no specific selection criteria or specific publication date limitations. Epidemiological data for Europe were also identified from the European Centre for Disease Prevention and Control (ECDC) Surveillance Atlas of Infectious Diseases tool [29] (last accessed 26 April 2023). In this, data for confirmed MenB case numbers were extracted for each year from 2011 onwards. As this tool presents data stratified in the following age groups (< 1 year; 1–4 years; 15–24 years; and ≥ 50 years), this format was retained in our reporting. For all other epidemiologic data, a broader range of age strata was used.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals.
Burden of MenB in Adults
The incidence of MenB is greatest in infants and young children < 5 years [2, 9, 10, 28]. Across Europe, surveillance data sourced from the ECDC Surveillance Atlas of Infectious Diseases tool indicate substantially higher MenB notification rates in infants > 1 year than other age groups [29] (Fig. 1). While the incidence of MenB is lower in the United States than in Europe, similar patterns are observed. The Centers for Disease Control and Prevention (CDC) report that between 2015 and 2019 the incidence of IMD due to MenB in infants > 1 year of age ranged from 0.42 to 0.82 per 100,000 (and 0.19 per 100,000 in 2020) [30,31,, 31, 69,70,71,72]. Although specific rates vary in different countries, incidence rates in other age strata are far lower (and lowest in adults). In the United Kingdom, with a relatively greater prevalence of MenB compared to most other countries, MenB notification rates in those > 50 years range from 0.29 per 100,000 in 2015 to 0.32 per 100,000 in 2019) [29]. In the United States, MenB incidence in adults is lower, between 0.01 and 0.04 per 100,000 in recent years [30,31,, 31, 69,70,71,72] (Fig. 1).
a Notification rates for IMD due to MenB across Europe (EU/EEA), and in the United Kingdom and France across 2011–2020 stratified by age [29]. For the United Kingdom, data for 2020 are not available. b MenB incidence rates in the United States (2015–2020) stratified by age [30,31,, 31, 69,70,71,72]. EU European Union, EEA European Economic Area, IMD invasive meningococcal disease, MenB N. meningitidis serogroup B
Despite this relatively lower incidence a substantial number of MenB cases occur in adults. This is illustrated by appraising data for confirmed MenB cases in regions/countries with robust surveillance reporting. In Europe, where a decline in MenB incidence between 2008 and 2017 has been observed across all age groups, this decline was greatest in younger children, less so in adolescents, and with the lowest relative decline observed in adults ≥ 50 years (where rates have remained relatively stable) [28]. This decline, and the recent implementation of routine infant 4CMenB immunisation in some countries, has led to changing patterns in the overall MenB case burden, with a high proportion of the overall case burden now observed in the adult population (Fig. 2). Surveillance data from the ECDC indicates that this pattern is seen across Europe, and in countries with established MenB infant immunisation such as the United Kingdom, and also in those countries (e.g. France) where the impact of more recent implementation of infant MenB immunisation (in 2022) remains to be established [29]. In 2019, approximately 20% of all MenB cases involved individuals > 50 years of age (Fig. 2). In the United States, data indicate that, of the 698 MenB cases reported by the CDC between 2015 and 2020, those involving individuals ≥ 24 years accounted for 43% of the cases, and with 26% of all MenB cases occurring in adults aged ≥ 45 years [30,31,, 31, 69,70,71,72] (Fig. 3). In Australia, of the 313 MenB cases reported across 2018–2021, 27% occurred in adults ≥ 25 years of age (and 16% in those aged ≥ 45 years) [33,34,35,36]. These data highlight the increasing contribution of adult MenB disease to the overall burden within populations, and the potential value of adult MenB immunisation in reducing adult MenB disease. Although not the focus of this article, it should be recognised that, as well as the proportionately greater burden of MenB disease in adults, substantial increases in the incidence of IMD due to MenW and MenY are also apparent globally in recent years in adults (especially in adults > 50 years) [2, 73, 74].
a Confirmed case numbers for IMD due to MenB across Europe (EU/EEA), and in the United Kingdom and France across 2011–2020 stratified by age [29]. For the United Kingdom, data for 2020 are not available. b Proportion of all MenB cases in different age groups in 2019. EU European Union, EEA European Economic Area, IMD invasive meningococcal disease, MenB N. meningitidis serogroup B
MenB Vaccines
Development of MenB Vaccines
In contrast to the MenA, MenC, MenW and MenY serogroups, the MenB capsular polysaccharide has poorer immunogenicity, requiring novel approaches to identify and target alternative highly immunogenic, subcapsular and surface-expressed protein antigens. Using ‘reverse vaccinology’ or immunologic/protein screening approaches, development of MenB recombinant protein vaccines has been directed against one or more of these subcapsular targets [45, 75, 76]. Four key target antigens were chosen; human factor H binding protein (fHbp, with two immunologically distinct subfamilies, A and B), Neisserial heparin binding antigen (NHBA), Neisseria adhesin A (NadA) and the porin A protein (PorA P1.4), the latter the immunodominant antigen of the MenB outer membrane vesicle (OMV) [45, 75, 76]. These target proteins are widely expressed by most pathogenic MenB strains, with some also expressed by other serogroups offering potential for broader IMD protection [77,78,79,80]. 4CMenB targets four proteins (fHbp [subfamily B], NHBA, NadA and PorA P1.4) while MenB-FHbp targets two distinct FHbp variants (one each from subfamilies A and B) [45, 76].
Initially, development and subsequent regulatory approval of 4CMenB and MenB-FHbp was on the basis of responses towards specific test MenB strains, chosen for their expression of one or more of these four main vaccine target antigens, and to accommodate meningococcal genetic diversity and global distribution of key pathogenic strains [45, 81]. In 4CMenB development, these included strain 44/76-SL (fHbp), strain 5/99 (NadA), strain NZ 98/254 (PorA P1.4), with the later inclusion of strain M10713 as a specific test strain for NHBA [45, 81, 82,83,]. For MenB-FHbp, four core strains were identified on the basis of fHbp subfamily A and B peptides (strains PMB80, PMB2001, PMB2948 and PMB2707) with ten additional strains expressing diverse fHbp peptides also used in selected studies [83].
Immunogenicity against these strains was assessed using specific bactericidal antibody assays with human complement (hSBA), with protective antibody responses against vaccine antigens considered as hSBA titres ≥ 4 or ≥ 5 observed for both 4CMenB and MenB-FHbp vaccines [43, 81, 83]. As circulating MenB strains expressing target antigens show substantial diversity within different populations, novel platforms to define the extent of predicted coverage of 4CMenB and MenB-FHbp against MenB strains are now in established use [44, 83]. For 4CMenB, the meningococcal antigen typing system (MATS) is used in isolates from culture confirmed cases, and the complementary genomic system (gMATS) can predict coverage in non-culture PCR-confirmed cases, while an alternative antigen sequence type (BAST) platform can predict coverage for both culture-confirmed and PCR-confirmed cases [84, 85]. For MenB-FHbp, the Meningococcal Antigen Surface Expression (MEASURE) assay can quantify fHbp expression on isolates from culture-confirmed cases [43, 45, 84]. Global studies utilising these platforms against extensive IMD isolate panels indicate relatively high predicted strain coverages for both 4CMenB and MenB-FHbp [75, 84, 86,87,88], and both 4CMenB and MenB-FHbp induce robust responses towards MenB outbreak strains [43, 89,90,91,92,93]. In addition, as these vaccine target antigens are capsule-independent, with antigens conserved across different capsular serogroups, 4CMenB and MenB-FHbp offer potential protection against non-B serogroups, with studies reporting high predicted coverage for both vaccines against MenC, MenW and MenY isolates from infants and adolescents [77,78,79], while predicted activity against other Neisseria species such as N. gonorrhoeae has also been reported [94, 95].
MenB Vaccine Clinical Trial Programmes
Licensure of 4CMenB followed an extensive clinical trial programme evaluating immunogenicity and safety/reactogenicity in more than 7000 infants, toddlers, adolescents and adults [44, 81, 96]. Most studies have focused on those age groups at greatest risk (infants, toddlers, and adolescents) with studies demonstrating robust immune responses to each of the three subcapsular target antigens, NadA, fHbp and NHBA, and also towards the OMV PorA1.4 protein [44, 81, 96]. As in other meningococcal vaccine development, as IMD is relatively uncommon, the initial licensure was on the basis of immunogenicity, with an accepted surrogate of protection (hSBA ≥ 1:4), with subsequent post-licensure studies required to demonstrate effectiveness in targeted populations [81].
Immunisation in the first year of life generates protective titres (hSBA ≥ 1:4) against test strains in 84–100% of infants after three doses, with marked increases in hSBA geometric mean titres (GMTs) and in the percentage of children with hSBA titres ≥ 4 observed after a subsequent booster dose [43, 61, 97,98,99]. While waning of immunogenicity is observed, persistence of protective titres for up to 36 months has been reported [98]. A 2 + 1 primary vaccination schedule with vaccinations at the age of 2, 4 and 12 months is now the preferred schedule in most routine immunisation programmes using infant 4CMenB vaccination [43, 61]. For children > 2 years and adolescents, a two-dose schedule is immunogenic and persistence is longer. Protective antibody responses to at least one of the test antigens are sustained for up to 7.5 years after primary vaccination in > 80% of adolescents, with subsequent booster vaccination generating rapid, robust responses against all four vaccine antigens [98, 100, 101]. Studies in adult populations also demonstrate robust responses (described in more detail below) [100, 102,103,104].
Clinical development of MenB-FHbp has focused on use in individuals aged ≥ 10 years (and usually 10–25 years) [43]. Protective titres against MenB test strains were observed in 72–99% of individuals after a 3-dose schedule (and in 69–98% after a 2-dose schedule) [43, 45]. Persistence for up to 4 years is reported in over 50% of subjects, with booster vaccination eliciting robust responses [105, 106]. A 2-dose schedule is preferred for routine adolescent MenB-FHbp vaccination [43]. Studies of MenB-FHbp and 4CMenB in adult populations are described below.
MenB Vaccine Approval
Both 4CMenB and MenB-FHbp are now widely available, although approval for use in specific age groups varies within different countries, with more restricted use in North America (Table 1). 4CMenB was first licensed in the European Union (EU) in 2013 [47] (and such approval continues within the United Kingdom following departure from the EU) [48], and also in Australia [49] and Canada [55] the same year, and then in the United States in 2015 [53]. 4CMenB is currently approved in 50 countries worldwide, including Argentina, Brazil, Chile, Hong Kong, Israel, Saudi Arabia, New Zealand, Switzerland, Turkey, the United Arab Emirates and Uruguay [57]. Recommended dosing in infants and toddlers is 2 or 3 doses given 1 or 2 months apart, and with a booster dose at 12–23 months of age [43, 57]. In older children, adolescents and adults, a 2-dose schedule is recommended [43, 57].
MenB-FHbp was initially approved in the United States in 2014 [54], and in the EU [50, 51], Australia [52] and Canada [56] in 2017. MenB-FHbp is currently approved in over 50 countries worldwide, including Argentina, Brazil, Chile, Colombia, Hong Kong, Israel, Kazakhstan, Kuwait and Singapore [57]. Recommended dosing in individuals aged from 10 through 25 years is 2 doses given at least 6 months apart, or where more rapid immunity is desired, e.g. in outbreak situations, as a 3-dose schedule with ≥ 1 month between the first and second doses and the third dose given ≥ 4 months after the second dose [43, 57]. Of some importance is that, unlike other meningococcal vaccines (e.g. quadrivalent MenACWY), 4CMenB and MenB-FHbp are not interchangeable, and so the same vaccine must be used for the complete vaccination series [43, 57].
Implementation of MenB Vaccines
Inclusion of MenB vaccines in National Immunisation Programmes (NIPs) continues to evolve, in response to both shifting epidemiology and the introduction and availability of MenB vaccines at a national level [57,58,59]. While most countries have a single national policy, implementation of MenB vaccine use may also be devolved on a regional/state basis, with individuals in some regions eligible for MenB vaccines out of national recommendations (e.g. in Australia) [107, 108]. At present, despite widespread approval and availability, inclusion of routine MenB immunisation within NIPs remains relatively restricted, and generally focused on infant/toddler immunisation (in Europe) and in adolescents and younger adults in the US [43, 57, 59]. In Europe, fully funded infant/toddler MenB immunisation (using 4CMenB) is included in the NIPs of the United Kingdom (since 2015), and then Italy, Portugal, Ireland, Lithuania, Malta, and more recently in the Czech Republic [57,58,59]. In France, following expert recommendations in 2021 [59], infant 4CMenB was formally included within the NIP in 2022 [109]. In Spain, while infant/toddler 4CMenB immunisation was previously only implemented in some regions (Castilla y Leon, the Canary Islands, Catalonia and Andalusia), this policy will be implemented nationwide from 2023 [110]. Other countries such as Austria and Hungary include infant 4CMenB immunisation in their NIP, but these are not funded [57]. In most of these settings, infant/toddler dosing is usually in a 2 + 1 schedule (although in some countries, e.g. Italy and Hungary, a 3 + 1 schedule is used) [57, 59]. Adolescent MenB immunisation is also included in the Czech Republic [15, 59], while vaccination of those aged 11–12 years is funded in the Puglia region of Italy (since 2018), where adolescent and adult vaccination has also been offered (chiefly through MenB-FHbp) [111].
Outside Europe, MenB vaccines have a more limited place in NIPs. In the United States, MenB immunisation (via either 4CMenB or MenB-FHbp) is recommended for those aged 16–23 years (on the basis of shared clinical decision-making), where the preferred age for vaccination is 16–18 years [13]. In Canada, routine MenB immunisation is not included in either national or regional immunisation programmes [57, 112]. In Australia, while all infants and toddlers in at-risk ethnic populations (Aboriginal or Torres Strait Islander children) should be offered 4CMenB [107, 108], broader routine infant 4CMenB vaccination is restricted to those residing in South Australia state (since 2018), where a funded school-based 4CMenB vaccination programme targeting adolescents aged 15–16 years also exists, and where catch-up immunisation for children aged up to 3 years and for those aged 17–20 years are also in place [57, 113]. While New Zealand has previously had no routine MenB vaccination policy, this will be implemented in 2023 for all infants up to 12 months of age, and also for people aged 13–25 years who are entering into or in their first year of specific close-living situations (boarding school hostel, tertiary education halls of residence, military barracks or prison) [114]. In Brazil, although a MenB immunisation policy for infants, toddlers and adolescents is recommended by the Brazilian Pediatric Society, this is not publicly funded and remains out with the NIP [57, 115].
Beyond these age-based recommendations, MenB is also offered to individuals considered ‘at risk’ within NIPs (and indeed were usually in place before the introduction of any age-based recommendations) [57,58,59]. This spans a wide range of at-risk medical conditions or situational risk, and applies across all age groups, including adults, although what constitutes at risk varies widely across countries (reviewed in [57]). For example, in some countries, HIV infection is not a specific indication for MenB vaccination [57]. In the United States and in the United Kingdom, persons considered at high risk due to pre-existing medical conditions include individuals with asplenia or splenic dysfunction (including sickle-cell disease) and those with persistent complement component deficiencies or receiving a complement component inhibitor (e.g. eculizumab) [13, 116]. Other individuals considered at situational risk include those with occupational health exposure (e.g. laboratory personnel) or living in specific high-risk environments (e.g. college dormitory students, military recruits) [13]. In addition, immunisation of close contacts of MenB cases is also recommended. In these circumstances, either 4CMenB or MenB-FHbp can be used, depending on age [13]. In Canada (where no routine MenB immunisation is recommended), a similarly broad range of at-risk categories where MenB immunisation should be considered also exist [117]. While in North America, these recommendations would include adults out with the licensed age-based indication, such off-label use is considered appropriate based on expert opinion [13, 117].
In Australia and New Zealand, national recommendations include a similar broad range of at-risk categories or situations where MenB immunisation should be considered [107, 108, 118]. Within Europe, while individuals with underlying medical conditions associated with increased risk are included in most NIPs, specific eligibility criteria differ across countries [57]. While some countries recommend immunisation of close contacts of affected MenB IMD cases, such policies also vary in different countries [57, 59].
Effectiveness of MenB Vaccines in the Real-World
Post-licensure studies of 4CMenB evaluating effectiveness following its introduction in NIPs have demonstrated substantial reductions in MenB disease in vaccine-eligible populations (chiefly those < 5 years of age) [61]. In the United Kingdom, after 3 years of use within the NIP, a surveillance-based study across 2015–2018 in England reported a 75% reduction in the incidence of IMD due to MenB in children fully eligible for vaccination [119], with evidence of continued impact and with further reductions in MenB disease in subsequent years in children aged < 5 years [120]. Case–control and surveillance studies conducted in Australia, Portugal, Italy and Spain also report robust VE against MenB in children < 5 years of age (ranging from 71.0% to 94.2%) [113, 121,122,123]. Data are limited for older populations. In South Australia, a cluster randomised trial reported that, following 4CMenB immunisation at 15–16 years of age, a 71% (95% CI 15–90%) overall reduction in the incidence of MenB in vaccinated subjects (aged 16–19 years) in the subsequent two years (2018–2019) compared with historical incidence (between 2003 and 2016) [124].
In addition to impact on MenB disease, some data indicate an impact of 4CMenB on other meningococcal serogroups (and also on other important Neisseria pathogens such as N. gonorrhoeae) [95]. Cohort and case–control studies conducted in Australia and the United States have reported significant reductions in incident gonorrhoea (of 32.7% and 46%, respectively) in adolescents and young adults receiving 4CMenB compared to controls [113, 125]. These findings, allied with previous data indicating the potential impact of 4CMenB on gonorrhoea are now the subject of ongoing clinical studies [95].
At present, there are no data for the effectiveness of MenB vaccines in adults in reducing IMD due to MenB, and this remains a barrier to decision-making regarding broader recommendations for more routine use in older adults.
MenB Immunisation in Outbreak Control
Both 4CMenB or MenB-FHbp have been successfully used in outbreak control in a range of different settings and populations [17, 61, 126]. Following outbreaks of MenB disease in university campuses in both the east and west coasts of the US (Princeton, New Jersey and UC Santa Barbara, California) in 2013/2014, 4CMenB was administered to more than 14,000 adults aged 18–65 years (comprising students, staff and adult family members), given in a 2-dose (0, 1 month) schedule [127,128,129]. In both settings, no safety issues were identified, and no MenB cases occurred in those individuals who received at least one dose of 4CMenB [127,128,129]. While this is reassuring, it should be noted that immunogenicity responses towards MenB strains were highly variable. An immunogenicity study evaluating protective serum antibody responses (hSBA ≥ 4) in 499 individuals from Princeton (mean age 20.5 ± 1.3 years) receiving two 4CMenB doses reported that only 66.1% (95% CI 61.8–70.3) of individuals were seropositive for the outbreak strain [89]. Geometric mean titres (GMTs) were also low (7.6, 95% CI 6.7–8.5) [89]. In contrast, 86.9% were seropositive for the 44/76-SL strain (with 96% genetic similarity to the outbreak strain) and 100% for the 5/99 strain, and GMTs were higher (17.4 and 256.3, respectively, against the 44/76-SL and 5/99 strains) [89].
Focused use of 4CMenB in outbreak control has also been observed in response to a local school outbreak in France (in Brittany) in 2016/2017, where mass vaccination of students and local individuals aged 11–19 years was implemented, and with no further cases reported [130]. In Canada, following a prolonged increase in the incidence of MenB disease in the Saguenay-Lac-Saint-Jean region of Quebec province between 2006 and 2013, 4CMenB vaccination of individuals aged between 2 months and 20 years was implemented in 2014 [131]. In this campaign, 82% of this targeted age group received at least one 4CMenB dose [131]. Between June 2014 and July 2018, five MenB cases were reported, one in a vaccinated child and four in unvaccinated adults (including three elderly adults). The estimated overall impact of the vaccination campaign was an 86% (95% CI –2 to 98%) decrease in risk of MenB disease [132].
MenB-FHbp has also been effective in outbreak control, notably following a college campus outbreak in Rhode Island in 2015 [133, 134].
Supportive Immunogenicity and Safety Data for the use of MenB Vaccination in Adults
Licensure of 4CMenB followed an extensive clinical trial programme evaluating immunogenicity and safety/reactogenicity in more than 7,000 infants, toddlers, adolescents and adults [44, 81, 96]. While most studies focused on those age groups at greatest risk (infants, toddlers, and adolescents), a number of studies within the 4CMenB development programme specifically evaluated immunogenicity in healthy adults [81, 96, 100, 102,103,104] (Table 2 and Fig. 4).
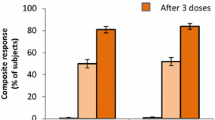
Percentages of adult participants in 4CMenB vaccine studies with hSBA titres ≥ 4 or ≥ 1:4 against target antigens at baseline and at 1 month after second dose of 4CMenB [100, 102,103,104, 138, 139]. * Study evaluated two different vaccination schedules (and reported hSBA titres ≥ 1:4). 4CMenB 4-component meningococcal serogroup B vaccine, hSBA human serum bactericidal assay, UK United Kingdom
A phase 3b extension study (NCT02446743) conducted in Australia, Canada and Chile assessed persistence of bactericidal activity at 4–7.5 years after a 2-dose primary series of 4CMenB and the response to a third (booster) dose in adolescents and young adults [100, 101]. This was a follow-up to earlier studies evaluating immunogenicity and safety of a 2-dose schedule in adolescents aged 11–17 years (reported in [135, 136]). In this extension study, as well as reporting on immunogenicity persistence and on booster responses at 4–7.5 years in previously primed individuals (now aged from 15–24 years), immunogenicity and safety of a 2-dose schedule (at 0, 1 months) were also evaluated in 255 vaccine-naïve subjects of a similar age, thus providing data on immunogenicity and safety of a 2-dose schedule in younger adults from these three countries [100]. Another phase 3 study conducted in the United Kingdom (NCT01214850) evaluated the effect of 2 doses of 4CMenB given 1 month apart in young adults aged 18–24 years (additional responses to MenACWY conjugate vaccine were also assessed [137]. While the primary outcome was the impact on N. meningitidis oropharyngeal carriage [133], an immunogenicity subset (n = 193) was included, in which antibody responses to 4CMenB and antibody persistence at 11 months after the 2-dose primary series were evaluated and subsequently reported [102]. An earlier phase 1 study (conducted in Switzerland) evaluating the safety and immunogenicity of 3 doses of 4CMenB (and alternative vaccine formulations with different antigenic content) given at 0, 1 and 2 months in adults aged 18–40 years (with 28 subjects receiving the 4CMenB formulation) [103]. A subsequent phase 2 study conducted in Germany and Italy assessed the safety and immunogenicity of 3 doses of 4CMenB (at 0, 2 and 6 months) in at-risk adults aged from 18 to 50 years (n = 46 laboratory workers exposed to a variety of meningococcal strains) [104].
Across these studies, 2 doses of 4CMenB administered either 1 or 2 months apart resulted in robust immune responses, with hSBA titres ≥ 4 (or hSBA titres ≥ 1:4) towards fHBP and NadA observed in 98–100% of subjects 1 month after the second dose, and with hSBA titres ≥ 4 (or ≥ 1:4) against PorA P1.4 and NHBA observed in 79–96% and 91–95%, respectively (Table 2; Fig. 4). Prior to any vaccination, up to 78% of participants had baseline hSBA titres ≥ 4 (or ≥ 1:4) towards one or more of the reference strains, indicating some level of antigenic priming.
Other studies in adults have also reported immunogenicity data [138, 139]. An early phase 2 study (NCT00962624) was conducted in the United Kingdom which evaluated co-administration of 4CMenB with a quadrivalent MenACWY vaccine in adult laboratory workers aged between 18 and 65 years (mean age, 34 years, range 23–55 years), with 32 subjects receiving two doses of 4CMenB and with available immunogenicity data [138]. Immunogenicity was assessed against the same indicator strains and target antigens as previously described; H44/76 (fHbp), 5/99 (NadA) and NZ98/254 (PorA P1.4). While baseline antibody titres were high (with hSBA titres ≥ 4 against indicator strains apparent in more than 60% of subjects), after the second dose the proportion of subjects with hSBA titres ≥ 4 increased to 100%, with robust increases in GMTs also observed. Furthermore, co-administration with MenACWY vaccine was safe and well tolerated [138]. A subsequent post-licensure study conducted in Canada (NCT02583412) evaluated immunogenicity in adolescents and young adults aged 17–26 years of age (mean age 20.6 ± 2.9 years) randomised to receive conventional (0, 2 months) and accelerated (with dose 2 given at 21 days) 4CMenB vaccination schedules in 121 healthy volunteers [139]. This study found protective hSBA titres ≥ 1:4 to fHbp, NadA and PorA P1.4 antigens in 98–100% of all participants 21 days after receiving the first 4CMenB dose, providing supportive evidence for use of a 1-dose schedule in outbreak control [139] (Table 2 and Fig. 4).
4CMenB was generally safe and well tolerated in adults in these studies (and broadly comparable to those observed in younger patients). After any 4CMenB dose, most subjects experienced reactogenicity, with the most common reaction being pain at the injection site. Most reported solicited local adverse events (AEs) i.e. local pain, erythema, induration, were mild to moderate in intensity with onset within 3 days after vaccination (after either dose), and the majority resolved within 7 days. The most common solicited systemic AEs included fatigue, headache, myalgia, and malaise [81, 96, 100, 102,103,104, 139]. Unsolicited AEs were collected for 1 month after each vaccination. Again, most unsolicited AEs were mild to moderate in intensity, with comparable rates reported in vaccinated and unvaccinated subjects, and most resolved before study completion. No serious AEs considered to be related to 4CMenB vaccination were reported [81, 96, 100, 102,103,104, 139].
As well as these studies, a number of additional smaller studies have evaluated immunogenicity and safety of two doses of 4CMenB in adult populations. One conducted in Germany (NCT01911221) assessed the immune response and safety to two doses in 13 adults aged 18–65 years (mean age, 38.5 ± 12.2 years), all laboratory workers at increased risk due to occupational exposure to MenB samples. At 1 month after the second dose, there were protective hSBA titres of ≥ 5 against all four vaccine target antigens, and with a robust increase in GMTs from baseline [140]. Another study (NCT01478347) evaluated safety in 18 adults (mean age, 34.5 ± 5.7 years) with similar occupational risk conducted in Italy [141]. In addition, a safety study (NCT02305446) assessed safety in 55 adults aged 18–50 years in Poland (mean age, 27.2 ± 6.9 years) [142]. Across these studies, 2 doses of 4CMenB were found to be well tolerated, with a safety profile in line with that seen in other trials in adult populations.
For MenB-FHbp, data in adults are chiefly derived from a single large phase 3 study (NCT01352845) evaluating immunogenicity and safety of a 3-dose schedule (0, 2 and 6 months) in 2471 adults aged 18−25 years [143]. Subjects were recruited from the United States, Canada and mainland Europe (Denmark, Finland, Poland and Spain [143]. After dose 2, a ≥ fourfold rise in hSBA titres against the four primary representative test strains was observed in 54.6–85.6% of subjects, and in 78.9–89.7% after the third dose. In addition, antibody titres against other strains were supportive of a broader protection against diverse MenB strains [45, 143].
Earlier studies provide some data supportive of the use of MenB-FHbp in a slightly broader adult population. One phase 1 study (NCT00879814) evaluated different MenB-FHbp doses in 48 adults aged 18–40 years, again via a 3-dose schedule (0, 2 and 6 months), with 12 subjects (mean age 31.0 ± 7.6 years) receiving the subsequently licensed dose [144]. In this study, which evaluated serum immunoglobulin G (IgG) titres against fHbp subfamilies A and B proteins, high IgG titres were observed after the second and third doses [144]. A subsequent phase 2 study in Australia (NCT00780806) evaluated the licensed dose in 60 adults aged 18–40 years [145]. After 3 doses, hSBA titres ≥ 1:4 against the target stain expressing the vaccine-homologous fHbp subfamily A variant was observed in 94.3% of subjects, with hSBA titres ≥ 1:4 against strains expressing other putative target fHbp variants achieved in 70.0–94.7% [145].
Both 4CMenB and MenB-FHbp are components of pentavalent MenABCWY meningococcal vaccines under development [146, 147]. While clinical studies evaluate immunogenicity and safety in younger adults (up to 25 years), these are reported as pooled data incorporating data from adolescents [148, 149]. As such, at the present time, no additional insight into specific adult immunogenicity data can be drawn from these programmes. It should also be recognised that, while the immunogenicity data for 4CMenB and MenB-FHbp we describe above are informative, some caution is warranted and limitations exist. The data we describe are chiefly from younger adults; indeed, product monographs make clear that there are no immunogenicity data for adults > 50 years of age for 4CMenB [47], and that data are limited for MenB-FHbp in 40- to 65-year-olds with no data in those > 65 years [50]. There remains a need for additional data in older adults to inform decision-making for greater use in older adults.
One aspect of responses to MenB vaccines in adults that remains largely unknown is the duration of protection that may be achieved, and the need for subsequent boosting. In the persistence/booster study conducted in Australia, Canada and Chile reported by Nolan et al. (described above) there was evidence of waning of protection induced by 4CMenB, although, following a booster dose, protective hSBA titres ≥ 4 were then observed in 93–100% of subjects [100]. Evidence of waning may also be derived from follow-up data from the use of 4CMenB in outbreak control in Canada, where VE against MenB disease declined from 100% during the first 2 post-campaign years to 50% (95% CI − 453, to 95) over an adjusted 5-year period [61, 131, 132]. This would indicate that periodic boosting is necessary to maintain protection. Given the paucity of data in older adults > 50 years of age, the immunogenicity and persistence of protective responses in older adults is unclear. In addition, as studies evaluating immunogenicity involved healthy individuals, the impact of comorbidity or frailty on vaccine responses is also unknown, and it may be anticipated that these are poorer. In some countries recommending MenB vaccine for at-risk adults (including those with immune dysfunction and laboratory workers), booster immunisation every 5 years is recommended [13, 57]. While data are required to define the duration of protection in older adults (and in those with comorbidity or frailty), it would seem that a similar booster vaccination approach would be required. Inevitably, this would increase programmatic costs associated with any broader adult immunisation policy.
Towards a Broader Adult MenB Vaccination Approach
These data in adults receiving either 4CMenB or MenB-FHbp indicate that a robust immune response to key antigens expressed by most pathogenic MenB serogroups are generated in adults across a diverse range of geographies and populations (and broadly comparable to that seen in children and adolescents). While most of the data were derived from use in younger adults (aged 18–26 years), equally robust hSBA responses were evident in those studies evaluating use in older subjects. As indicated earlier, it should be recognised that there remain important knowledge gaps as to immunogenicity in adults > 50 years of age, persistence of protective responses, and impact of comorbidity/frailty in older adults. Further studies specifically examining this are welcome.
As we describe, present surveillance data indicate that there exists a substantial burden of MenB disease in many adult populations. It could be anticipated that direct protection through greater routine use of MenB vaccination in adult immunisation strategies would also have a clinical impact on IMD due to MenB, and help to reduce the case burden and adverse clinical outcomes (associated mortality and long-term sequelae in survivors) observed in adults. The current focus on predominantly age-based MenB immunisation recommendations towards younger at-risk populations (infants, toddlers and adolescents) is such that most adults remain at continued risk.
Greater efforts are needed to ensure access to and uptake of MenB vaccines in those adults at greater risk due to medical comorbidity. While data for vaccine uptake in at-risk individuals are limited, available data from the United States indicate relatively low coverage in at-risk populations, with uptake in individuals with complement deficiencies or with asplenia of 2.2% and 9.7%, respectively [150, 151]. In Australia, between 2014 and 2019 the reported MenB vaccine coverage in adults > 50 years with at-risk medical comorbidities ranged from 12% to 14%, and was lower in those with lower socioeconomic status [152]. Efforts to increase vaccine uptake in at-risk adults where MenB immunisation is recommended should be a priority.
It can be argued that a broader, more comprehensive MenB immunisation policy for adults could also be considered. This is in keeping with a lifelong approach to immunisation in which equitable access to vaccines throughout the lifespan is an essential element of healthy aging [67, 153,154,155]. Although the focus in this approach is on immunisation against other vaccine-preventable disease (notably influenza, pneumococcal infection, pertussis and herpes zoster in older adults), this could also encompass a broader approach with adult meningococcal MenB immunisation (in those countries with broad age-based approval for use across adults of all ages). A broader adult immunisation approach would also be supportive of the aims of the World Health Organization’s ‘Defeating meningitis by 2030 global road map’, with a target of the near-elimination of bacterial meningitis (including IMD due to MenB) by this date [156, 157]. However, there exist significant challenges to this. Immunisation recommendations by public health agencies must also accommodate other priorities and are subject to financial constraints, and any broader adult MenB immunisation policy would have substantial impact on healthcare budgets. While MenB in adults constitutes as much as 20% of the overall disease burden in a number of countries, the low incidence rate in adults is such that any MenB vaccine policy would not be considered cost-effective under existing criteria. These aspects pose considerable challenges when trying to balance healthcare financial resources with a more equitable approach to access to MenB vaccination. There remains a need to pursue additional studies to support policy decision-making.
Change history
04 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40121-023-00858-2
References
Acevedo R, Bai X, Borrow R, et al. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines. 2019;18:15–30. https://doi.org/10.1080/14760584.2019.1557520.
Parikh SR, Campbell H, Bettinger JA, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81:483–98. https://doi.org/10.1016/j.jinf.2020.05.079.
Wang B, Santoreneos R, Giles L, Haji Ali Afzali H, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37:2768–82. https://doi.org/10.1016/j.vaccine.2019.04.020.
Viner RM, Booy R, Johnson H, et al. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol. 2012;11:774–83. https://doi.org/10.1016/S1474-4422(12)70180-1.
Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30(Suppl 2):B3-9. https://doi.org/10.1016/j.vaccine.2011.12.062.
Olbrich KJ, Muller D, Schumacher S, Beck E, Meszaros K, Koerber F. Systematic review of invasive meningococcal disease: sequelae and quality of life impact on patients and their caregivers. Infect Dis Ther. 2018;7:421–38. https://doi.org/10.1007/s40121-018-0213-2.
Schiess N, Groce NE, Dua T. The impact and burden of neurological sequelae following bacterial meningitis: a narrative review. Microorganisms. 2021;9:900. https://doi.org/10.3390/microorganisms9050900.
Shen J, Bouee S, Aris E, Emery C, Beck EC. Long-term mortality and state financial support in invasive meningococcal disease-real-world data analysis using the french national claims database (SNIIRAM). Infect Dis Ther. 2022;11:249–62. https://doi.org/10.1007/s40121-021-00546-z.
Borrow R, Alarcon P, Carlos J, et al. The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16:313–28. https://doi.org/10.1080/14760584.2017.1258308.
Jafri RZ, Ali A, Messonnier NE, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11:17. https://doi.org/10.1186/1478-7954-11-17.
Parikh SR, Campbell H, Gray SJ, et al. Epidemiology, clinical presentation, risk factors, intensive care admission and outcomes of invasive meningococcal disease in England, 2010–2015. Vaccine. 2018;36:3876–81. https://doi.org/10.1016/j.vaccine.2018.02.038.
Taha MK, Weil-Olivier C, Bouee S, et al. Risk factors for invasive meningococcal disease: a retrospective analysis of the French national public health insurance database. Hum Vaccin Immunother. 2021;17:1858–66. https://doi.org/10.1080/21645515.2020.1849518.
Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1–41. https://doi.org/10.15585/mmwr.rr6909a1.
Weiss D, Varma JK. Control of recent community-based outbreaks of invasive meningococcal disease in men who have sex with men in Europe and the United States. Euro Surveill. 2013;18(28):20522. https://doi.org/10.2807/1560-7917.es2013.18.28.20522.
Taha MK, Martinon-Torres F, Kollges R, et al. Equity in vaccination policies to overcome social deprivation as a risk factor for invasive meningococcal disease. Expert Rev Vaccines. 2022;21:659–74. https://doi.org/10.1080/14760584.2022.2052048.
Dubey H, Oster P, Fazeli MS, et al. Risk factors for contracting invasive meningococcal disease and related mortality: a systematic literature review and meta-analysis. Int J Infect Dis. 2022;119:1–9. https://doi.org/10.1016/j.ijid.2022.03.032.
Soumahoro L, Abitbol V, Vicic N, Bekkat-Berkani R, Safadi MAP. Meningococcal disease outbreaks: a moving target and a case for routine preventative vaccination. Infect Dis Ther. 2021;10:1949–88. https://doi.org/10.1007/s40121-021-00499-3.
Muttalif AR, Presa JV, Haridy H, Gamil A, Serra LC, Cane A. Incidence and prevention of invasive meningococcal disease in global mass gathering events. Infect Dis Ther. 2019;8:569–79. https://doi.org/10.1007/s40121-019-00262-9.
Badur S, Khalaf M, Ozturk S, et al. Meningococcal disease and immunization activities in Hajj and Umrah pilgrimage: a review. Infect Dis Ther. 2022;11:1343–69. https://doi.org/10.1007/s40121-022-00620-0.
Rudmann KC, Brown NE, Rubis AB, et al. Invasive meningococcal disease among people experiencing homelessness-United States, 2016–2019. J Infect Dis. 2022;226:S322–6. https://doi.org/10.1093/infdis/jiac230.
Dinleyici EC, Borrow R. Meningococcal infections among refugees and immigrants: silent threats of past, present and future. Hum Vaccin Immunother. 2020;16:2781–6. https://doi.org/10.1080/21645515.2020.1744979.
Pilat EK, Stuart JM, French CE. Tobacco smoking and meningococcal disease in adolescents and young adults: a systematic review and meta-analysis. J Infect. 2021;82:135–44. https://doi.org/10.1016/j.jinf.2021.02.018.
Peterson ME, Li Y, Bita A, et al. Meningococcal serogroups and surveillance: a systematic review and survey. J Glob Health. 2019;9:010409. https://doi.org/10.7189/jogh.09.010409.
Purmohamad A, Abasi E, Azimi T, et al. Global estimate of Neisseria meningitidis serogroups proportion in invasive meningococcal disease: a systematic review and meta-analysis. Microb Pathog. 2019;134:103571. https://doi.org/10.1016/j.micpath.2019.103571.
Agnememel A, Hong E, Giorgini D, Nunez-Samudio V, Deghmane AE, Taha MK. Neisseria meningitidis Serogroup X in Sub-Saharan Africa. Emerg Infect Dis. 2016;22:698–702. https://doi.org/10.3201/eid2204.150653.
Villena R, Safadi MAP, Valenzuela MT, Torres JP, Finn A, O’Ryan M. Global epidemiology of serogroup B meningococcal disease and opportunities for prevention with novel recombinant protein vaccines. Hum Vaccin Immunother. 2018;14:1042–57. https://doi.org/10.1080/21645515.2018.1458175.
Whittaker R, Dias JG, Ramliden M, et al. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004–2014. Vaccine. 2017;35:2034–41. https://doi.org/10.1016/j.vaccine.2017.03.007.
Nuttens C, Findlow J, Balmer P, Swerdlow DL, Tin Tin Htar M. Evolution of invasive meningococcal disease epidemiology in Europe, 2008 to 2017. Euro Surveill. 2022;27:2002075. https://doi.org/10.2807/1560-7917.ES.2022.27.3.2002075.
European Centre for Disease Prevention and Control (ECDC). Surveillance atlas of infectious diseases. Stockholm. https://ecdc.europa.eu/en/surveillance-atlas-infectious-diseases. Accessed 26 Apr 2023.
US Centers for Disease Control and Prevention (CDC). Enhanced Meningococcal Disease Surveillance Report. 2018. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2018.pdf. Accessed 26 Apr 2023.
US Centers for Disease Control and Prevention (CDC). Enhanced Meningococcal Disease Surveillance Report. 2019. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2019.pdf. Accessed 26 Apr 2023.
Tsang RSW, Law DKS, De Paola R, et al. Culture-confirmed invasive meningococcal disease in Canada, 2010 to 2014: characterization of serogroup B Neisseria meningitidis strains and their predicted coverage by the 4CMenB vaccine. mSphere. 2020;5:e00883-e919. https://doi.org/10.1128/mSphere.00883-19.
Lahra MM, Enriquez RP, Hogan TP, National Neisseria Network A. Australian Meningococcal Surveillance Programme annual report, 2018. Commun Dis Intell (2018). 2020. https://doi.org/10.33321/cdi.2020.44.10.
Lahra MM, Hogan TR, National Neisseria Network A. Australian Meningococcal Surveillance Programme annual report, 2019. Commun Dis Intell (2018). 2020. https://doi.org/10.33321/cdi.2020.44.62.
Lahra MM, George CRR, Shoushtari M, Hogan TR. Australian Meningococcal Surveillance Programme Annual Report, 2020. Commun Dis Intell (2018). 2021. https://doi.org/10.33321/cdi.2021.45.46.
Lahra MM, George CR, Hogan TR. Australian Meningococcal Surveillance Programme Annual Report, 2021. Commun Dis Intell (2018). 2022. https://doi.org/10.33321/cdi.2022.46.46.
Yang Z, Ren X, Davies H, et al. Genomic surveillance of a globally circulating distinct group W clonal complex 11 meningococcal variant, New Zealand, 2013–2018. Emerg Infect Dis. 2021;27:1087–97. https://doi.org/10.3201/eid2704.191716.
Borrow R, Caugant DA, Ceyhan M, et al. Meningococcal disease in the Middle East and Africa: findings and updates from the Global Meningococcal Initiative. J Infect. 2017;75:1–11. https://doi.org/10.1016/j.jinf.2017.04.007.
Taha MK, Presa J, Serra L. A review of the epidemiology of invasive meningococcal disease and vaccination strategies in North Africa. Int J Infect Dis. 2021;104:189–97. https://doi.org/10.1016/j.ijid.2020.11.162.
Li J, Shao Z, Liu G, et al. Meningococcal disease and control in China: findings and updates from the Global Meningococcal Initiative (GMI). J Infect. 2018;76:429–37. https://doi.org/10.1016/j.jinf.2018.01.007.
Xu J, Chen Y, Yue M, et al. Prevalence of Neisseria meningitidis serogroups in invasive meningococcal disease in China, 2010–2020: a systematic review and meta-analysis. Hum Vaccin Immunother. 2022;18:e2071077. https://doi.org/10.1080/21645515.2022.2071077.
Chiou CS, Liao YS, Chen BH, et al. Demographic features of invasive meningococcal disease in Taiwan, 1993 to 2020, and genetic characteristics of Neisseria meningitidis isolates, 2003 to 2020. Microbiol Spectr. 2022;10:e0088222. https://doi.org/10.1128/spectrum.00882-22.
Safadi MAP, Martinon-Torres F, Serra L, Burman C, Presa J. Translating meningococcal serogroup B vaccines for healthcare professionals. Expert Rev Vaccines. 2021;20:401–14. https://doi.org/10.1080/14760584.2021.1899820.
Watson PS, Novy PL, Friedland LR. Potential benefits of using a multicomponent vaccine for prevention of serogroup B meningococcal disease. Int J Infect Dis. 2019;85:22–7. https://doi.org/10.1016/j.ijid.2019.05.019.
Perez JL, Absalon J, Beeslaar J, et al. From research to licensure and beyond: clinical development of MenB-FHbp, a broadly protective meningococcal B vaccine. Expert Rev Vaccines. 2018;17:461–77. https://doi.org/10.1080/14760584.2018.1483726.
Pizza M, Bekkat-Berkani R, Rappuoli R. Vaccines against meningococcal diseases. Microorganisms. 2020;8:1521. https://doi.org/10.3390/microorganisms8101521.
European Medicines Agency Public Assessment Report (EPAR) for Bexsero. 2012. https://www.ema.europa.eu/en/medicines/human/EPAR/bexsero. Accessed 5 Aug 2022.
Electronic Medicines Compendium (UK). Bexsero meningococcal group B vaccine for injection in pre-filled syringe. 2021. https://www.medicines.org.uk/emc/product/5168. Accessed 5 Aug 2022.
Therapeutic Goods Administration. Australian Product Information: BEXSERO® suspension for injection. Multicomponent meningococcal group B vaccine (recombinant, adsorbed). 2019. https://gskpro.com/content/dam/global/hcpportal/en_AU/assets/pdfs/products/bexsero/bexsero_australian_product_information.pdf. Accessed 5 Aug 2022.
European Medicines Agency Public Assessment Report (EPAR) for Trumenba. 2017. https://www.ema.europa.eu/en/medicines/human/EPAR/trumenba. Accessed 5 Aug 2022.
Electronic Medicines Compendium (UK). Trumenba. 2021. https://www.medicines.org.uk/emc/product/2670. Accessed 5 Aug 2022.
Therapeutic Goods Administration. Australian Product Information: Trumenba® (Meningococcal group B vaccine) suspension for injection pre-filled syringe. 2019. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2017-PI-02674-1&d=20220818172310101. Accessed 5 Aug 2022.
US Food & Drug Administration (FDA). BEXSERO® (Meningococcal Group B Vaccine). https://www.fda.gov/media/90996/download. Accessed 5 Aug 2022.
US Food & Drug Administration (FDA). Trumenba® (Meningococcal Group B Vaccine). https://www.fda.gov/media/89936/download. Accessed 5 Aug 2022.
GSK Canada. Bexsero. Multicomponent Meningococcal B Vaccine (recombinant, adsorbed). 2022. https://ca.gsk.com/media/6328/bexsero.pdf. Accessed 5 Aug 2022.
Pfizer Canada. Trumenba. Meningococcal group B vaccine [Bivalent recombinant lipoprotein (rLP2086)]. 2019.https://www.pfizer.ca/en/our-products/trumenba-meningococcal-group-b-vaccine. Accessed 5 Aug 2022.
Sulis G, Horn M, Borrow R, Basta NE. A comparison of national vaccination policies to prevent serogroup B meningococcal disease. Vaccine. 2022;40:3647–54. https://doi.org/10.1016/j.vaccine.2022.04.101.
Martinon-Torres F, Taha MK, Knuf M, et al. Evolving strategies for meningococcal vaccination in Europe: overview and key determinants for current and future considerations. Pathog Glob Health. 2022;116:85–98. https://doi.org/10.1080/20477724.2021.1972663.
Sohn WY, Tahrat H, Novy P, Bekkat-Berkani R. Real-world implementation of 4-component meningococcal serogroup B vaccine (4CMenB): implications for clinical practices. Expert Rev Vaccines. 2022;21:325–35. https://doi.org/10.1080/14760584.2022.2021881.
Burman C, Alderfer J, Snow VT. A review of the immunogenicity, safety and current recommendations for the meningococcal serogroup B vaccine, MenB-FHbp. J Clin Pharm Ther. 2020;45:270–81. https://doi.org/10.1111/jcpt.13083.
Martinon-Torres F, Banzhoff A, Azzari C, et al. Recent advances in meningococcal B disease prevention: real-world evidence from 4CMenB vaccination. J Infect. 2021;83:17–26. https://doi.org/10.1016/j.jinf.2021.04.031.
Marshall HS, McMillan M, Koehler AP, et al. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N Engl J Med. 2020;382:318–27. https://doi.org/10.1056/NEJMoa1900236.
McMillan M, Chandrakumar A, Wang HLR, et al. Effectiveness of meningococcal vaccines at reducing invasive meningococcal disease and pharyngeal Neisseria meningitidis carriage: a systematic review and meta-analysis. Clin Infect Dis. 2021;73:e609–19. https://doi.org/10.1093/cid/ciaa1733.
McMillan M, Marshall HS, Richmond P. 4CMenB vaccine and its role in preventing transmission and inducing herd immunity. Expert Rev Vaccines. 2022;21:103–14. https://doi.org/10.1080/14760584.2022.2003708.
McMillan M, Koehler AP, Lawrence A, et al. B part of it school leaver study: a repeat cross-sectional study to assess the impact of increasing coverage with meningococcal b (4CMenb) vaccine on carriage of Neisseria meningitidis. J Infect Dis. 2022;225:637–49. https://doi.org/10.1093/infdis/jiab444.
Guedes S, Bricout H, Langevin E, Tong S, Bertrand-Gerentes I. Epidemiology of invasive meningococcal disease and sequelae in the United Kingdom during the period 2008 to 2017—a secondary database analysis. BMC Public Health. 2022;22:521. https://doi.org/10.1186/s12889-022-12933-3.
Bonanni P, Sacco C, Donato R, Capei R. Lifelong vaccination as a key disease-prevention strategy. Clin Microbiol Infect. 2014;20(Suppl 5):32–6. https://doi.org/10.1111/1469-0691.12537.
Bonanni P, Angelillo IF, Villani A, et al. Maintain and increase vaccination coverage in children, adolescents, adults and elderly people: Let’s avoid adding epidemics to the pandemic: appeal from the Board of the Vaccination Calendar for Life in Italy: maintain and increase coverage also by re-organizing vaccination services and reassuring the population. Vaccine. 2021;39:1187–9. https://doi.org/10.1016/j.vaccine.2020.10.024.
US Centers for Disease Control and Prevention (CDC). Enhanced Meningococcal Disease Surveillance Report. 2015. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2015.pdf. Accessed 26 Apr 2023.
US Centers for Disease Control and Prevention (CDC). Enhanced Meningococcal Disease Surveillance Report. 2016. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report.pdf. Accessed 26 Apr 2023.
US Centers for Disease Control and Prevention (CDC). Enhanced Meningococcal Disease Surveillance Report. 2017. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2017.pdf. Accessed 26 Apr 2023.
US Centers for Disease Control and Prevention (CDC). Enhanced Meningococcal Disease Surveillance Report. 2020. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2020.pdf. Accessed 26 Apr 2023.
Booy R, Gentile A, Nissen M, Whelan J, Abitbol V. Recent changes in the epidemiology of Neisseria meningitidis serogroup W across the world, current vaccination policy choices and possible future strategies. Hum Vaccin Immunother. 2019;15:470–80. https://doi.org/10.1080/21645515.2018.1532248.
Krone M, Gray S, Abad R, et al. Increase of invasive meningococcal serogroup W disease in Europe, 2013 to 2017. Euro Surveill. 2019;24:1800245. https://doi.org/10.2807/1560-7917.ES.2019.24.14.1800245.
Masignani V, Pizza M, Moxon ER. The development of a vaccine against meningococcus B using reverse vaccinology. Front Immunol. 2019;10:751. https://doi.org/10.3389/fimmu.2019.00751.
Rappuoli R, Pizza M, Masignani V, Vadivelu K. Meningococcal B vaccine (4CMenB): the journey from research to real world experience. Expert Rev Vaccines. 2018;17:1111–21. https://doi.org/10.1080/14760584.2018.1547637.
Biolchi A, De Angelis G, Moschioni M, et al. Multicomponent meningococcal serogroup B vaccination elicits cross-reactive immunity in infants against genetically diverse serogroup C, W and Y invasive disease isolates. Vaccine. 2020;38:7542–50. https://doi.org/10.1016/j.vaccine.2020.09.050.
Biolchi A, Tomei S, Brunelli B, et al. 4CMenB immunization induces serum bactericidal antibodies against non-serogroup B meningococcal strains in adolescents. Infect Dis Ther. 2021;10:307–16. https://doi.org/10.1007/s40121-020-00370-x.
Harris SL, Tan C, Andrew L, et al. The bivalent factor H binding protein meningococcal serogroup B vaccine elicits bactericidal antibodies against representative non-serogroup B meningococci. Vaccine. 2018;36:6867–74. https://doi.org/10.1016/j.vaccine.2018.05.081.
Beeslaar J, Absalon J, Anderson AS, et al. MenB-FHbp vaccine protects against diverse meningococcal strains in adolescents and young adults: post hoc analysis of two phase 3 studies. Infect Dis Ther. 2020;9:641–56. https://doi.org/10.1007/s40121-020-00319-0.
O’Ryan M, Stoddard J, Toneatto D, Wassil J, Dull PM. A multi-component meningococcal serogroup B vaccine (4CMenB): the clinical development program. Drugs. 2014;74:15–30. https://doi.org/10.1007/s40265-013-0155-7.
Findlow J, Borrow R. Does post-implementation vaccine effectiveness data support pre-implementation predictions of 4CMenB utility? Pathog Dis. 2017;75:ftx025. https://doi.org/10.1093/femspd/ftx025.
Findlow J, Borrow R, Stephens DS, et al. Correlates of protection for meningococcal surface protein vaccines: current approaches for the determination of breadth of coverage. Expert Rev Vaccines. 2022;21:753–69. https://doi.org/10.1080/14760584.2022.2064850.
Borrow R, Taha MK, Giuliani MM, Pizza M, Banzhoff A, Bekkat-Berkani R. Methods to evaluate serogroup B meningococcal vaccines: From predictions to real-world evidence. J Infect. 2020;81:862–72. https://doi.org/10.1016/j.jinf.2020.07.034.
Brehony C, Rodrigues CMC, Borrow R, et al. Distribution of Bexsero(R) Antigen Sequence Types (BASTs) in invasive meningococcal disease isolates: implications for immunisation. Vaccine. 2016;34:4690–7. https://doi.org/10.1016/j.vaccine.2016.08.015.
Muzzi A, Brozzi A, Serino L, et al. Genetic Meningococcal Antigen Typing System (gMATS): a genotyping tool that predicts 4CMenB strain coverage worldwide. Vaccine. 2019;37:991–1000. https://doi.org/10.1016/j.vaccine.2018.12.061.
McNeil LK, Donald RGK, Gribenko A, et al. Predicting the susceptibility of meningococcal serogroup B isolates to bactericidal antibodies elicited by bivalent rLP2086, a novel prophylactic vaccine. MBio. 2018;9:e00036–18. https://doi.org/10.1128/mBio.00036-18.
Bettinger JA, Liberator P, Halperin SA, et al. Estimated susceptibility of Canadian meningococcal B isolates to a meningococcal serogroup B vaccine (MenB-FHbp). Vaccine. 2020;38:2026–33. https://doi.org/10.1016/j.vaccine.2019.12.051.
Basta NE, Mahmoud AA, Wolfson J, et al. Immunogenicity of a meningococcal B vaccine during a university outbreak. N Engl J Med. 2016;375:220–8. https://doi.org/10.1056/NEJMoa1514866.
Lujan E, Winter K, Rovaris J, Liu Q, Granoff DM. Serum bactericidal antibody responses of students immunized with a meningococcal serogroup B vaccine in response to an outbreak on a university campus. Clin Infect Dis. 2017;65:1112–9. https://doi.org/10.1093/cid/cix519.
Harris SL, Donald RG, Hawkins JC, et al. Neisseria meningitidis serogroup B vaccine, bivalent rLP2086, induces broad serum bactericidal activity against diverse invasive disease strains including outbreak strains. Pediatr Infect Dis J. 2017;36:216–23. https://doi.org/10.1097/INF.0000000000001399.
Taha MK, Hawkins JC, Liberator P, et al. Bactericidal activity of sera from adolescents vaccinated with bivalent rLP2086 against meningococcal serogroup B outbreak strains from France. Vaccine. 2017;35:1530–7. https://doi.org/10.1016/j.vaccine.2017.01.066.
Biolchi A, Tomei S, Santini L, et al. Four-component meningococcal serogroup B vaccine induces antibodies with bactericidal activity against diverse outbreak strains in adolescents. Pediatr Infect Dis J. 2021;40:e66–71. https://doi.org/10.1097/INF.0000000000002957.
Semchenko EA, Tan A, Borrow R, Seib KL. The serogroup B meningococcal vaccine Bexsero elicits antibodies to Neisseria gonorrhoeae. Clin Infect Dis. 2019;69:1101–11. https://doi.org/10.1093/cid/ciy1061.
Ruiz Garcia Y, Sohn WY, Seib KL, et al. Looking beyond meningococcal B with the 4CMenB vaccine: the Neisseria effect. NPJ Vaccines. 2021;6:130. https://doi.org/10.1038/s41541-021-00388-3.
Deghmane AE, Taha MK. Product review on the IMD serogroup B vaccine Bexsero(R). Hum Vaccin Immunother. 2022;18:2020043. https://doi.org/10.1080/21645515.2021.2020043.
Martinon-Torres F, Carmona Martinez A, Simko R, et al. Antibody persistence and booster responses 24–36 months after different 4CMenB vaccination schedules in infants and children: a randomised trial. J Infect. 2018;76:258–69. https://doi.org/10.1016/j.jinf.2017.12.005.
Martinon-Torres F, Nolan T, Toneatto D, Banzhoff A. Persistence of the immune response after 4CMenB vaccination, and the response to an additional booster dose in infants, children, adolescents, and young adults. Hum Vaccin Immunother. 2019;15:2940–51. https://doi.org/10.1080/21645515.2019.1627159.
Davis K, Valente Pinto M, Andrews NJ, et al. Immunogenicity of the UK group B meningococcal vaccine (4CMenB) schedule against groups B and C meningococcal strains (Sched3): outcomes of a multicentre, open-label, randomised controlled trial. Lancet Infect Dis. 2021;21:688–96. https://doi.org/10.1016/S1473-3099(20)30600-9.
Nolan T, Santolaya ME, de Looze F, et al. Antibody persistence and booster response in adolescents and young adults 4 and 7.5 years after immunization with 4CMenB vaccine. Vaccine. 2019;37:1209–18. https://doi.org/10.1016/j.vaccine.2018.12.059.
European Medicines Agency. Assessment report for paediatric studies submitted in accordance with article 46 of regulation (EC) No1901/2006, as amended. Bexsero. Procedure no.: EMA/H/C/002333 /P46/026. https://www.ema.europa.eu/en/documents/variation-report/bexsero-h-c-2333-p46-026-epar-assessment-report_en.pdf. Accessed 5 Aug 2022.
Read RC, Dull P, Bai X, et al. A phase III observer-blind randomized, controlled study to evaluate the immune response and the correlation with nasopharyngeal carriage after immunization of university students with a quadrivalent meningococcal ACWY glycoconjugate or serogroup B meningococcal vaccine. Vaccine. 2017;35:427–34. https://doi.org/10.1016/j.vaccine.2016.11.071.
Toneatto D, Ismaili S, Ypma E, Vienken K, Oster P, Dull P. The first use of an investigational multicomponent meningococcal serogroup B vaccine (4CMenB) in humans. Hum Vaccin. 2011;7:646–53. https://doi.org/10.4161/hv.7.6.15482.
Kimura A, Toneatto D, Kleinschmidt A, Wang H, Dull P. Immunogenicity and safety of a multicomponent meningococcal serogroup B vaccine and a quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135, and Y in adults who are at increased risk for occupational exposure to meningococcal isolates. Clin Vaccine Immunol. 2011;18:483–6. https://doi.org/10.1128/CVI.00304-10.
Marshall HS, Richmond PC, Beeslaar J, et al. Meningococcal serogroup B-specific responses after vaccination with bivalent rLP2086: 4 year follow-up of a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2017;17:58–67. https://doi.org/10.1016/S1473-3099(16)30314-0.
Vesikari T, Ostergaard L, Beeslaar J, et al. Persistence and 4-year boosting of the bactericidal response elicited by two- and three-dose schedules of MenB-FHbp: a phase 3 extension study in adolescents. Vaccine. 2019;37:1710–9. https://doi.org/10.1016/j.vaccine.2018.11.073.
Australian Technical Advisory Group on Immunisation (ATAGI). Australian immunisation handbook 2020. https://www.health.gov.au/health-topics/immunisation/immunisation-throughout-life/national-immunisation-program-schedule. Accessed 5 Aug 2022.
Sharma K, Chiu C, Wood N. Meningococcal vaccines in Australia: a 2019 update. Aust Prescr. 2019;42:131–5. https://doi.org/10.18773/austprescr.2019.042.
Ministre des Solidarités et de la Santé [France]. Calendrier simplifié des vaccinations 2022 (April 2022). https://solidarites-sante.gouv.fr/prevention-en-sante/preserver-sa-sante/vaccination/calendrier-vaccinal. Accessed 5 Aug 2022.
Álvarez García FJ, Cilleruelo Ortega MJ, Álvarez Aldeán J, et al. Calendario de inmunizaciones de la Asociación Española de Pediatría: recomendaciones 2023. Anal Pediatr. 2023;98:58e1–e10. https://doi.org/10.1016/j.anpedi.2022.10.002.
Stefanizzi P, Bianchi FP, Martinelli A, et al. Safety profile of MenB-FHBp vaccine among adolescents: data from surveillance of Adverse Events Following Immunization in Puglia (Italy), 2018–2020. Hum Vaccin Immunother. 2022;18:2041359. https://doi.org/10.1080/21645515.2022.2041359.
Robinson JL. Update on invasive meningococcal vaccination for Canadian children and youth. Paediatr Child Health. 2018;23:e1–4. https://doi.org/10.1093/pch/pxx162.
Wang B, Giles L, Andraweera P, et al. Effectiveness and impact of the 4CMenB vaccine against invasive serogroup B meningococcal disease and gonorrhoea in an infant, child, and adolescent programme: an observational cohort and case-control study. Lancet Infect Dis. 2022;22:1011-20. https://doi.org/10.1016/S1473-3099(21)00754-4.
Pharmaceutical Management Agency (PHARMAC). Decision to widen access to the meningococcal B vaccine and secure supply of the shingles vaccine, 8 December 2022. https://pharmac.govt.nz/news-and-resources/consultations-and-decisions/2022-12-08-meningococcal-b-vaccine-notification. Accessed 12 Dec 2022.
Chicuto LAD, de Moraes C, de Moraes JC, Safadi MAP. A critical analysis of serogroup B meningococcal disease burden in Brazil (2001–2015): implications for public health decisions. Hum Vaccin Immunother. 2020;16:1945–50. https://doi.org/10.1080/21645515.2019.1700710.
Public Health England. The Green Book. Chapter 7: Immunisation of individuals with underlying medical conditions. https://www.gov.uk/government/publications/immunisation-of-individuals-with-underlying-medical-conditions-the-green-book-chapter-7. Accessed 12 Dec 2022.
National Advisory Committee on Immunization (NACI). Canadian Immunization Guide. https://www.canada.ca/en/public-health/services/canadian-immunization-guide.html. Accessed 5 Aug 2022.
Manatū Hauora Ministry of Health. Immunisation Handbook 2020. Chapter 13. Meningococcal disease. https://www.health.govt.nz/our-work/immunisation-handbook-2020/13-meningococcal-disease. Accessed 12 Dec 2022.
Ladhani SN, Andrews N, Parikh SR, et al. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med. 2020;382:309–17. https://doi.org/10.1056/NEJMoa1901229.
Isitt C, Cosgrove CA, Ramsay ME, Ladhani SN. Success of 4CMenB in preventing meningococcal disease: evidence from real-world experience. Arch Dis Child. 2020;105:784–90. https://doi.org/10.1136/archdischild-2019-318047.
Rodrigues FMP, Marlow R, Simoes MJ, Danon L, Ladhani S, Finn A. Association of use of a meningococcus group B vaccine with group B invasive meningococcal disease among children in Portugal. JAMA. 2020;324:2187–94. https://doi.org/10.1001/jama.2020.20449.
Azzari C, Moriondo M, Nieddu F, et al. Effectiveness and impact of the 4CMenB vaccine against group B meningococcal disease in two Italian regions using different vaccination schedules: a five-year retrospective observational study (2014–2018). Vaccines (Basel). 2020;8:469. https://doi.org/10.3390/vaccines8030469.
Castilla J, Garcia Cenoz M, Abad R, et al. Effectiveness of a meningococcal group B vaccine (4CMenB) in children. N Engl J Med. 2023;388:427–38. https://doi.org/10.1056/NEJMoa2206433.
McMillan M, Wang B, Koehler AP, Sullivan TR, Marshall HS. Impact of meningococcal B vaccine on invasive meningococcal disease in adolescents. Clin Infect Dis. 2021;73:e233–7. https://doi.org/10.1093/cid/ciaa1636.
Bruxvoort KJ, Lewnard JA, Chen LH, et al. Prevention of Neisseria gonorrhoeae with meningococcal B vaccine: a matched cohort study in Southern California. Clin Infect Dis. 2023;76:e1341–9. https://doi.org/10.1093/cid/ciac436.
Alderfer J, Isturiz RE, Srivastava A. Lessons from mass vaccination response to meningococcal B outbreaks at US universities. Postgrad Med. 2020;132:614–23. https://doi.org/10.1080/00325481.2020.1766265.
McNamara LA, Shumate AM, Johnsen P, et al. First use of a serogroup B meningococcal vaccine in the US in response to a university outbreak. Pediatrics. 2015;135:798–804. https://doi.org/10.1542/peds.2014-4015.
Soeters HM, McNamara LA, Blain AE, et al. University-based outbreaks of meningococcal disease caused by serogroup B, United States, 2013–2018. Emerg Infect Dis. 2019;25:434–40. https://doi.org/10.3201/eid2503.181574.
Banzhoff A. Multicomponent meningococcal B vaccination (4CMenB) of adolescents and college students in the United States. Ther Adv Vaccines. 2017;5:3–14. https://doi.org/10.1177/2051013616681365.
Pivette M, Taha MK, Barret AS, et al. Targeted vaccination campaigns of teenagers after two clusters of B invasive meningococcal disease in Brittany, France, 2017. BMC Public Health. 2020;20:1382. https://doi.org/10.1186/s12889-020-09487-7.
De Wals P, Deceuninck G, Lefebvre B, et al. Impact of an immunization campaign to control an increased incidence of serogroup B meningococcal disease in one region of Quebec, Canada. Clin Infect Dis. 2017;64:1263–7. https://doi.org/10.1093/cid/cix154.
Deceuninck G, Lefebvre B, Tsang R, Betala-Belinga JF, De Serres G, De Wals P. Impact of a mass vaccination campaign against Serogroup B meningococcal disease in the Saguenay-Lac-Saint-Jean region of Quebec four years after its launch. Vaccine. 2019;37:4243–5. https://doi.org/10.1016/j.vaccine.2019.06.021.
Fiorito TM, Bornschein S, Mihalakos A, et al. Rapid response to a college outbreak of meningococcal serogroup B disease: Nation’s first widespread use of bivalent rLP2086 vaccine. J Am Coll Health. 2017;65:294–6. https://doi.org/10.1080/07448481.2017.1285772.
Fiorito TM, Baird GL, Alexander-Scott N, et al. Adverse events following vaccination with bivalent rLP2086 (Trumenba(R)): an observational, longitudinal study during a college outbreak and a systematic review. Pediatr Infect Dis J. 2018;37:e13–9. https://doi.org/10.1097/INF.0000000000001742.
Perrett KP, McVernon J, Richmond PC, et al. Immune responses to a recombinant, four-component, meningococcal serogroup B vaccine (4CMenB) in adolescents: a phase III, randomized, multicentre, lot-to-lot consistency study. Vaccine. 2015;33:5217–24. https://doi.org/10.1016/j.vaccine.2015.06.103.
Santolaya ME, O’Ryan ML, Valenzuela MT, et al. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet. 2012;379:617–24. https://doi.org/10.1016/S0140-6736(11)61713-3.
Read RC, Baxter D, Chadwick DR, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384:2123–31. https://doi.org/10.1016/S0140-6736(14)60842-4.
Findlow J, Bai X, Findlow H, et al. Safety and immunogenicity of a four-component meningococcal group B vaccine (4CMenB) and a quadrivalent meningococcal group ACWY conjugate vaccine administered concomitantly in healthy laboratory workers. Vaccine. 2015;33:3322–30. https://doi.org/10.1016/j.vaccine.2015.05.027.
Langley JM, Gantt S, Quach C, et al. Randomized trial of 2 schedules of meningococcal B vaccine in adolescents and young adults, Canada. Emerg Infect Dis. 2020;26:454–62. https://doi.org/10.3201/eid2603.190160.
A Phase 3b, Single-center, open-label study to assess the immunogenicity and safety of Novartis meningococcal B recombinant vaccine when administered at a 0, 2-month schedule in healthy at-risk adults aged 18 to 65 years inclusive. https://clinicaltrials.gov/ct2/show/results/NCT01911221. Accessed 5 Aug 2022.
A Phase 3b study to assess the safety of Novartis meningococcal B recombinant vaccine when administered in healthy at-risk adults. https://clinicaltrials.gov/ct2/show/results/NCT01478347. Accessed 5 Aug 2022.
Safety and blood donations in adults vaccinated with rMenB+OMV NZ. https://clinicaltrials.gov/ct2/show/results/NCT02305446. Accessed 5 Aug 2022.
Ostergaard L, Vesikari T, Absalon J, et al. A bivalent meningococcal B vaccine in adolescents and young adults. N Engl J Med. 2017;377:2349–62. https://doi.org/10.1056/NEJMoa1614474.
Sheldon EA, Schwartz H, Jiang Q, Giardina PC, Perez JL. A phase 1, randomized, open-label, active-controlled trial to assess the safety of a meningococcal serogroup B bivalent rLP2086 vaccine in healthy adults. Hum Vaccin Immunother. 2012;8:888–95. https://doi.org/10.4161/hv.19983.
Marshall HS, Richmond PC, Nissen MD, et al. A phase 2 open-label safety and immunogenicity study of a meningococcal B bivalent rLP2086 vaccine in healthy adults. Vaccine. 2013;31:1569–75. https://doi.org/10.1016/j.vaccine.2013.01.021.
Bekkat-Berkani R, Fragapane E, Preiss S, et al. Public health perspective of a pentavalent meningococcal vaccine combining antigens of MenACWY-CRM and 4CMenB. J Infect. 2022;85:481–91. https://doi.org/10.1016/j.jinf.2022.09.001.
Marshall GS, Fergie J, Presa J, Peyrani P. Rationale for the development of a pentavalent meningococcal vaccine: a US-focused review. Infect Dis Ther. 2022;11:937–51. https://doi.org/10.1007/s40121-022-00609-9.
Beran J, Drazan D, Enweonye I, Bhusal C, Toneatto D. Immunogenicity and safety of investigational MenABCWY Vaccine and of 4CMenB and MenACWY vaccines administered concomitantly or alone: a phase 2 randomized study of adolescents and young adults. mSphere. 2021;6:e0055321. https://doi.org/10.1128/mSphere.00553-21.
Drazan D, Czajka H, Maguire JD, et al. A phase 3 study to assess the immunogenicity, safety, and tolerability of MenB-FHbp administered as a 2-dose schedule in adolescents and young adults. Vaccine. 2022;40:351–8. https://doi.org/10.1016/j.vaccine.2021.11.053.
Marshall GS, Ghaswalla PK, Bengtson LGS, et al. Low meningococcal vaccination rates among patients with newly diagnosed complement component deficiencies in the United States. Clin Infect Dis. 2022;75:155–8. https://doi.org/10.1093/cid/ciab917.
Ghaswalla PK, Bengtson LGS, Marshall GS, et al. Meningococcal vaccination in patients with newly diagnosed asplenia in the United States. Vaccine. 2021;39:272–81. https://doi.org/10.1016/j.vaccine.2020.11.068.
de Oliveira CJ, Gianacas C, Beard F, et al. Cumulative annual coverage of meningococcal B vaccination in Australian general practice for three at-risk groups, 2014 to 2019. Hum Vaccin Immunother. 2021;17:3692–701. https://doi.org/10.1080/21645515.2021.1923349.
Philip RK, Attwell K, Breuer T, Di Pasquale A, Lopalco PL. Life-course immunization as a gateway to health. Expert Rev Vaccines. 2018;17:851–64. https://doi.org/10.1080/14760584.2018.1527690.
Bonanni P, Villani A, Scotti S, et al. The recommended lifetime immunization schedule from the board of vaccination calendar for life in Italy: a continuing example of impact on public health policies. Vaccine. 2021;39:1183–6. https://doi.org/10.1016/j.vaccine.2021.01.019.
Laupeze B, Del Giudice G, Doherty MT, Van der Most R. Vaccination as a preventative measure contributing to immune fitness. NPJ Vaccines. 2021;6:93. https://doi.org/10.1038/s41541-021-00354-z.
WHO. World Health Organisation. Defeating meningitis by 2030: a global road map. April 2020. https://www.who.int/publications/i/item/9789240026407. Accessed 4 Mar 2022.
Greenwood B, Sow S, Preziosi MP. Defeating meningitis by 2030—an ambitious target. Trans R Soc Trop Med Hyg. 2021;115:1099–101. https://doi.org/10.1093/trstmh/trab133.
Acknowledgements
Funding
This work and the article processing charge, including the journal’s Rapid Service fee, were sponsored by GlaxoSmithKline Biologicals SA.
Medical Writing, Editorial, and Other Assistance
The authors would like to thank Adriana Guzman Holst, Lamine Soumahoro and Pavitra Deeywadaa, employees of GSK, for their contribution to this manuscript. The authors would also like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, and design support for the digital illustrations, on behalf of GSK. Iain O’Neill (freelance, on behalf of GSK) provided medical writing support.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conception and design: George Kassianos, Osamah Barasheed, Victoria Abbing-Karahagopian, Angelika Banzhoff, Mansour Khalaf, Serdar Ozturk and Selim Badur. Data collection: Serdar Ozturk and Selim Badur. Statistical analysis: Not applicable. Drafting and revising for intellectual content: Selim Badur, Serdar Ozturk, Victoria Abbing-Karahagopian, Angelika Banzhoff, Mansour Khalaf, Serdar Ozturk, Osamah Barasheed and George Kassianos. Agreed to be accountable for the integrity of the work: George Kassianos, Osamah Barasheed, Victoria Abbing-Karahagopian, Angelika Banzhoff, Mansour Khalaf, Serdar Ozturk and Selim Badur.
Disclosures
Victoria Abbing-Karahagopian and Selim Badur are employed by GSK. Angelika Banzhoff, Serdar Ozturk and Mansour Khalaf are employed by and hold shares in GSK. George Kassianos is the Royal College of General Practitioners National Immunisation Lead, and British Global and Travel Health Association President; and participated in advisory boards or lectured at meetings organized by vaccine manufacturers (GSK, MSD, Sanofi Pasteur, AstraZeneca, Pfizer, Janssen, Valneva, Moderna and CSL Seqirus). George Kassianos, Victoria Abbing-Karahagopian, Angelika Banzhoff, Selim Badur, Serdar Ozturk and Mansour Khalaf declare no other financial and non-financial relationships and activities. Osamah Barasheed declares no financial and non-financial relationships and activities and no conflicts of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies performed by any of the authors with human participants or animals.
Data Availability
All data generated or analysed are included in this published article or in the original data sources.
Trademark Statement
Bexsero is a trademark owned by or licensed to GSK. Trumenba is a trademark of Pfizer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kassianos, G., Barasheed, O., Abbing-Karahagopian, V. et al. Meningococcal B Immunisation in Adults and Potential Broader Immunisation Strategies: A Narrative Review. Infect Dis Ther 12, 2193–2219 (2023). https://doi.org/10.1007/s40121-023-00836-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00836-8