Abstract
Introduction
In addition to significant morbidity and mortality, the coronavirus disease (COVID-19) has strained health care systems globally. This study investigated the cost-effectiveness of remdesivir + standard of care (SOC) for hospitalized COVID-19 patients in the USA.
Methods
This cost-effectiveness analysis considered direct and indirect costs of remdesivir + SOC versus SOC alone among hospitalized COVID-19 patients in the US. Patients entered the model stratified according to their baseline ordinal score. At day 15, patients could transition to another health state, and on day 29, they were assumed to have either died or been discharged. Patients were then followed over a 1-year time horizon, where they could transition to death or be rehospitalized.
Results
Treatment with remdesivir + SOC avoided, per patient, a total of 4 hospitalization days: two general ward days and a day for both the intensive care unit and the intensive care unit plus invasive mechanical ventilation compared to SOC alone. Treatment with remdesivir + SOC presented net cost savings due to lower hospitalization and lost productivity costs compared to SOC alone. In increased and decreased hospital capacity scenarios, remdesivir + SOC resulted in more beds and ventilators being available versus SOC alone.
Conclusions
Remdesivir + SOC alone represents a cost-effective treatment for hospitalized patients with COVID-19. This analysis can aid in future decisions on the allocation of healthcare resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
While the COVID-19 healthcare paradigm has evolved, infections and hospitalizations continue to occur. |
What was learned from the study? |
Treatment with remdesivir, in addition to standard of care, reduced time spent in hospital and presented net cost savings compared to standard of care alone. |
Across various hospital capacity scenarios, compared to standard of care alone, treatment with remdesivir resulted in more beds and ventilators being available. |
In the context of increasingly constrained healthcare resources, remdesivir is a cost-effective treatment for hospitalized patients with COVID-19 and provides good value for health systems. |
Introduction
Severe acute respiratory syndrome corona virus type 2 (SARS-CoV-2) emerged at the end of 2019 as a novel coronavirus disease (COVID-19) and spread rapidly worldwide. Since the virus was first detected in the USA in March 2020, over 1 million Americans have died [1]. Though multiple vaccines are available, the pandemic is far from over, particularly given ongoing immune evasiveness from humoral immunity, even as cellular immunity from vaccination and/or prior recovery alters the relative risk [2]. Despite approximately two thirds of the American population being considered fully vaccinated, > 120,000 new cases are reported daily, resulting in > 400 daily deaths and > 4500 patients hospitalized in the intensive care unit [1]. Strains on the health system resources have resulted in postponed elective inpatient surgical admissions and workforce shortages [3, 4]. While many services have resumed with lower COVID-19 hospitalizations, local surges still cause disruption.
Patients infected with COVID-19 can experience a variety of symptoms and, in some cases, can be managed in the outpatient setting or at home. However, for others, disease progression can occur rapidly, requiring hospitalization and the potential for invasive mechanical ventilation (IMV), leading to substantial clinical burden. Remdesivir (RDV), an antiviral therapy that received emergency use authorization in May 2020 from the Food and Drug Administration, has since received full approval as a treatment for hospitalized and non-hospitalized COVID-19 patients, including pediatric patients aged ≥ 28 days, weighing at least 3 kg [5]. The inpatient approval was primarily based on the pivotal ACTT-1 trial [6], which compared RDV plus standard of care (SOC) versus placebo plus SOC and found that treatment with RDV led to a shorter time to recovery (15 versus 10 days), with an increased recovery rate of 29%. Furthermore, in a post hoc analysis, RDV was associated with a 70% reduction in mortality for patients who required low-flow oxygen support at baseline [6]. The findings of the ACTT-1 trial have been supported by similar results from other trials [7] and in real-world comparative effectiveness studies [8, 9]. More recently, efficacy has been demonstrated for the early use of remdesivir for patients with one or more risk factors for progression [10].
The COVID-19 pandemic has strained health care systems globally, impacting staffing, supplies, and space [11]. In the context of increasingly constrained healthcare resources, the relative value of treatments needs to be considered. Furthermore, as the pandemic continues, new evidence emerges for novel and existing treatments, with guidelines being constantly reviewed and revised [12]. This study aims to investigate the cost-effectiveness of RDV + SOC for hospitalized COVID-19 patients in the US.
Methods
Model Overview
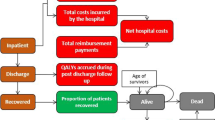
To assess the cost-effectiveness of treatment with RDV + SOC, a health economic model was developed in Microsoft Excel 2016 following the International Society for Pharmacoeconomic and Outcomes Research Modelling Good Practice Guidelines [13] from a societal perspective (Fig. 1). Hospitalized COVID-19 patients enter a decision tree model, stratified according to their baseline status, as defined by the World Health Organization ordinal score (OS), as follows: 0, no clinical or virological evidence of infection; 1, ambulatory, no activity limitation; 2, ambulatory, activity limitation; 3, hospitalized, no oxygen therapy; 4, hospitalized, oxygen mask or nasal prongs; 5, hospitalized, non-invasive ventilation (NIV) or high-flow oxygen; 6, hospitalized, intubation with IMV; 7, hospitalized, IMV + additional support such as pressors, renal replacement therapy, or extracorporeal membrane oxygenation (ECMO); 8, deceased [14]. In this model, on day 15, patients could then transition into one of the following health states: discharged, ward with no supplemental oxygen (OS 3), ward on low-flow oxygen (OS 4), intensive care unit (ICU) with NIV or high-flow oxygen (OS 5), or ICU with IMV (OS 6) or ECMO (OS 7). On day 29 in this model, patients are assumed to have either died or been discharged to rehabilitation or home. Patients can also be re-hospitalized post-discharge, incurring hospital costs and outcomes. After day 29, patients enter a Markov model, subject to age- and sex-adjusted mortality derived from US-specific lifetables. Patients can transition to death at any time. Long-term effects of COVID-19 are not considered as part of this model due to a lack of information on the impact of treatment over time. The model time horizon was 1 year; thus, no discounting for costs or outcomes was applied. No ethics or patient consent was necessary for this study; this study is based on previously conducted studies and does not contain any new studies with human participants performed by the authors.
Population Inputs
The number of COVID-19 cases in the model base case is calculated using national epidemiological data in adults for 2022 [15]. The average age and gender split were taken from a recent retrospective cohort analysis of over 850,000 patients from Premier Healthcare Data [16]. Based on surveillance data, a hospitalization rate of 10% was applied to the total number of confirmed COVID-19 cases [15], recognizing that this rate may differ based on the evolving virus.
Clinical Inputs
The clinical distribution by OS at baseline was informed by the retrospective cohort analysis of Premier Healthcare Data, which included adult patients, with first admission to the hospital between May 2020 and December 2021 and with primary or secondary discharge diagnosis of COVID-19 [16]. The proportion of patients with OS4, OS5, OS6, and OS7 was determined to be 60.0%, 21.0%, 14.0%, and 5.0% over the time period of the study [16]. As transition probabilities for the two treatment arms (RDV + SOC and SOC alone) were not available from Premier Healthcare Data, they were calculated using data from Table 2 from Beigel et al. of the ACTT-1 trial [6], which provided a breakdown of participants by OS at baseline and their respective improvements by day 15 (see Table S1 Supplementary Material).
On day 29, the proportion of patients alive in the base case was derived from the OS-specific hazard ratios reported in Table 2 from Beigel et al. of the ACTT-1 trial (Table 1). It was assumed that patients with an OS of between 1 and 3 at day 29 were at no higher risk than the general population for mortality; thus, post-discharge, US-specific background mortality rates apply to these patients [17].
Length of stay (LOS) for each OS was derived from an analysis of the Premier Healthcare Database, which evaluated hospital costs, LOS and discharge status among adult COVID-19 patients between April 1 and December 31, 2020 (Table 1) [18]. LOS for rehospitalization was assumed to be 5.0 days. The rate of rehospitalization was elicited from an internal analysis and was verified by clinical experts; this rate is in line with other published sources [19]. Rate of rehospitalization in the SOC arm was 12.0% [20] with a RDV rate ratio of rehospitalization of 1.67 taken from the ACTT-1 trial [6].
Cost Inputs
Costs are reported in 2021 US dollars (USD). The base case model considered both direct costs to the health care setting, including drug costs and inpatient stays, and indirect costs (Table 1). It was assumed that a treatment course of RDV would consist of six vials. No cost was assigned to SOC. The cost per hospital day by OS was obtained from a study on COVID-19 hospital admissions using national data from the Premier Health Database, which stratified costs by level of care and ward; the overall cost per day for the hospital (general ward) and ICU informed this analysis [18]. For the cost of ICU including IMV, an average of the overall hospital and ICU costs for OS 7 was used [18]. Costs included health care professional time, tests and monitoring, and hotel costs. The cost per day of a hospital readmission was assumed to be the same as the general ward.
Productivity losses were included in the base case. From the US Bureau of Labor Statistics, it was assumed that 96.0% of the population would be economically active and working [21]. For those working, a loss cost per day due to hospitalization/rehabilitation of $221.20 was applied, based on the average hourly wage for all employees in the US [22].
Utilities
Utility decrements associated with hospitalization were incorporated by level of care, based on the highest level of care received (Table 1). It was assumed that the utility decrement for the general ward was equivalent to “COVID-19 symptoms only” and, for ICU, was equivalent to “oxygen support without ventilation,” based on a cost-effectiveness framework that evaluated acute treatments for hospitalized patients with COVID-19 [23]. This model assumed that upon discharge from the hospital (and upon entering the Markov model), patients are subject to background age-adjusted US population utility norms from the EQ-5D [24].
Outcomes
The number of hospital days avoided (by ward, ICU, and ICU + IMV) and the number needed to treat to avoid 1 day in the general ward, the ICU, or ICU + IMV (derived by determining how many patients needed to receive RDV + SOC to avoid 1 day in hospital) were estimated and presented on a per patient basis. Treatment costs, hospitalization costs, and the cost per quality-adjusted life year (QALY) were calculated.
Sensitivity and Scenario Analyses
The model estimated parameter uncertainty through both one-way and probabilistic sensitivity analyses. For one-way analyses, all parameters were varied individually by ± 10% to determine the top ten most influential inputs on the model. The probabilistic sensitivity analysis, where all inputs are varied simultaneously across pre-specified distributions, was repeated over 5000 iterations. For the probabilistic analysis, population settings, clinical inputs, and costs were varied using the gamma distribution; proportions and utility decrements were varied using the beta distribution.
In addition, various scenarios were investigated. Scenarios included the use of the ACTT-1 clinical trial data to inform OS baseline distribution [6], surge capacity for peak infection periods where bed (general, ICU and ICU + IMV) increased to 90.0%, increased hospital costs of 30%, and a scenario where only direct costs are considered (payer perspective).
As caring for COVID-19 patients has downstream effects on the resource use for hospitals, the model also considered the implications of the capacity of hospital resources used to treat COVID-19 patients as a scenario analysis. Treatment capacity was assessed based on the total population, and the number of general ward and ICU beds, along with mechanical ventilators, was taken from a survey done by the American Hospital Association on capacity in 2020 [25]. Of the total available resources, it was assumed that 64.0% of general ward beds and 63.0% of both ICU beds and mechanical ventilators would be available for COVID-19 patients at baseline [3].
Results
In the base case, the model estimated that treatment with RDV + SOC avoided, per patient, a total of 4 hospitalization days: 2 general ward days and 1 day for both the ICU and ICU + IMV compared to SOC alone (Fig. 2). The number needed to treat to avoid 1 general ward day, 1 ICU day, and 1 ICU + MIV day was 0.55, 1.05, and 1.10, respectively. Per patient estimated cost outcomes are presented in Table 2. Treatment with RDV + SOC resulted in incremental costs for treatment and rehabilitation as well as cost savings for hospitalization and lost productivity. There were net total savings for treatment with RDV + SOC.
The life years (LYs) and QALYs of treatment with RDV + SOC versus SOC along with the cost-effectiveness results are presented in Table 3. Treatment with RDV + SOC was less costly and more effective than SOC alone and thus is considered dominant.
Sensitivity and Scenario Analyses
The top ten drivers of the incremental cost-effectiveness ratio are displayed in Figure S1, Supplementary Material. One-way sensitivity analyses found that the model was most sensitive to the relative risk reduction associated with LOS across the OS 4–8, along with the cost per day of IMV. The probabilistic sensitivity analysis indicated that RDV + SOC was dominant (cost less and was more effective) in 99.8% of the 5000 iterations versus SOC alone (see Figure S2, Supplementary Material).
Scenario analyses exploring the alternate assumptions for using the baseline distribution in ACTT-1, increased hospital costs, and considering direct costs only are presented in Table 4. Across all scenarios, RDV + SOC remained dominant (less costly and more effective).
Scenario analysis of the impact of RDV + SOC with varying proportions of hospital capacity being used for COVID-19, estimated at a total population level, is presented in Table S2, Supplementary Material. In the base case, treatment with RDV + SOC requires 10% more general ward bed days versus 47% more when treated with SOC alone to manage patients with COVID-19. Across both increased and decreased treatment capacity scenarios, treatment with RDV + SOC compared to SOC alone results in more general ward and ICU bed days along with more ventilators available.
Discussion
This study demonstrated the cost-effectiveness of inpatient RDV + SOC versus SOC alone for the treatment of hospitalized COVID-19 patients from a societal perspective. We found that under base case assumptions, treatment with RDV + SOC reduced the LOS and avoided days in hospital, resulting in cost savings compared to SOC alone. The results were robust across multiple scenarios, and RDV + SOC was cost-effective in almost all iterations in the probabilistic sensitivity analysis. Treatment with RDV + SOC, under these assumptions, is a cost-effective option for US health payers, while also minimizing lost productivity due to illness.
This study contributes to the growing body of literature reporting that remdesivir is cost-effective for the treatment of hospitalized patients with COVID-19. Furthermore, these results can be considered highly generalizable given the various sources used to populate the inputs in the model [26,27,28,29,30]. Although the direct quantification of the impact of hospitalizations due to COVID-19 on lost productivity costs and absenteeism is not yet available, the impact of COVID-19 on the global economic and financial markets has been significant, with reductions in income and a rise in unemployment [31]. Furthermore, RDV reduces the number of days in hospital and thus increases the capacity in the ICU by freeing up bed space [32, 33]. Not only does this allow patients to return to work faster following discharge from hospital, but this may also aid in offsetting losses in revenue due to reduced elective surgeries [34]. This study may underestimate the benefit on RDV on reducing healthcare resource use as it focuses on the use of RDV in hospitalized patients only. Given the recent data on the use of RDV in preventing progression to severe COVID-19 among outpatients [10], the cost-effectiveness of RDV in other settings should be explored to fully understand the benefit of treatment.
While this model is informed by multiple inputs from the literature, the COVID-19 pandemic is continually evolving. Clinical estimates may vary based on regional/seasonal surges and new COVID variants within the US. Furthermore, as observed over the last 3 years, the impact on hospitalizations and severity of disease changes as new variants emerge. This has wide-reaching health system impacts: when hospitals approach and exceed capacity because of COVID bed utilization, even hospital transfers for non-COVID-related healthcare needs are affected; these impacts are not yet well quantified. However, while recent data have shown that LOS, a key driver in the current model, has not changed significantly over time across the observed variants [16], the science and understanding of COVID-19 continue to evolve, which may impact other inputs in the model. While we explored an increase of 30% in costs in a scenario analysis, this may not fully address the actual increases in costs that hospitals are currently facing. As of this writing, inflation has hit a 40-year high in the US, and in the face of rising costs and labor shortages, many hospitals have turned to contract workers (travel nurses) to fill the gaps at significant additional costs [35, 36].
While the model should be interpreted in the context of its limitations and assumptions, many of the assumptions were conservative. The transition probabilities informing the model for the current analysis were taken from an earlier time in the pandemic, and disease transmission rates may be different as new variants emerge. Furthermore, these disease transmission rates were taken from a time before vaccinations emerged. The long-term effects of COVID-19 (post-discharge) were not considered, as data in this area are not well understood and were thus considered too uncertain to be included at this time. While further work on identifying the risk factors, groups disproportionately affected, and health and financial costs associated with post-COVID would help inform these inputs in the future, preliminary data indicate that treatment with RDV leads to a reduction in long-term COVID-19 symptoms [37]. While the base case analysis considered the societal perspective, this model did not explicitly consider burnout—physical and mental exhaustion—among healthcare workers during the pandemic [38]. In addition to the significant impact on the mental well-being of healthcare workers, burnout can generate inefficiencies in the healthcare organizations and lead to staffing shortages. Finally, our model did not estimate the number of deaths avoided when patients were treated with RDV + SOC. Given the benefit in survival observed with treatment with RDV + SOC [6, 39], this model may underestimate the benefit of RDV in the inpatient setting.
Conclusions
Despite the availability of multiple vaccines and treatments, the health and economic burden of the COVID-19 pandemic remain significant, and the ongoing need to deploy effective interventional therapies to treat patients remains an urgent need. This model found that RDV + SOC is a cost-effective treatment for hospitalized patients with COVID-19, both reducing the disease burden of patients and representing good value for health systems. This model can aid in guiding future decisions for the allocation of healthcare resources.
References
The New York Times. Coronavirus in the US: latest map and case count. 2022. Available from: https://www.nytimes.com/interactive/2021/us/covid-cases.html. 18 Jul 2022.
Zhang Y, et al. Immune evasive effects of SARS-CoV-2 variants to COVID-19 emergency used vaccines. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.771242.
Tsai TC, Jacobson BH, Jha AK. American hospital capacity and projected need for COVID-19 patient care. 2022. Available from: https://www.healthaffairs.org/do/https://doi.org/10.1377/forefront.20200317.457910/full/. 18 July 2020.
Smallwood N, et al. COVID-19 infection and the broader impacts of the pandemic on healthcare workers. Respirology. 2022;27(6):411–26.
US Food & Drug Administration. Remdesivir (Veklury) approval for the treatment of COVID-19—the evidence for safety and efficacy. 2020; Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19. Cited 22 Oct 2021.
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D. Remdesivir for the treatment of Covid-19—final report. N Eng J Med. 2020. https://doi.org/10.1056/NEJMoa2007764.
Goldman JD, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Eng J Med. 2020. https://doi.org/10.1056/NEJMoa2015301.
Mozaffari E, et al. Remdesivir treatment in hospitalized patients with COVID-19: a comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab875.
Chokkalingam AP et al. Comparative effectiveness of remdesivir treatment in patients hospitalized with COVID-19, in World Microbe Forum; 2021.
Gottlieb RL, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2021;386(4):305–15.
Myers LC, Liu VX. The COVID-19 pandemic strikes again and again and again. JAMA Netw Open. 2022;5(3):e221760–e221760.
World Health Organization. Update to living WHO guideline on drugs for covid-19. BMJ. 2022;377:o1005.
Weinstein MC, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR task force on good research practices-modeling studies. Value Health. 2003;6(1):9–17.
Rubio-Rivas M, et al. WHO ordinal scale and inflammation risk categories in COVID-19. Comparative study of the severity scales. J Gen Intern Med. 2022;37(8):1980–7.
Couture A, et al. Estimating COVID-19 hospitalizations in the united states with surveillance data using a Bayesian hierarchical model: modeling study. JMIR Public Health Surveill. 2022;8(6): e34296.
Chen L, et al. COVID-19 treatment and outcomes in over 850,000 hospitalized patients in the United States: May 2020–December 2021. In ECCMID. 2022. Lisbon, Portugal.
Centers for Disease Control and Prevention, United States Life Tables 2017. 2017. Available at: https://www.cdc.gov/nchs/products/life_tables.htm. Accessed July 2022.
Ohsfeldt RL, et al. Inpatient hospital costs for COVID-19 patients in the united states. Adv Ther. 2021;38(11):5557–95.
Loo WK, et al. Systematic review on COVID-19 readmission and risk factors: future of machine learning in COVID-19 readmission studies. Front Public Health. 2022. https://doi.org/10.3389/fpubh.2022.898254.
Gilead Sciences Inc., Data on file. 2022.
US Bureau of Labor Statistics. A-10. Unemployment rates by age, sex, and marital status, seasonally adjusted. 2021/2022. Available from: https://www.bls.gov/web/empsit/cpseea10.htm. Accessed Aug 2022.
Statista. Real average hourly earnings for all employees in the United States from June 2021 to June 2022. 2022. Available from: https://www.statista.com/statistics/216259/monthly-real-average-hourly-earnings-for-all-employees-in-the-us/. Accessed Sept 2022.
Sheinson D, et al. A Cost-Effectiveness Framework for COVID-19 Treatments for Hospitalized Patients in the United States. Adv Ther. 2021;38(4):1811–31.
Janssen B, Szende A. Population norms for the EQ-5D. In: Szende A, Janssen B, Cabases J, editors. Self-reported population health: an international perspective based on EQ-5D. Dordrecht: Springer Netherlands; 2014. p. 19–30.
American Hospital Association. Fast Facts on U.H. Hospitals, 2022. 2022. Available from: https://www.aha.org/statistics/fast-facts-us-hospitals. 18 Jul 2022.
Rafia R, et al. A cost-effectiveness analysis of remdesivir for the treatment of hospitalized patients with COVID-19 in England and wales. Value Health. 2022;25(5):761–9.
Oksuz E, et al. Cost-effectiveness analysis of remdesivir treatment in covid-19 patients requiring low-flow oxygen therapy: payer perspective in Turkey. Adv Ther. 2021;38(9):4935–48.
Ruggeri M, et al. Modello di stima dei costi sanitari e della capacity delle terapie intensive in Italia nel trattamento di pazienti affetti da COVID-19: valutazione dell’impatto di remdesivir. AboutOpen. 2020;2020:1–8.
Jo Y, et al. Cost-effectiveness of remdesivir and dexamethasone for COVID-19 treatment in South Africa. Open Forum Infect Dis. 2021;8(3):ofa040.
Béraud G, Timsit J-F, Leleu H. Remdesivir and dexamethasone as tools to relieve hospital care systems stressed by COVID-19: a modelling study on bed resources and budget impact. PLoS One. 2022;17(1): e0262462.
Pak A, et al. Economic consequences of the COVID-19 outbreak: the need for epidemic preparedness. Front Public Health. 2020;8:241.
Nichols BE, et al. The role of remdesivir in south Africa: preventing COVID-19 deaths through increasing intensive care unit capacity. Clin Infect Dis. 2021;72(9):1642–4.
Andreß S, et al. Deferral of non-emergency cardiac procedures is associated with increased early emergency cardiovascular hospitalizations. Clin Res Cardiol. 2022. https://doi.org/10.1007/s00392-022-02032-z.
Bose SK, et al. The cost of quarantine: projecting the financial impact of canceled elective surgery on the Nation’s Hospitals. Ann Surg. 2021. https://doi.org/10.1097/SLA.0000000000004766.
Yang YT, Mason DJ. COVID-19’s impact on nursing shortages, the rise of travel nurses, and price gouging. 2022. Available from: https://www.healthaffairs.org/do/https://doi.org/10.1377/forefront.20220125.695159/. 19 Jul 2022.
Pollack R. Perspective: climbing costs and rising inflation challenge hospitals’ ability to provide care. 2022. Available from: https://www.aha.org/news/perspective/2022-05-13-perspective-climbing-costs-and-rising-inflation-challenge-hospitals. 19 July 2022.
Boglione L, et al. Risk factors and incidence of long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect? QJM. 2022;114(12):865–71.
Leo CG, et al. Burnout among healthcare workers in the COVID 19 era: a review of the existing literature. Front Public Health. 2021;9: 750529.
Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet. 2022; 399(10339): 1941–1953.
Acknowledgements
Funding
This study was supported by Gilead Sciences; Gilead Sciences funded the rapid service fee. The Maple Health Group received consulting fees from Gilead Sciences for this work.
Author Contributions
Alice Hsiao and James Jarrett conceptualized the work; Rachel Beckerman, Sushanth Jeyakumar, Alice Hsiao, James Jarrett and Robert L. Gottlieb participated in the methodology; Lianne Barnieh, Rachel Beckerman, Sushanth Jeyakumar were involved in the analysis and investigation. Lianne Barnieh was responsible for preparation of the original draft. All authors participated in the review and editing of the final manuscript.
Disclosures
Robert L. Gottlieb was on Scientific Advisory Boards with Eli Lilly, Hoffman-La Roche, Gilead Sciences, and GlaxoSmithKline and was on the Academic Steering Committee with Kinevant Sciences. Robert L. Gottlieb’s institution has received an in-kind gift of medication from Gilead Sciences to facilitate unrelated research. Alice Hsiao and James Jarrett are employees of Gilead Sciences. Lianne Barnieh, Rachel Beckerman, and Sushanth Jeyakumar have no competing interests to declare.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants performed by any of the authors.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Barnieh, L., Beckerman, R., Jeyakumar, S. et al. Remdesivir for Hospitalized COVID-19 Patients in the United States: Optimization of Health Care Resources. Infect Dis Ther 12, 1655–1665 (2023). https://doi.org/10.1007/s40121-023-00816-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00816-y