Abstract
Introduction
This randomized, double-blind, placebo-controlled, phase 2a trial was conducted to evaluate the safety and immunogenicity of the ID93 + glucopyranosyl lipid adjuvant (GLA)-stable emulsion (SE) vaccine in human immunodeficiency virus (HIV)-negative, previously Bacillus Calmette–Guérin (BCG)-vaccinated, and QuantiFERON-TB-negative healthy adults in South Korea.
Methods
Adults (n = 107) with no signs or symptoms of tuberculosis were randomly assigned to receive three intramuscular injections of 2 μg ID93 + 5 μg GLA-SE, 10 μg ID93 + 5 μg GLA-SE, or 0.9% normal saline placebo on days 0, 28, and 56. For safety assessment, data on solicited adverse events (AEs), unsolicited AEs, serious AEs (SAEs), and special interest AEs were collected. Antigen-specific antibody responses were measured using serum enzyme-linked immunosorbent assay. T-cell immune responses were measured using enzyme-linked immunospot and intracellular cytokine staining.
Results
No SAEs, deaths, or AEs leading to treatment discontinuation were found. The solicited local and systemic AEs observed were consistent with those previously reported. Compared with adults administered with the placebo, those administered with three intramuscular vaccine injections exhibited significantly higher antigen-specific antibody levels and Type 1 T-helper cellular immune responses.
Conclusion
The ID93 + GLA-SE vaccine induced antigen-specific cellular and humoral immune responses, with an acceptable safety profile in previously healthy, BCG-vaccinated, Mycobacterium tuberculosis-uninfected adult healthcare workers.
Trial Registration
This clinical trial was retrospectively registered on 16 January 2019 at Clinicaltrials.gov (NCT03806686).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Tuberculosis (TB) is a major infectious disease inflicting more than 10 million patients and 1.5 million deaths worldwide each year. Given the inconsistent effectiveness of Bacillus Calmette–Guérin (BCG) vaccination against adult pulmonary TB, it is imperative to develop new safe and effective TB vaccine candidates capable of inducing robust and long-term protection against all forms of TB in diverse populations and geographic regions. |
Although the ID93 + GLA-SE TB vaccine candidate was reported safe and immunogenic in people in the USA and South Africa, no report is available yet in Asian countries. |
This study aimed to evaluate the safety and immunogenicity of the ID93 + GLA-SE TB vaccine candidate among the BCG-vaccinated healthy adults in South Korea. |
What was learned from the study? |
The study showed the ID93 + GLA-SE vaccine candidate was safe among the BCG-vaccinated healthy adults of South Korea and induced the antigen-specific antibody and type 1 T-helper cellular immune responses. |
This is the first report on the safety and immunogenicity of ID93 + GLA-SE in an Asian population, positively supporting the future efficacy trials of ID93 + GLA-SE (QTP101) in human immunodeficiency virus (HIV)-negative, previously BCG-vaccinated, healthy adults. |
INTRODUCTION
Both severe acute respiratory syndrome coronavirus 2, the causative agent of coronavirus disease 2019, and Mycobacterium tuberculosis (Mtb), which causes chronic tuberculosis (TB), are the leading infectious agents worldwide, ranking above human immunodeficiency virus (HIV) infection [1]. A recent meta-analysis of interferon-γ (IFN-γ) release assays and tuberculin skin test surveys showed that one-fourth of the world’s population is infected with Mtb [2]. Individuals who come in contact with patients with active TB are at risk of developing latent TB infection (LTBI), whereas 5–10% of those with LTBI develop active TB during their lifetime [1]. Furthermore, nearly 50% of individuals with active TB progression from a recent infection occurs within the first 2 years [2].
In South Korea, TB is a major public health concern, despite high infant Bacillus Calmette–Guérin (BCG) immunization coverage and improvement in controlling TB under the National Tuberculosis Program (NTP). A recent annual report from the surveillance system indicated a TB incidence rate of 46.4 per 100,000 people (approximately 23,821 new cases), with 1610 cases of TB-related deaths [3]. To achieve their goal of reducing TB, the Korean NTP has strengthened a latent TB management program since 2016 with various health policies, such as “mandatory LTBI diagnosis” and “full-subsidy for LTBI treatment costs” for specific groups of individuals, including those eligible for conscription, kindergarten teachers, inmates, and healthcare workers. However, as individuals with LTBI do not feel sick and appear healthy, they often hesitate to start or complete preventive treatment, which requires steady medication for 3–6 months with possible side-effects. To complement such limitations of current NTP policies to control TB infection, initiatives through innovative strategies in diagnosis, treatments, and vaccine development remain essential. The development of effective vaccines against infectious TB is the most successful approach to protect against TB infection and transmission globally. However, the only commercially available vaccine against TB is the live attenuated BCG vaccine, which has some disadvantages, such as variable and partial effectiveness for pulmonary TB in adolescents and adults (0–80%), and is influenced by population, region, and exposure to environmental factors [4,5,6,7]. The benefits and pitfalls of the BCG vaccine remain debatable, while international efforts continue to develop a novel vaccine effective against all forms of TB and in all age groups, regardless of environmental factors [8]. In countries where BCG prevents childhood TB for up to a minimum of 10 years, the most efficient strategy to control TB might be the development of booster vaccines to repair vaccine-induced immune failure of BCG instead of developing a better neonatal TB vaccine to replace BCG [9]. Encouraging outcomes from recent clinical trials also support a strategy to combine BCG vaccination with subunit vaccine candidates, designed almost exclusively as BCG boosters to enhance vaccine activity against TB [8]. These subunit vaccine candidates are composed of recombinant protein components with adjuvants, generally requiring multiple doses to induce an effective immune response.
ID93 is composed of four Mtb antigens (Rv2608, Rv3619, Rv3620, and Rv1813) associated with virulence or latency; glucopyranosyl lipid adjuvant (GLA)-stable emulsion (SE) is a synthetic Toll-like receptor 4 agonist developed as an adjuvant formulated with an oil-in-water emulsion [10, 11]. This adjuvant system has been successfully combined with recombinant protein antigens to yield high antibody titers [12, 13] and induce T-helper type 1 (Th1) cellular immune responses associated with Mtb and Leishmania infection models [12,13,14]. The safety, immunogenicity, and pharmacodynamics of the ID93 + GLA-SE vaccine have been previously demonstrated in various animal models [15, 16]. Previous clinical studies have also demonstrated that the ID93 + GLA-SE vaccine is safe and immunogenic in different populations, including HIV-negative healthy adults, adults with LTBI, and treated patients with TB in the USA and South Africa [17,18,19]. In addition to its use with the ID93 vaccine antigen, GLA-SE was used in clinical trials for vaccines against schistosomiasis, malaria, leishmaniasis, and influenza [20,21,22,23,24]. Injections of vaccines containing GLA-SE have generally been well tolerated, and adverse events (AEs) have been mostly mild, with no treatment-related serious AEs (SAEs) reported [17,18,19]. Along with the previous studies that evaluated adults with LTBI or treated patients with TB, this trial focuses on healthy adults not previously infected with Mtb. We evaluated the safety and immunogenicity of two dosages of the ID93 + GLA-SE vaccine compared with those of placebo after three intramuscular (IM) injections in HIV-negative, previously BCG-vaccinated QuantiFERON-TB (QFT)-negative, healthy healthcare workers who are considered at higher risk of TB exposure than the general population in South Korea [25,26,27].
METHODS
Study Design and Participants
In this randomized, double-blind, placebo-controlled, parallel phase 2a trial, we enrolled HIV-negative, previously BCG-vaccinated, QFT-negative healthy adults (age 19–65 years), with no evidence of historical or current TB, among healthcare workers currently employed in three hospitals located in Seoul and Suwon: Yonsei University Severance Hospital, Ajou University Hospital, and Chung-Ang University Hospital. The study procedures, including inclusion and exclusion criteria, are described in detail in Supplementary Material Appendix 1.1 and 1.2. This study was approved by the Institutional Review Board and Ethical Committee of Yonsei University Severance Hospital (IRB# 4-2018-0230), Ajou University Hospital (IRB# AJIRB-MED-CT2-18-078), and Chung-Ang University Hospital (1833-001-320). Written informed consent was obtained from all study participants. This study was conducted in accordance with the Declaration of Helsinki.
Randomization and Masking
Each participant was sequentially assigned a unique randomization number generated by a randomization manager using the SAS program (SAS v9.4 or higher). Randomization of participants was performed in a 1:1:1 ratio to the three treatment cohorts. Participants who were QFT-negative and BCG-vaccinated were sorted into cohort 1, 2, or 3 and received injections on days 0, 28, and 56. The investigational product (IP: ID93 + GLA-SE) was administered by an unblinded pharmacist, according to the participant’s randomization number. The syringes used for injection were blinded. Unblinded study personnel were not involved in any other duties that could have broken the double-blind setting. Double blinding of the study was maintained until database lock and data analysis were completed at the end of the study. The treatment assignments were disclosed to the participants and the investigator at the end of the study.
Procedures
The IP was purchased from the Infectious Disease Research Institute/Access to Advanced Health Institute (IDRI/AAHI; Seattle, WA, USA) and supplied to pharmacists at the study sites. Study participants were randomized to receive a 0.5 ml IM injection of 2 μg ID93 + 5 μg GLA-SE (cohort 1), 10 μg ID93 + 5 μg GLA-SE (cohort 2), or 0.9% normal saline placebo (cohort 3) on days 0, 28, and 56. All participants were then followed up for 12 months after the final vaccination. Acute AEs were assessed 30 min after each vaccination. Solicited AEs (local and systemic) were assessed 7 days after each vaccination, and unsolicited AEs were assessed 28 days after each vaccination on the basis of their severity, causality, and seriousness. Data on AEs were collected from participant voluntary reporting, monitoring, and interviews at site visits, phone call follow-ups, and participant diaries. For long-term safety assessment, SAEs and adverse events of special interest (AESIs) were monitored for up to 12 months after the final vaccination. Further definitions of AEs are provided in Supplementary Material Appendix 1.3.
Other safety assessments included clinical laboratory tests (clinical chemistry and hematology), collection of vital signs (blood pressure, pulse, and body temperature), and physical examination, including height and weight measurements, chest radiography, pregnancy testing, and virus screening tests that included HIV, hepatitis B, hepatitis C, and sputum culture tests (only if clinically indicated).
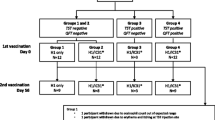
Immunogenicity assessment was performed using blood samples collected from the study participants on days 0 (baseline), 28, 56, 84, and 421. Immunogenicity analysis was conducted at central laboratories [Seegene Medical Foundation, Seoul, South Korea; IDRI/AAHI, Seattle, WA, USA; and the Center for Global Infectious Disease Research (CGIDR) of Seattle Children’s Research Institute, Seattle, WA, USA]. To measure humoral immune responses, serum IgG enzyme-linked immunosorbent assay (ELISA) was performed with recombinant ID93 antigen as described in Supplementary Material Appendix 1.4.1. To measure cell-mediated immune responses induced by vaccination, peripheral blood mononuclear cell (PBMC)-based enzyme-linked immunospot (ELISpot) and intracellular cytokine staining were performed according to the method described in Supplementary Material Appendix 1.4.2 and 1.4.3. The overall study schedule for follow-up and blood collection is shown in Fig. 1a.
Study design and trial profile. a Study design and scheduled follow-ups and blood collections. Safety assessment of ID93 + GLA-SE recipients (cohorts 1 and 2) compared with placebo recipients (cohort 3) was conducted following three administrations at days 0, 28, and 56, and follow-ups for 12 months (421 days) from the final vaccination. Blood samples for immunogenicity assessment of ID93 + GLA-SE recipients compared with placebo recipients were collected on days 0 (baseline), 28, 56, 84, and 421. b Eligible participants were randomly assigned in a 1:1:1 ratio to one of three treatment groups to receive either 2 μg ID93 + 5 μg GLA-SE (cohort 1), 10 μg ID93 + 5 μg GLA-SE (cohort 2), or 0.9% normal saline placebo (cohort 3) on days 0, 28, and 56
Outcomes
The safety of ID93 + GLA-SE as primary outcomes included solicited AEs up to 7 days and unsolicited AEs up to 28 days after each vaccination, which were based on their severity, causality, and seriousness. SAEs and AESIs were followed for up to 12 months. AEs were classified according to severity (i.e., mild, moderate, or severe) and causality (i.e., related or unrelated). The immunogenicity of ID93 + GLA-SE as secondary outcomes were identified by the antigen-specific total IgG antibody titer, IFN-γ secreting cells, and CD4+ T cells expressing any Th1 cytokine(s). A safety analysis was performed using a safety set, while immunogenicity analysis was performed using a per-protocol set. This trial was retrospectively registered at ClinicalTrials.gov (NCT03806686).
Statistical Analysis
On the basis of previous phase 1 and phase 2 studies [17,18,19], the sample size was determined to be 35 participants per cohort, with an estimated dropout rate of 15%. The number of events along with the frequency, incidence rate, and 95% confidence intervals (CIs) were calculated for local and systemic solicited AEs observed for 7 days following each vaccination, unsolicited AEs observed 28 days following each vaccination, and SAEs/AESIs observed up to day 421. The AEs were classified according to their severity and causal relationship to the vaccine, and the frequency of events, number of people, and incidence rate were presented. For severity analysis, the severity of local and systemic solicited AEs was graded according to the upper limit of their severity. All AEs other than local and systemic solicited AEs were classified following the Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class (SOC) and Preferred Terms (PT).
Differences in immune responses between study groups were compared using the Mann–Whitney test (two groups), the Kruskal–Wallis test (three or more groups), and Fisher’s exact test (two nominal variables). In addition, differences in paired immune responses that occurred between baseline and post-vaccination visits within the study group were compared using Friedman’s test (three or more groups) or the Wilcoxon matched-pair signed-rank test. A p value < 0.05 between the compared study groups was considered statistically different. Statistical analyses and graphical representation were performed with SAS version 9.4 and GraphPad Prism version 9.4.1.
RESULTS
Study Participants
As shown in Fig. 1, 107 of the 123 participants screened were eligible. Of these, 16 participants were excluded on the basis of screening criteria: 4 refused before randomization, 9 had QFT-positive results, 1 had an influenza vaccination before study participation, 1 visited outside the window period for randomization, and 1 had a fever > 38 °C. All eligible participants were randomly allocated to three study cohorts: cohort 1 received 2 μg ID93 + 5 μg GLA-SE (n = 35), cohort 2 received 10 μg ID93 + 5 μg GLA-SE (n = 36), and cohort 3 received 0.9% normal saline placebo (n = 36) (Fig. 1b). The median age of study participants was 34 years (23–58 years). The proportion of female participants (73.8%) was higher than that of male participants (26.2%), as there are more female healthcare workers than male. Most participants (63.9–72.2%) worked in a department of the hospital with medium risk of TB exposure, followed by departments of high risk (19.4–22.9%) and low risk of TB exposure (5.7–13.9%) (Table 1). Overall, demographics and baseline characteristics were comparable across the study cohorts.
All eligible participants who received at least one study injection were included in the safety analysis. Of these participants, 28 (80.0%), 32 (88.9%), and 31 (86.1%) participants in cohorts 1, 2, and 3, respectively, completed the study as per the protocol. Sixteen (14.9%) participants discontinued during the study, mostly because they did not consent to continue study follow-up (withdrawal of consent). No participants were withdrawn due to any treatment-related SAEs. In cohort 1, seven participants were excluded owing to the withdrawal of consent, major protocol deviation, and QFT-positive conversion at day 421. In cohort 2, four participants discontinued the study owing to noncompliance with the protocol, withdrawal of consent, loss to follow-up, and QFT-positive conversion at day 84. Five participants in cohort 3 were withdrawn from the study owing to noncompliance with the protocol, withdrawal of consent, loss to follow-up, and QFT-positive conversion on day 421 (Fig. 1b).
Safety Evaluation
Safety analysis indicated that ID93 + GLA-SE was safe and well tolerated at the two dosages assessed. The most frequently reported solicited local and systemic AEs were pain, tenderness at the injection site, headache, fatigue, and myalgia in all three cohorts (Table 2). In the vaccine groups (cohorts 1 and 2), 83.7% participants reported local AEs and 56.9% participants reported systemic AEs after all three injections. In the placebo group, 23.3% participants reported local AEs and 19.4% participants reported systemic AEs during the study. Most solicited local and systemic AEs were mild in severity in all treatment groups. The overall frequencies of participants (%) with solicited local and systemic AEs after each dose of IP, according to symptoms and severity, are presented in Supplementary Material Tables S1 and S2. The most frequently reported (≥ 3.0% of total participants) AEs sorted by PT were nasopharyngitis (6.5%), dyspepsia (4.7%), and headache (3.7%). Of these, the most frequently reported vaccine-related AE was dyspepsia (3.7%) (Table 3); however, most of the unsolicited AEs were mild in severity in the vaccine groups. In total, 24.3% of the participants reported at least one AE that was related to the vaccine (Supplemental Table S3). The most frequently reported (≥ 3.0% of total participants) unsolicited AEs sorted via SOC and PT are presented with their severity and causality in Supplementary Material Table S3.
During this study, eight participants (7.5%) reported SAEs unrelated to IP, as determined by study clinicians. One (2.9%) participant in cohort 1 reported an ovarian cyst, four (11.1%) participants in cohort 2 reported SAEs: tooth impaction, ankle fracture, colon cancer, and facial paralysis, and three (8.3%) participants in the placebo group reported SAEs: facial bone fracture, spinal column stenosis, and spontaneous abortion. One AESI unrelated to IP was reported in cohort 2 (2.8%), which was also assessed as an SAE (facial paralysis). In summary, 51 (47.7%) participants reported 91 AEs during the study. Cohort 2 (63.9%) had the highest proportion of participants who reported any AEs compared with cohort 1 (40.0%) and cohort 3 (placebo) (38.9%). Approximately half of the reported AEs were related to the vaccine [43 AEs reported in 26 participants (24.3%)], but the most frequently occurring AEs were local injection reactions. One participant in each group reported an AE of grade 3 (severe); however, none of these SAEs were related to the vaccine (Table 4). Moreover, none of the SAEs or AESIs were related to the vaccine and no deaths or AEs associated with vaccine discontinuation were reported.
Immunogenicity Evaluation
Humoral Immune Response: Antigen-Specific IgG Antibody Titer
To determine the vaccine-mediated humoral immune response, serum total IgG antibody levels specific to the ID93 fusion protein as detected using ELISA with geometric mean titer (GMT), geometric mean fold rise (GMFR), and seroresponse rates (SRR) at post-vaccination timepoints (days 28, 56, 84, and 421) were compared between the three study cohorts. As shown in Table 5, the GMT in cohorts 1 and 2 increased consistently from day 28 and peaked at day 84 (4 weeks after the third IP dose), before decreasing at day 421. At day 421 (12 months after the third IP dose), the GMT in both cohorts decreased to levels close to those at day 28, but remained higher than the baseline. Across the three cohorts, baseline antigen-specific IgG responses were low and equivalent. The increase in GMT over time was reflected in the GMFR, which increased from 5.59 at day 28 to 143.07 at day 84 in cohort 1 and from 8.06 at day 28 to 183.15 at day 84 in cohort 2. The GMFR over time in cohort 2 was slightly higher than that in cohort 1; however, this difference was not significant. From day 84 to day 421, the GMFR decreased just as GMT decreased in cohorts 1 and 2. The GMFR over time in cohort 3 (placebo group) remained unchanged or was lower than 1.00. After the first IP dose (day 28), the SRRs of cohorts 1 and 2 were 57.14% (95% CI 37.18–75.54) and 68.75% (95% CI 49.99–83.88), respectively, with a higher but statistically insignificant SRR in cohort 2. After the second and third IP doses (days 56 and 84), the SRR increased to 100% equally in both vaccine groups. However, after 12 months from the third IP dose, the SRR decreased to 42.86% (95% CI 2.46–62.82) in cohort 1 and 59.38% (95% CI 40.64–76.30) in cohort 2, remaining slightly, but insignificantly, higher in cohort 2. However, all antigen-specific IgG antibody responses in terms of GMT, GMFR, and SRR in vaccine cohorts were significantly higher than those in cohort 3 (placebo) after the first dose and lasted up to 12 months after the third dose (Table 5).
Antigen-Specific IFN-γ-Secreting Cells
The ELISpot assay was used to measure the level of antigen-specific IFN-γ-secreting cells in blood samples compared with PBS and phytohemagglutinin as negative and positive controls, respectively. The antigen-specific IFN-γ-secreting T cells were comparable at baseline in all three cohorts, whose mean values were under the limit of detection [< 25 spot-forming cells (SFCs)]. Both cohorts 1 and 2 showed increased antigen-specific IFN-γ-secreting cells on days 28, 56, and 84, with cohort 2 showing slightly greater responses than cohort 1 (Fig. 2). The peaks of IFN-γ-secreting cells in cohort 1 (mean 151.10, 95% CI 209.40–92.8) and cohort 2 (mean 194.10, 95% CI 263.60–124.60) were observed on day 84. The IFN-γ-secreting cells in both vaccine groups dropped to an equivalent level at 12 months after the third IP dose (cohort 1: 62.80 SFC per 106 PBMCs, cohort 2: 91.15 SFC per 106 PBMCs). The mean SFCs in the placebo group remained low (< 25 SFCs) throughout the study period (Fig. 2a). For cohorts 1 and 2, the level of antigen-specific IFN-γ-secreting cells gradually increased from the first to third IP doses with significant changes from the baseline. From 12 months after the third IP dose, the level of SFCs decreased, but was significantly higher than the baseline. For cohort 3, there was no change in the level of SFCs between baseline and each post-vaccination timepoint (Fig. 2b).
Immunogenicity assessment of antigen-specific interferon-γ (IFN-γ)-secreting cells. ID93-specific IFN-γ producing T cells using ELISpot. PBMCs from blood samples obtained before vaccination (day 0) and on days 28, 56, 84, and 421 after vaccination were stimulated with ID93 fusion protein in vitro. Results are shown for subjects vaccinated with 2 μg ID93 + 5 μg GLA-SE (cohort 1), 10 μg ID93 + 5 μg GLA-SE (cohort 2), or a saline placebo (cohort 3). a Data are represented as the mean and standard deviation and b each box extends from the 25th to 75th percentile, and the line in the middle of the box represents the median value. The whiskers go down to the minimum value and extend to the maximum value. Values were considered significantly different if p < 0.05 within the group, as indicated by *p < 0.05, **p < 0.01, or ****p < 0.0001
Antigen-Specific Th1 CD4+ Cell-Mediated Immune Response
T-cell immune responses induced by ID93 + GLA-SE were determined via PBMC-based intracellular cytokine staining (ICS) analysis using flow cytometry. The gating strategy and representative scatterplots for the ID93 stimulation are shown in Supplementary Material Figure S1. As shown in Fig. 3a, the percentage of ID93 antigen-specific CD4+ T cells that produced at least one, two, or three cytokine(s) [IFN-γ, tumor necrosis factor-alpha (TNF-α), and/or interleukin-2 (IL-2)] were measured on days 0 (baseline), 28, 56, 84, and 421. ID93 antigen-specific CD4+ T-cell responses were low before vaccination, but significantly increased in both vaccine groups after vaccination at all visits. Both cohort 1 and cohort 2 showed increased frequencies of cytokine-secreting CD4+ T-cell responses on days 28, 56, and 84. On day 421, significantly greater cytokine-producing CD4+ T-cell frequencies were observed with cohort 2 than cohort 1 (p = 0.0116), showing a higher magnitude of ID93-specific CD4+ T cells with 10 μg ID93 + 5 μg GLA-SE. Medians for cohort 2 are slightly higher but statistically insignificant at days 56 and 84. Cytokine-secreting CD4+ T-cell responses in the placebo group remained low (< 0.1%) throughout the study. Meanwhile, there were no differences in antigen-specific CD8+ T-cell responses among the three cohorts or timepoints, indicating no CD8+ T-cell responses were observed in this study (Fig. 3b). Similar frequencies of polyfunctional CD4+ T-cell responses on days 56 and 84 were observed in cohorts 1 and 2 (Fig. 3c and d, respectively). Pie charts representing day 84 versus day 421 samples show fewer 4+ and 3+ cytokine-producing CD4+ T cells at day 421 compared with day 84 samples, demonstrating the contraction of the ID93-specific population. Cohort 2 had proportionately more 1+ and fewer 3+ than cohort 1; we speculate that the statistical significance for day 421 (see above) was because of the enhanced retention of single cytokine producers.
Antigen-specific cytokine(s) positive CD4+ T cells from stimulated cryopreserved peripheral blood mononuclear cells. Blood samples were obtained before each vaccination (days 0, 28, and 56) and at 4 weeks and 12 months after the final vaccination (days 84 and 421). a The percentages of ID93-specific CD4+ T cells producing any of the three T-helper type 1 (Th1) cytokines, interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-2 (IL-2) (single producer, double producers, and triple producers) were measured in PBMC stimulated with ID93 antigen using intracellular cytokine staining and flow cytometry from each study participant. b The percentages of ID93 specific CD8+ T-cell responses. Values were considered significantly different if p < 0.05, as indicated by *p < 0.05 or ****p < 0.0001. For distribution of multifunctional CD4+ T cells by visits, the data is displayed from the two cohorts administered ID93 + GLA-SE; c cohort 1 and d cohort 2. Data are presented as the percentage frequency of ID93-specific CD4+ T cells expressing either 4, 3, 2, or 1 immune marker combination(s) including IFN-γ, IL-2, TNF-α, and CD40L at days 0, 28, 56, 84, and 421 for each cohort. Pie charts represent the mean proportions of cells expressing (after in vitro stimulation) any single marker and combination of IFN-γ, IL-2, TNF-α, and CD40L marker-positive CD4+ T cells of the total immune marker-expressing CD4+ T-cell response, at days 84 and 421 after vaccination. Paired responses of immune marker-positive CD4+ T cells on days 0, 28, 56, 84, and 421 after vaccination are shown in c cohort 1 and d cohort 2
DISCUSSION
This study provides the first data to evaluate the safety and immunogenicity of the ID93 + GLA-SE vaccine on over 70 previously BCG-vaccinated and QFT-negative individuals of an Asian population. Previously, a phase 1b study in South Africa was conducted in 12 QFT-negative adults who were less likely to be latently infected with Mtb, using a dose reported to be safe by the US phase 1 study [17, 18]. Similar to the phase 1 and phase 1b clinical studies conducted previously, no safety issues were reported in the present study, which selected ID93 (antigen) and GLA-SE (adjuvant) doses and an administration route that were based on the initial safety data from clinical studies in South Africa and the USA [17,18,19], and clinical trials on other vaccines with GLA-SE as adjuvants [23, 24, 28]. In the US phase 1, vaccination of BCG-naive, QuantiFERON-negative, healthy adults with 2 μg ID93 plus 2 μg GLA-SE, 2 μg ID93 plus 5 μg GLA-SE, 10 μg ID93 plus 2 μg GLA-SE, or 10 μg ID93 plus 5 μg GLA-SE induced a significantly higher antibody response than ID93 alone, which peaked after two injections in 100% of recipients and did not differ between varying antigen and adjuvant doses [17]. A preferential increase in IgG1 and IgG3 subclasses was observed, along with a multifaceted Fc-mediated effector function response, and enhanced magnitude and polyfunctional cytokine profile of CD4+ T cells. In the phase 1b in South Africa, vaccination of BCG-immunized, QuantiFERON-negative and positive, healthy adults showed that vaccine dose (10 μg ID93 plus 2 μg GLA-SE, 2 μg ID93 plus 2 μg GLA-SE, and 10 μg ID93 plus 5 μg GLA-SE) did not affect frequency or severity of adverse events and vaccination induced durable antigen-specific IgG and Th1 cellular responses, which peaked after two administrations. Vaccine dose also did not affect magnitude, kinetics, or profile of antibody and cellular responses in these participants [18]. With an acceptable safety profile, three injections of ID93 + GLA-SE induced strong ID93-specific antibody and cellular immune responses in both vaccine cohorts that were significantly higher than those with placebo, and the responses were maintained for a 12 month duration. This result was consistent with a previous study showing an antigen-specific antibody response predominantly composed of IgG1 and IgG3 subclasses, suggestive of strong major histocompatibility complex class II T-cell activity, and CD4+ T-cell responses elicited from all four ID93 antigen components that persisted for a 6 month study period [19].
In this study, there were peaks in both antibody and T-cell responses to the ID93 antigens 1 month after the third doses, which were somewhat different from those in the previous studies. In the phase 1 US study, a linear dose–response relationship was not observed and differences in CD4+ T-cell responses in the whole blood ICS assay were not statistically significant [17]. While responses peaked after two injections in 100% of recipients, responses did not decrease after the third vaccine administration. Vaccine dose or dosage did not affect magnitude, kinetics, or profile of antibody and cellular responses in the phase 1 or phase 1b participants [17, 18]. One of the reasons for differences in a peak time of immune responses to the ID93 antigen may be the BCG vaccination and latent TB infection histories of participants and vaccination intervals among the clinical studies. Further studies are required to select vaccination frequencies and intervals to reach the maximum efficacy in the target populations.
While there was no difference in immune responses and safety between vaccine doses and both ID93 and GLA-SE concentrations in the previous clinical studies [17,18,19], a fixed dose of 5 μg GLA-SE was chosen in this study on the basis of the adjuvant formulation results [10]. Antigen doses of 2 μg ID93 (cohort 1) and 10 μg ID93 (cohort 2), which were used in the previous studies, were also compared to select a dose in this study. Although there was a tendency of higher antibody and IFN-γ ELISpot responses in cohort 2, there was no statistical significant difference in immune responses to the ID93 antigen, except in higher ID93-specific cytokine(s) positive CD4+ T cells 1 year after the last vaccination in cohort 2 compared with those in cohort 1. Therefore, three intramuscular injections of a dose of 10 μg ID93 + 5 μg GLA-SE are desirable for further studies including efficacy evaluation in the future.
Healthcare workers were selected as the study population since the public health service has the highest distribution of occupational infectious diseases among all industries, with approximately one-third of the diseases being LTBI [29]. According to the Rules for the Prevention of TB, in ordinance of the Ministry of Health and Welfare of the Republic of Korea, the head of a medical institution should periodically conduct screenings for active and latent TB infections in healthcare workers to detect early-stage infection. As per these rules, TB screening is carried out at least once a year for healthcare providers who examine and treat patients with TB, for medical technicians who have diagnosed patients with TB, and for any other healthcare workers who are at high risk of respiratory infection [30]. The results of this study are from a population with a higher risk of Mtb infection and who would be expected to generate data that allows for subsequent studies to evaluate the safety and exploratory outcomes of this vaccine in the wider public, non-healthcare workers who are BCG-vaccinated, and QFT-negative or QFT-positive adults and adolescents.
Despite a confirmation of safety and immunogenicity of the ID93 + GLA-SE TB vaccine candidate in the BCG-vaccinated healthy adults in South Korea, this study had limitations such as more than two-thirds of enrolled participants in all three cohorts with female. Considering that more than 60% of TB patients reported in the country in 2019 were male, the gender ratio appears to be significantly skewed in this study [3]. In addition, this study employed a voluntary reporting procedure of AEs by filling up the daily diary between study visits, which may vary depending on the participants’ understanding of the characteristics and severity of AEs. While their impacts are not known yet, these two limitations need to be addressed in the upcoming phase 2b study by balancing sexes of participants and by employing information technology tools for monitoring AEs between study visits, respectively.
For further applications of ID93 + GLA-SE, we intend to characterize the preexisting and underlying non-vaccine Mtb-specific responses and compare them with the profile of ID93 + GLA-SE-induced and non-vaccine Mtb-specific responses during immunization using an MTB300 megapool without ID93 antigens in future studies. Combinatorial Polyfunctionality Analysis of Antigen-Specific T-cell Subsets (COMPASS) can also be used to evaluate vaccine-induced CD4+ T-cell subsets with unique and/or overlapping profiles compared with preexisting Mtb-specific responses [19].
CONCLUSIONS
This study showed the selected ID93 + GLA-SE regimens had an acceptable safety profile and were clinically well tolerated after three IM injections in BCG-vaccinated, QFT-negative, healthy healthcare workers. This study showed that a higher proportion of participants administered active IP doses reported AEs than those administered placebo. The solicited local and systemic AEs observed in the current study were consistent with those reported in previous studies, with no reported SAEs related to IP or death, or AEs that caused treatment discontinuation. The immunogenicity data in the current study showed an immunogenic profile comparable to those of the previous studies for ID93 + GLA-SE. Participants administered ID93 + GLA-SE showed higher humoral and Th1 cellular immune responses, which persisted throughout the 12 month study period, compared with those who received placebo.
REFERENCES
World Health Organization. Tuberculosis (TB): fact sheets. 2022. https://www.who.int/news-room/fact-sheets/detail/tuberculosis. Accessed 30 Nov 2022.
Cohen A, Mathiasen VD, Schön T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2019;54:1900655. https://doi.org/10.1183/13993003.00655-2019.
Korea Disease Control and Prevention Agency. Annual report on the notified tuberculosis patients in Korea 2019. 2020. http://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=1&pblctDtaSn=2088. Accessed 30 Nov 2022.
Clemens JD, Chuong JJ, Feinstein AR. The BCG controversy. A methodological and statistical reappraisal. JAMA. 1983;249:2362–9. https://doi.org/10.1001/jama.1983.03330410048027.
Menzies R, Vissandjee B. Effect of bacille Calmette-Guérin vaccination on tuberculin reactivity. Am Rev Respir Dis. 1992;145:621–5. https://doi.org/10.1164/ajrccm/145.3.621.
Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, et al. The efficacy of bacillus Calmette-Guérin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96:29–35. https://doi.org/10.1542/peds.96.1.29.
Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–45. https://doi.org/10.1016/s0140-6736(95)92348-9.
Woodworth JS, Clemmensen HS, Battey H, Dijkman K, Lindenstrøm T, Laureano RS, et al. A Mycobacterium tuberculosis-specific subunit vaccine that provides synergistic immunity upon co-administration with Bacillus Calmette-Guérin. Nat Commun. 2021;12:6658. https://doi.org/10.1038/s41467-021-26934-0.
Andersen P, Doherty TM. The success and failure of BCG—implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–62. https://doi.org/10.1038/nrmicro1211.
Anderson RC, Fox CB, Dutill TS, Shaverdian N, Evers TL, Poshusta GR, et al. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf B Biointerfaces. 2010;75:123–32. https://doi.org/10.1016/j.colsurfb.2009.08.022.
Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6:e16333. https://doi.org/10.1371/journal.pone.0016333.
Baldwin SL, Shaverdian N, Goto Y, Duthie MS, Raman VS, Evers T, et al. Enhanced humoral and Type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine. 2009;27:5956–63. https://doi.org/10.1016/j.vaccine.2009.07.081.
Lousada-Dietrich S, Jogdand PS, Jepsen S, Pinto VV, Ditlev SB, Christiansen M, et al. A synthetic TLR4 agonist formulated in an emulsion enhances humoral and Type 1 cellular immune responses against GMZ2–a GLURP-MSP3 fusion protein malaria vaccine candidate. Vaccine. 2011;29:3284–92. https://doi.org/10.1016/j.vaccine.2011.02.022.
Bertholet S, Goto Y, Carter L, Bhatia A, Howard RF, Carter D, et al. Optimized subunit vaccine protects against experimental leishmaniasis. Vaccine. 2009;27:7036–45. https://doi.org/10.1016/j.vaccine.2009.09.066.
Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2:53ra74. https://doi.org/10.1126/scitranslmed.3001094.
Baldwin SL, Reese VA, Larsen SE, Beebe E, Guderian J, Orr MT, et al. Prophylactic efficacy against Mycobacterium tuberculosis using ID93 and lipid-based adjuvant formulations in the mouse model. PLoS One. 2021;16:e0247990. https://doi.org/10.1371/journal.pone.0247990.
Coler RN, Day TA, Ellis R, Piazza FM, Beckmann AM, Vergara J, et al. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: first-in-human trial. NPJ Vaccines. 2018;3:34. https://doi.org/10.1038/s41541-018-0057-5.
Penn-Nicholson A, Tameris M, Smit E, Day TA, Musvosvi M, Jayashankar L, et al. Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: a randomised, double-blind, placebo-controlled phase 1 trial. Lancet Respir Med. 2018;6:287–98. https://doi.org/10.1016/S2213-2600(18)30077-8.
Day TA, Penn-Nicholson A, Luabeya AKK, Fiore-Gartland A, Du Plessis N, Loxton AG, et al. Safety and immunogenicity of the adjunct therapeutic vaccine ID93 + GLA-SE in adults who have completed treatment for tuberculosis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Respir Med. 2021;9:373–86. https://doi.org/10.1016/S2213-2600(20)30319-2.
Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS, et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One. 2010;5:e13677. https://doi.org/10.1371/journal.pone.0013677.
Coler RN, Duthie MS, Hofmeyer KA, Guderian J, Jayashankar L, Vergara J, et al. From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clin Transl Immunol. 2015;4:e35. https://doi.org/10.1038/cti.2015.6.
Singh K, Mukherjee P, Shakri AR, Singh A, Pandey G, Bakshi M, et al. Malaria vaccine candidate based on Duffy-binding protein elicits strain transcending functional antibodies in a Phase I trial. NPJ Vaccines. 2018;3:48. https://doi.org/10.1038/s41541-018-0083-3.
Santini-Oliveira M, Coler RN, Parra J, Veloso V, Jayashankar L, Pinto PM, et al. Schistosomiasis vaccine candidate Sm14/GLA-SE: Phase 1 safety and immunogenicity clinical trial in healthy, male adults. Vaccine. 2016;34:586–94. https://doi.org/10.1016/j.vaccine.2015.10.027.
Sirima SB, Richert L, Chêne A, Konate AT, Campion C, Dechavanne S, et al. PRIMVAC vaccine adjuvanted with alhydrogel or GLA-SE to prevent placental malaria: a first-in-human, randomised, double-blind, placebo-controlled study. Lancet Infect Dis. 2020;20:585–97. https://doi.org/10.1016/S1473-3099(19)30739-X.
Field MJ, editor. Tuberculosis in the workplace. Washington (DC): National Academies Press; 2001.
Jung DH, Jo KW, Shim TS. Prevalence of latent tuberculosis infection among medical students in South Korea. Tuberc Respir Dis (Seoul). 2012;73:219–23. https://doi.org/10.4046/trd.2012.73.4.219.
Youakim S. The occupational risk of tuberculosis in a low-prevalence population. Occup Med (Lond). 2016;66:466–70. https://doi.org/10.1093/occmed/kqw040.
Tendler M, Almeida MS, Vilar MM, Pinto PM, Limaverde-Sousa G. Current status of the Sm14/GLA-SE schistosomiasis vaccine: overcoming barriers and paradigms towards the first anti-parasitic human(itarian) vaccine. Trop Med Infect Dis. 2018;3:121. https://doi.org/10.3390/tropicalmed3040121.
Chung YK, Ahn YS, Jeong JS. Occupational infection in Korea. J Korean Med Sci. 2010;25:S53-61. https://doi.org/10.3346/jkms.2010.25.S.S53.
Joint committee for the revision of Korean guidelines for tuberculosis. Korean guidelines for tuberculosis. 2nd ed. Cheongju: Korea Centers for Disease Control & Prevention; 2016
ACKNOWLEDGEMENTS
We would like to thank the volunteers who participated in this study. We also thank Dr. Tracey A. Day, who provided us with the ID93 protein for the immunogenicity assessment. Dr. Tracey A. Day’s support was not funded.
Funding
This study was funded by Quratis Inc. and supported by a grant from the Korea Health Technology R and D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI14C1324). Quratis Inc. financially supported the study and was involved in the study design, data interpretation, data analysis, review of the report, and the journal’s rapid service fee. The corresponding authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Author Contributions
Yu Hwa Choi and Kwan Goo Cho designed the study. Young Ae Kang, Kwang Joo Park, and Jae Chol Choi developed and recruited the cohorts that were used in the study and provided clinical help. Yu Hwa Choi, Kwan Goo Cho, Young Ae Kang, Kwang Joo Park, Jae Chol Choi, Da Yeon Ko, Jun Ho Ahn, Yun Ju Woo, Kwangsoo Jung, Steven G. Reed, Boram Lee, Eunsol Ahn, Nan Yul Kim, Valerie A. Reese, Sasha E. Larsen, Susan L. Baldwin, Rhea N. Coler, Hyejon Lee, and Sang-Nae Cho analyzed the data and interpreted the results. Da Yeon Ko, Jun Ho Ahn, and Yun Ju Woo provided clinical and regulatory support. Da Yeon Ko, Jun Ho Ahn, and Yun Ju Woo, Nan Yul Kim, and Kwangsoo Jung conducted study oversight, study management activities, and study monitoring. Hyejon Lee, Sang-Nae Cho, Rhea N. Coler, Yu Hwa Choi, Young Ae Kang, Kwang Joo Park, Jae Chol Choi, Kwan Goo Cho, Jun Ho Ahn, Kwangsoo Jung, Boram Lee, Eunsol Ahn, Yun Ju Woo, Nan Yul Kim, Valerie A. Reese, Sasha E. Larsen, Susan L. Baldwin, Steven G. Reed, and Da Yeon Ko wrote the manuscript. All authors fully reviewed and revised the manuscript before submission. All authors read and approved the final manuscript.
Disclosures
Yu Hwa Choi, Kwan Goo Cho, Da Yeon Ko, Jun Ho Ahn, Yun Ju Woo, Kwangsoo Jung, Eunsol Ahn, Boram Lee, Nan Yul Kim, Hyejon Lee, and Sang-Nae Cho are employees of Quratis at the time of the study or analysis. Yu Hwa Choi, Kwan Goo Cho, Sang-Nae Cho, Da Yeon Ko, and Jun Ho Ahn own shares or options to shares in Quratis. Valerie A. Reese, Sasha E. Larsen, Susan L. Baldwin, and Rhea N. Coler received grants from Quratis for the immunoassays of the study. All other authors report no competing interests.
Compliance with Ethics Guidelines
This study was approved by the Institutional Review Board and Ethical Committee of Yonsei University Severance Hospital (IRB# 4-2018-0230), Ajou University Hospital (IRB# AJIRB-MED-CT2-18-078), and Chung-Ang University Hospital (1833-001-320). The study was performed in line with the principles of the Declaration of Helsinki. The trial was retrospectively registered at clinicaltrials gov (NCT03806686). All study participants gave written informed consent to participate in the study and to publish the results of the clinical trial.
Data Availability
The data supporting the findings in this study are included in this manuscript and its electronic supplementary material.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Choi, Y.H., Kang, Y.A., Park, K.J. et al. Safety and Immunogenicity of the ID93 + GLA-SE Tuberculosis Vaccine in BCG-Vaccinated Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial. Infect Dis Ther 12, 1605–1624 (2023). https://doi.org/10.1007/s40121-023-00806-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00806-0