Abstract
Introduction
The hyperinflammation phase of severe SARS-CoV-2 is characterised by complete blood count alterations. In this context, the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) can be used as prognostic factors. We studied NLR and PLR trends at different timepoints and computed optimal cutoffs to predict four outcomes: use of continuous positive airways pressure (CPAP), intensive care unit (ICU) admission, invasive ventilation and death.
Methods
We retrospectively included all adult patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia admitted from 23 January 2020 to 18 May 2021. Analyses included non-parametric tests to study the ability of NLR and PLR to distinguish the patients’ outcomes at each timepoint. Receiver operating characteristic (ROC) curves were built for NLR and PLR at each timepoint (minus discharge) to identify cutoffs to distinguish severe and non-severe disease. Their statistical significance was assessed with the chi-square test. Collection of data under the SMACORE database was approved with protocol number 20200046877.
Results
We included 2169 patients. NLR and PLR were higher in severe coronavirus disease 2019 (COVID-19). Both ratios were able to distinguish the outcomes at each timepoint. For NLR, the areas under the receiver operating characteristic curve (AUROC) ranged between 0.59 and 0.81, and for PLR between 0.53 and 0.67. From each ROC curve we computed an optimal cutoff value.
Conclusion
NLR and PLR cutoffs are able to distinguish severity grades and mortality at different timepoints during the course of disease, and, as such, they allow a tailored approach. Future prospects include validating our cutoffs in a prospective cohort and comparing their performance against other COVID-19 scores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) are markers of inflammatory status that can be used as prognostic factors in COVID-19. |
We studied NLR and PLR trends at different timepoints and computed optimal cutoffs to predict use of continuous positive airways pressure (CPAP), ICU admission, invasive ventilation and death. |
NLR and PLR were higher in severe COVID-19 and were able to distinguish the outcomes at each timepoint. |
An optimal cutoff value was computed for NLR and PLR to predict severity outcomes. |
Introduction
Early in the SARS-CoV-2 pandemic, Siddiqi et al. proposed a model on the natural history of SARS-CoV-2 infection that comprises a viral and an inflammatory phase and three stages: early infection, which presents with systemic, mild symptoms, a pulmonary stage, when pneumonia and local inflammation develops, and systemic hyperinflammation that involves only the minority of patients who progress to severe illness with multiorgan involvement [1].
Several markers have been proposed to identify the progression towards this last stage, and C-reactive protein (CRP), D-dimer, lactate dehydrogenase (LDH), troponin I, leucocyte alterations and thrombocytopenia have already been confirmed to be of prognostic significance [2, 3]. Leucocyte alterations involve both the lymphocyte and the neutrophil populations. Neutrophils, in fact, possess several inflammatory and pro-thrombotic properties such as the production of neutrophil extracellular traps (NETs) and reactive oxygen species (ROS), which can lead to sustained inflammation during SARS-CoV-2 infection [4,5,6]. Lymphocytes, on the other hand, mainly exhibit the hallmarks of immune exhaustion with CD4 and CD8 T-cell loss, which has been linked to an amplified inflammatory response due to the persistent viral load and the consequent neutrophil stimulation [4, 5].
In this context, the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) could be used as prognostic factors. Thanks to their availability, low cost and reproducibility, they already have been proposed as inflammatory and prognostic markers in a variety of specialties (traumatic brain injury, cardiovascular diseases, hepatic and pancreatic diseases, solid cancer, rheumatic diseases and chronic obstructive pulmonary diseases) [7,8,9,10,11,12].
Several studies have shown a similar trend in COVID-19. Three small Chinese studies performed early in the pandemic demonstrated that a high NLR correlates with severity and risk of progression [13,14,15]. Subsequent studies confirmed this finding, adding that an upward trend correlates with mortality and that peak NLR is associated with mechanical ventilation [16,17,18]. A large meta-analysis including 30 studies and 5570 patients confirmed that NLR accurately determines COVID-19 severity [19].
Similarly, several studies have determined that a baseline low platelet count and a downward trend are associated with severe COVID-19 [20, 21]. Studies that focus specifically on PLR are scant, but demonstrate that a high PLR and a big PLR increase during treatment is associated with a longer hospital stay and more severe disease [22, 23].
Our aims are:
-
1.
To describe NLR and PLR trends at different timepoints during the hospital stay of patients with COVID-19;
-
2.
To compute for each timepoint an optimal cutoff that predicts the risk of four outcomes: use of mechanical non-invasive ventilation (in the form of continuous positive airways pressure—CPAP), ICU admission, invasive ventilation and death. For each cutoff, we will also produce a receiver operating characteristic (ROC) curve and we will compute the area under the receiver operating characteristic curve (AUROC) as well as the sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV);
-
3.
To compare NLR and PLR performance as predictors.
Methods
Design and Setting
This is a cohort retrospective study, conducted at the Fondazione IRCCS Policlinico San Matteo (Pavia, Italy), an academic hospital. All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Eligibility
All adult patients (older than 18 years) with pneumonia and a positive RT-PCR SARS-CoV-2 nasopharyngeal swab who were admitted to the hospital from 21 February 2020 to 18 May 2021 were included in the study. Patients without at least one value of neutrophils, lymphocytes and platelets were excluded. No patients were excluded on the basis of comorbidities that could impact their complete blood count, in an effort to improve generalisation of our results.
Ethics Compliance
All patients provided informed consent for the use of clinical data for scientific purpose according to hospital policy. Administrative data about our hospital’s patients with COVID-19 are collected in a registry (SMACORE), approved by the Fondazione IRCCS Policlinico San Matteo’s ethics committee with protocol number 20200046877, within the framework of the PERISCOPE (Pan-European Response to the ImpactS of COVID-19 and future Pandemics and Epidemics) European project, funded by Horizon 2020 (grant agreement 101016233).
Data and Variables
Data extracted from the SMACORE registry include epidemiological data (age and gender), duration of hospital stay and wards of stay. Comorbidities data were extracted using ICD-9-CM codes. We selected diabetes mellitus (250), chronic obstructive pulmonary disease (from 490 to 496), hypertension (from 401 to 405) and ischaemic heart disease (from 410 to 414).
Outcome data include ICU stay, death, use of intubation/tracheostomy/invasive ventilation and non-invasive mechanical ventilation (CPAP). ICD-9-PCS codes were used to identify patients who underwent intubation (96.04, 96.05), tracheostomy (31.1), invasive ventilation (96.70, 96.71, 96.72) and non-invasive mechanical ventilation (93.90). In the context of this study, intubation, tracheostomy and invasive ventilation are considered under a single outcome called “invasive ventilation”. No missing data were detected in all the extracted variables.
Laboratory data, extracted from the patients’ records, include neutrophils, lymphocytes and platelet count. Laboratory data were extracted at four timepoints: admission (0–24 h), 48 h (24–72 h), 7 days (120–216 h) and discharge (within the last 48 h before discharge). If more than one value of neutrophils, lymphocytes and platelets was available at each timepoint, the mean value was used to compute NLR and PLR.
Data were anonymised before use.
Statistical Analyses
Categorical data are presented as absolute frequencies and proportions. Continuous data are presented by mean and standard deviation if normally distributed or by median and interquartile range if not (Shapiro test is used to assess the normality hypothesis).
To study the ability of NLR and PLR to distinguish the patients’ outcomes at each timepoint, a non-parametric (Mann–Whitney U) test was used (univariate analysis). We performed a subset of univariate analyses to assess the statistical difference in NLR and PLR values according to the comorbidities.
ROC curves were built for NLR and PLR at each timepoint (minus discharge) for males and females in order to identify a cutoff to distinguish severe and non-severe disease. We defined our optimal cutoff as the NLR or PLR value with the highest sensibility and a minimum specificity of 0.5, to have the smallest possible number of false negatives. The statistical significance of the cutoff values was assessed with the chi-square test, applied to 2 × 2 adjacency tables from which classification metrics, such as sensitivity and specificity, were extracted.
We also compared NLR and PLR ROC curves with DeLon’s test to define which is the best predictor of each outcome at the four timepoints.
All statistical tests were two-sided, and a p value of 0.05 was used.
Data pre-processing, descriptive analysis and univariate analyses were handled with Python 3.7.
ROC curves and cutoff analyses were performed on R 4.0.5.
Results
Population
We found 2204 eligible patients. Readmissions were excluded, generating a total of 2169 patients. The mean age at admission was 68 ± 16 years; 1317 were males (60.7%). Figure 1 correlates the number of admitted patients with age and gender. Hypertension was the most common comorbidity, presenting in 33.5% of patients. The clinical characteristics of the patients population are reported in Table 1.
Distribution of length of stay (LOS) is shown in Fig. 2. The mean LOS was 12 ± 13 days; the maximum was 141.
Patients were treated according to the latest evidence available at the time of their admission (e.g. lopinavir/ritonavir and remdesivir early in the pandemic, and remdesivir and steroids later).
Outcomes
The prevalence of each outcome (CPAP, ICU admission, invasive ventilation and death) in the population is presented in Table 2.
Neutrophil, Lymphocyte, Platelet, NLR and PLR Trend
In Fig. 3 we report the mean trend at the four timepoints of neutrophils, lymphocytes and platelets, while Fig. 4 shows the mean trend of NLR and PLR. Females experience lower mean levels of neutrophils and higher mean levels of lymphocytes. Both NLR and PLR are higher in males than in females, but while NLR decreases during hospitalisation in both genders, PLR decreases in males but remains stable in females.
Ratio trends at the four timepoints. NLR (A) and PLR (B) mean trends (with 95% confidence intervals) from the total population are presented in grey with a dashed line; the blue and red lines represent the mean trend for male and female patients, respectively. NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio
The trends at the four timepoints for each outcome are shown in Fig. 5. The orange lines show the trend of patients who had severe COVID-19 (patients who underwent invasive or non-invasive ventilation, patients who were admitted to the ICU and patients who died), while the blue lines show the trend of patients who had non-severe COVID-19.
Ratio trends for each outcome at the four timepoints. NLR (A) and PLR (B) mean trends (with 95% confidence intervals) are in orange for the patients with the outcome and in blue for the patients without the outcome. P values are reported for each outcome and express the significance of the difference in the NLR or PLR values for each timepoint (Mann–Whitney test). NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio
Both NLR and PLR are always higher in severe COVID-19 at all the timepoints, with a gap that gets bigger with more severe disease. From admission, both NLR and PLR show a progressive increase reaching a peak at 7 days. This trend is steeper for more severe outcomes. There are two exceptions to this: NLR and PLR trends for the CPAP outcome, which increase from admission to 48 h, but decrease slightly after the 48 h timepoint and plummet at discharge. This could be explained by the fact that CPAP therapy is usually implemented in the first hours after admission but rarely as late as the seventh day of hospitalisation. The second exception is the NLR trend for the death outcome: the orange line continually increases, diverging from the blue line that shows the normalisation of NLR of patients who survived.
Univariate Analyses
In Table 3 we report the number of patients who had at least one available measure of NLR and PLR at each timepoint and the results of the first univariate analyses. NLR and PLR values for the first three timepoints were able to distinguish between the outcomes, with the exception of PLR at admission that cannot predict ICU admission. NLR and PLR at discharge distinguish between patients who died and patients who survived.
In addition, Table 4 presents the results of the univariate analysis conducted to test the difference in NLR and PLR values between patients with and without the considered comorbidity.
Cutoff Analyses (ROC Curves)
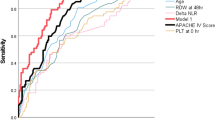
The results of ROC curve analyses for NLR and PLR values for males and females are presented in Tables 5 and 6. From each ROC curve we computed an optimal cutoff value, which is presented with sensitivity, specificity, accuracy, and positive and negative predictive values (PPV and NPV respectively). For example, the NLR cutoff for CPAP use at admission is 7.00 for males and 6.36 for females, and 239.22 for males and 233.00 for females for PLR. The cutoffs have been plotted in Figs. 6 and 7 in order of timepoints and outcome severity.
Figure 8 shows the composite ROC curves with area under the receiver operating curve (AUROC) values (a higher number indicates a better predictive performance). NLR performed better than PLR, and males performed better than females. For NLR, the lowest AUROC is 0.59 for CPAP and ICU admission at the admission timepoint for males, while the highest is 0.81 for both genders for death at 7 days. For PLR, the lowest AUROC is 0.53 for death at admission for males, while the highest is 0.67 for CPAP at 48 h for females and for invasive ventilation at 7 days for males.
NLR and PLR Comparison
The comparison of NLR and PLR ROC curves (Fig. 9) showed that NLR generally performs better, with comparable curves only for the CPAP outcome.
Discussion
We showed that NLR and PLR are able to distinguish between patients with mild/moderate COVID-19 and patients with severe disease (with need of invasive/non-invasive ventilation or ICU admission and patients who died). In fact, both ratio show higher values in the population with severe disease. An NLR that is continually increasing at successive timepoints, in particular, is able to predict death.
We also computed cutoffs for males and females for the four outcomes at each timepoint. Males’ NLR cutoffs are higher than females’ of at least 1 point. Cutoffs’ sensitivity is also higher in males. At each timepoint an “easy to remember” value (e.g. 6.3 and 7.0 at admission for females and males, respectively, or 5.3 and 7.3 at 48 h for males and females, respectively) could be quite good at predicting all the outcomes, with a sensitivity that increases progressively at successive timepoints (the minimum is 0.61 for females at admission for ICU admission; the maximum 0.91 for males at 7 days for invasive ventilation). The positive predictive values are low, but the negative predictive values range from 0.73 to 0.98, making our cutoffs efficacious in excluding patients at risk if their NLR is lower. The same can be said for PLR cutoffs, even though, differently from NLR, they start out similar for males and females. Moreover, at a single timepoint, PLR cutoffs can be quite different depending on the outcome (e.g. 250 versus 293 for males at 48 h for CPAP and death, respectively), possibly making it easier to distinguish different grades of severity.
Finally, we showed that NLR is generally better than PLR at predicting severity.
A Chinese study aimed at finding NLR and PLR reference values for healthy subjects showed that both are sex and age dependent [24]; successive studies confirmed that this distinction is maintained in disease [25], in line with our findings.
Four Chinese studies plotted NLR and PLR from admission to post-discharge and obtained trends similar to ours, with stable and lower values for patients with non-severe disease and higher and increasing values for patients with severe disease [16, 22, 26, 27].
Regarding the predictive value of the ratios and their cutoffs, several studies plotted ROC curves for NLR and PLR, finding that they perform well in predicting severity and death, with NLR performing better than PLR in those studies that compared them [14, 16, 17, 23, 27,28,29]. A Turkish study on 306 patients found that at admission NLR (but not PLR) significantly predicted COVID-19 pneumonia, and they computed a cutoff of 1.73 [28]. A similar study on 69 patients found a cutoff for severe disease of 3.3 for NLR and 180 for PLR, and another on 61 patients used 3.13 for NLR [13, 23]. All of these studies, however, did not aim at distinguishing patients with severe disease from those with non-severe disease, but patients with or without pneumonia, which could explain the difference between their cutoffs and ours, which are much higher. In fact, a study which tried to distinguish different grades of severity (common cases, severe-non-ICU patients and severe-ICU patients) found values of NLR and PLR that increase with severity, although they did not compute any cutoffs [27]. A cutoff was actually computed by Ye et al., who found a NLR cutoff of 7.13 for death and 7.28 for invasive ventilation, both values that are very similar to our own [16]. An Indian study limited to patients with severe disease found a cutoff of 5.1 for mortality, but it did not specify at which point during hospitalisation the NLR was extracted, and two Iranian ones have the same limitation and, moreover, are focused only on patients with specific characteristics [17, 30, 31]. Finally, Yldiz et al. computed a 5.94 NLR cutoff for mortality on a derivation cohort and subsequently validated it in a successive cohort [29].
All these studies have the limitation of being retrospective and limited to a single centre with no more than a few hundred patients. Our study presents the same limitations, but dealt with more than 2000 patients and avoided stringent exclusion criteria, thus limiting the confounding factors and outliers and expanding the generalisation of our results as much as possible. We also divided our results for males and females, which we consider a strength as it takes into consideration the physiologic differences of NLR and PLR and adds a further level of stratification. Moreover, our cutoffs not only deal with severity and mortality but allow for a more tailored approach, dividing patients with severe disease in those who can still be treated in an ordinary ward, possibly with the implementation of CPAP, and those who need early referral to the ICU.
Another limitation is selection bias, since we selected our patients and stratified them in severity outcomes based on ICD9 codes, which can be inaccurate.
Finally, another confounder could be the use of anti-inflammatory or myelotoxic drugs, but this effect should be corrected by the number and the periodicity of observations. In fact, the greatest usefulness of the cutoffs lies in the admission and 48 h timepoints, where the steroid-induced neutrophilia is not yet present.
Conclusion
Our study shows that NLR and PLR can distinguish mortality, need for ventilation (non-invasive and invasive), and ICU admission, and their trend correlates with disease severity. We also computed male and female cutoffs (with good sensitivity and negative predictive values) for the various stages of severity at different timepoints during hospitalisation. Our score is extremely handy and easy to compute, while many other proposed tools are difficult to measure or vary at different timepoints, which might explain their conflicting results. In fact, future prospects include validating our cutoffs in a prospective, possibly multi-centric cohort and comparing their performance against scores such as the Quick COVID-19 Severity Index (qCSI), and the 4C Mortality Score.
References
Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transpl. 2020;39(5):405–7. https://doi.org/10.1016/j.healun.2020.03.012.
Gallo Marin B, Aghagoli G, Lavine K, Yang L, Siff EJ, Chiang SS, Salazar-Mather TP, Dumenco L, Savaria MC, Aung SN, Flanigan T, Michelow IC. Predictors of COVID-19 severity: a literature review. Rev Med Virol. 2021;31(1):1–10. https://doi.org/10.1002/rmv.2146.
Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57(6):389–99. https://doi.org/10.1080/10408363.2020.1770685.
Melenotte C, Silvin A, Goubet AG, Lahmar I, Dubuisson A, Zumla A, Raoult D, Merad M, Gachot B, Hénon C, Solary E, Fontenay M, André F, Maeurer M, Ippolito G, Piacentini M, Wang FS, Ginhoux F, Marabelle A, Kroemer G, Derosa L, Zitvogel L. Immune responses during COVID-19 infection. Oncoimmunology. 2020;9(1):1807836. https://doi.org/10.1080/2162402X.2020.1807836.
Arcanjo A, Logullo J, Menezes CCB, de Souza Carvalho Giangiarulo TC, Dos Reis MC, de Castro GMM, da Silva Fontes Y, Todeschini AR, Freire-de-Lima L, Decoté-Ricardo D, Ferreira-Pereira A, Freire-de-Lima CG, Barroso SPC, Takiya C, Conceição-Silva F, Savino W, Morrot A. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19). Sci Rep. 2020;10(1):19630. https://doi.org/10.1038/s41598-020-76781-0.
Cicco S, Cicco G, Racanelli V, Vacca A. Neutrophil extracellular traps (NETs) and damage-associated molecular patterns (DAMPs): two potential targets for COVID-19 treatment. Mediators Inflamm. 2020;16(2020):7527953. https://doi.org/10.1155/2020/7527953.
Sabouri E, Majdi A, Jangjui P, Rahigh Aghsan S, Naseri Alavi SA. Neutrophil-to-lymphocyte ratio and traumatic brain injury: a review study. World Neurosurg. 2020;140:142–7. https://doi.org/10.1016/j.wneu.2020.04.185.
Delcea C, Buzea CA, Dan GA. The neutrophil to lymphocyte ratio in heart failure: a comprehensive review. Rom J Intern Med. 2019;57(4):296–314. https://doi.org/10.2478/rjim-2019-0018.
Bhat TM, Afari ME, Garcia LA. Neutrophil lymphocyte ratio in peripheral vascular disease: a review. Expert Rev Cardiovasc Ther. 2016;14(7):871–5. https://doi.org/10.1586/14779072.2016.1165091.
Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. 2019;39(4):345–57. https://doi.org/10.3343/alm.2019.39.4.345.
El-Gazzar AG, Kamel MH, Elbahnasy OKM, El-Naggar ME. Prognostic value of platelet and neutrophil to lymphocyte ratio in COPD patients. Expert Rev Respir Med. 2020;14(1):111–6. https://doi.org/10.1080/17476348.2019.1675517.
Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, Seruga B, Ocaña A, Tannock IF, Amir E. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1204–12. https://doi.org/10.1158/1055-9965.EPI-14-0146.
Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, Zhang M, Tan J, Xu Y, Song R, Song M, Wang L, Zhang W, Han B, Yang L, Wang X, Zhou G, Zhang T, Li B, Wang Y, Chen Z, Wang X. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18(1):206. https://doi.org/10.1186/s12967-020-02374-0.
Fu J, Kong J, Wang W, Wu M, Yao L, Wang Z, Jin J, Wu D, Yu X. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: a retrospective study in Suzhou China. Thromb Res. 2020;192:3–8. https://doi.org/10.1016/j.thromres.2020.05.006x.
Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, Luo M, Chen L, Zhao Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81(1):e6–12. https://doi.org/10.1016/j.jinf.2020.04.002.
Ye W, Chen G, Li X, Lan X, Ji C, Hou M, Zhang D, Zeng G, Wang Y, Xu C, Lu W, Cui R, Cai Y, Huang H, Yang L. Dynamic changes of D-dimer and neutrophil–lymphocyte count ratio as prognostic biomarkers in COVID-19. Respir Res. 2020;21(1):169. https://doi.org/10.1186/s12931-020-01428-7.
Ramesh J, Reddy SLS, Rajesh M, Varghese J. Evaluation of simple and cost-effective immuno-haematological markers to predict outcome in hospitalized severe COVID-19 patients, with a focus on diabetes mellitus – a retrospective study in Andhra Pradesh India. Diabetes Metab Syndr. 2021;15(3):739–45. https://doi.org/10.1016/j.dsx.2021.03.025.
Aly MM, Meshref TS, Abdelhameid MA, Ahmed SA, Shaltout AS, Abdel-Moniem AE, Hamad DA. Can hematological ratios predict outcome of COVID-19 patients? A multicentric study. J Blood Med. 2021;29(12):505–15. https://doi.org/10.2147/JBM.S316681.
Wang Y, Zhao J, Yang L, Hu J, Yao Y. Value of the neutrophil–lymphocyte ratio in predicting COVID-19 severity: a meta-analysis. Dis Markers. 2021. https://doi.org/10.1155/2021/2571912.
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022.
Liu Y, Sun W, Guo Y, Chen L, Zhang L, Zhao S, Long D, Yu L. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020;31(4):490–6. https://doi.org/10.1080/09537104.2020.1754383.
Qu R, Ling Y, Zhang YH, et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020;92(9):1533–41. https://doi.org/10.1002/jmv.25767.
Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. https://doi.org/10.1016/j.intimp.2020.106504.
Wang J, Zhang F, Jiang F, Hu L, Chen J, Wang Y. Distribution and reference interval establishment of neutral-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) in Chinese healthy adults. J Clin Lab Anal. 2021;35:e23935. https://doi.org/10.1002/jcla.23935.
Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. 2019;9:19673. https://doi.org/10.1038/s41598-019-56218-z.
Ding X, Yu Y, Lu B, Huo J, Chen M, Kang Y, Lou J, Liu Z. Dynamic profile and clinical implications of hematological parameters in hospitalized patients with coronavirus disease 2019. Clin Chem Lab Med. 2020;58(8):1365–71. https://doi.org/10.1515/cclm-2020-0411.
Sun S, Cai X, Wang H, et al. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou. China Clin Chim Acta. 2020;507:174–80. https://doi.org/10.1016/j.cca.2020.04.024.
Damar Çakırca T, Torun A, Çakırca G, Portakal RD. Role of NLR, PLR, ELR and CLR in differentiating COVID-19 patients with and without pneumonia. Int J Clin Pract. 2021. https://doi.org/10.1111/ijcp.14781.
Yildiz H, Castanares-Zapatero D, Pierman G, Pothen L, De Greef J, Aboubakar Nana F, Rodriguez-Villalobos H, Belkhir L, Yombi JC. Validation of neutrophil-to-lymphocyte ratio cut-off value associated with high in-hospital mortality in COVID-19 patients. Int J Gen Med. 2021;14:5111–7. https://doi.org/10.2147/IJGM.S326666.
Hosseninia S, Ghobadi H, Garjani K, Hosseini SAH, Aslani MR. Aggregate index of systemic inflammation (AISI) in admission as a reliable predictor of mortality in COPD patients with COVID-19. BMC Pulm Med. 2023;23(1):107. https://doi.org/10.1186/s12890-023-02397-5.
Ghobadi H, Mohammadshahi J, Javaheri N, Fouladi N, Mirzazadeh Y, Aslani MR. Role of leukocytes and systemic inflammation indexes (NLR, PLR, MLP, dNLR, NLPR, AISI, SIR-I, and SII) on admission predicts in-hospital mortality in non-elderly and elderly COVID-19 patients. Front Med (Lausanne). 2022;9:916453. https://doi.org/10.3389/fmed.2022.916453.
Acknowledgements
We thank the participants of the study.
Funding
This research was granted by the Pan-European Response to the ImpactS of COVID-19 and future Pandemics and Epidemics (PERISCOPE) H2020-SC1-PHE-CORONAVIRUS-2020-2 PERISCOPE Project (grant agreement 101016233). The rapid service publication fee will be paid by the Fondazione IRCCS Policlinico San Matteo’s Scientific Direction.
Author Contributions
Conceptualisation: Valentina Zuccaro, Federica Melazzini, Angioletta Lasagna, Andrea Peri and Paolo Sacchi; Methodology, Valentina Zuccaro, Paolo Sacchi, Giuseppe Albi and Erika Asperges; Formal Analysis, Giuseppe Albi; investigation: Margherita Sambo, Teresa C. Pieri, Matteo Calia, Marta Colaneri and Laura Maiocchi; Resources, Raffaele Bruno; data curation: Giuseppe Albi; writing – original draft preparation: Giuseppe Albi and Erika Asperges; writing – review and editing: Valentina Zuccaro, Paolo Sacchi, Federica Melazzini, Francesco Mojoli and Raffaele Bruno; visualisation: Giuseppe Albi; Supervision, Paolo Sacchi, Francesco Mojoli and Raffaele Bruno. All authors read and approved the final manuscript.
Prior Presentation
An abstract based on this study was presented at ECCMID 2022 in Lisbon (23–26 April 2022).
Disclosures
The authors Erika Asperges, Giuseppe Albi, Valentina Zuccaro, Margherita Sambo, Teresa C Pieri, Matteo Calia, Marta Colaneri, Laura Maiocchi, Federica Melazzini, Angioletta Lasagna, Andrea Peri, Francesco Mojoli, Paolo Sacchi and Raffaele Bruno have nothing to disclose.
Compliance with Ethics Guidelines
All patients provided informed consent for the use of clinical data for scientific purpose according to hospital policy. Collection of data under the SMACORE database was approved by the Fondazione IRCCS Policlinico San Matteo’s ethics committee (protocol number 20200046877). The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Data Availability
The published article includes all datasets generated or analysed during the study. Data sharing is available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Asperges, E., Albi, G., Zuccaro, V. et al. Dynamic NLR and PLR in Predicting COVID-19 Severity: A Retrospective Cohort Study. Infect Dis Ther 12, 1625–1640 (2023). https://doi.org/10.1007/s40121-023-00813-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00813-1