Abstract
Introduction
Gram-negative bacteria (GNB) have become prominent across healthcare and community settings due to factors including lack of effective infection control and prevention (ICP) and antimicrobial stewardship programs (ASPs), GNB developing antimicrobial resistance (AMR), and difficulty treating infections. This review summarizes available literature on healthcare-associated infections (HAIs) in Middle Eastern pediatric patients.
Methods
Literature searches were performed with PubMed and Embase databases. Articles not reporting data on GNB, HAIs, pediatric patients, and countries of interest were excluded.
Results
The searches resulted in 220 publications, of which 49 met the inclusion criteria and 1 additional study was identified manually. Among 19 studies across Egypt reporting GNB prevalence among pediatric patients, Klebsiella species/K. pneumoniae and Escherichia coli were typically the most common GNB infections; among studies reporting carbapenem resistance and multidrug resistance (MDR), rates reached 86% and 100%, respectively. Similarly, in Saudi Arabia, Klebsiella spp./K. pneumoniae and E. coli were the GNB most consistently associated with infections, and carbapenem resistance (up to 100%) and MDR (up to 75%) were frequently observed. In other Gulf Cooperation Council countries, including Kuwait, Oman, and Qatar, carbapenem resistance and MDR were also commonly reported. In Jordan and Lebanon, E. coli and Klebsiella spp./K. pneumoniae were the most common GNB isolates, and AMR rates reached 100%.
Discussion
This review indicated the prevalence of GNB-causing HAIs among pediatric patients in Middle Eastern countries, with studies varying in reporting GNB and AMR. Most publications reported antimicrobial susceptibility of isolated GNB strains, with high prevalence of extended-spectrum beta-lactamase-producing K. pneumoniae and E. coli isolates. A review of ASPs highlighted the lack of data available in the region.
Conclusions
Enhanced implementation of ICP, ASPs, and AMR surveillance is necessary to better understand the widespread burden of antimicrobial-resistant GNB and to better manage GNB-associated HAIs across Middle Eastern countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Review of available data related to healthcare-associated Gram-negative bacteria (GNB) indicated that GNB are major causes of healthcare-associated infections among Middle Eastern pediatric patients. |
Klebsiella spp./K. pneumoniae and Escherichia coli were the most common causes of infections in Egypt and Gulf Cooperation Council countries, where high multidrug resistance was reported in some studies. |
Publications from Jordan and Lebanon most commonly reported E. coli and Klebsiella spp./K. pneumoniae infections, with antimicrobial resistance (AMR) rates of up to 100%. |
Increased adoption of antimicrobial stewardship practices and AMR surveillance programs is needed to better understand the prominent healthcare-associated GNB infections across Middle Eastern countries. |
Introduction
Gram-negative bacteria (GNB) have become prevalent in causing disease across community and healthcare settings around the world [1, 2]. Antibiotic misuse, lack of effective infection control measures, and the ability of GNB to develop antimicrobial resistance (AMR) through a range of mechanisms are major factors underlying this rise in prominence [3, 4]. Development of antibiotic resistance among GNB is associated with poorer clinical outcomes and increased health costs, presenting a global public health threat [1]. Compared with Gram-positive bacteria, the distinctive outer membrane of GNB makes them more resistant to antibiotics, resulting in significant morbidity and mortality [4]. This greater resistance to antibiotics is highlighted by the 2017 World Health Organization global priority pathogens list, which includes 12 groups of pathogens in three priority categories (critical, high, medium); among the Gram-negative pathogens, carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Pseudomonas aeruginosa, and carbapenem-resistant extended-spectrum beta-lactamase (ESBL)–producing Enterobacterales, formerly Enterobacteriaceae, are classified as critical pathogens [4, 5].
GNB are often common causes of healthcare-associated infections (HAIs), such as pneumonia, urinary tract infections (UTIs), wound and surgical site infections, intra-abdominal infections, bloodstream infections (BSIs), and meningitis [1, 6, 7]. Patients in pediatric and neonatal intensive care units (PICUs and NICUs) are particularly vulnerable to HAIs because of their immature immune systems, presence of multiple comorbidities, and cross-infection from frequent close contact with care team members [8]. Of paramount concern, HAIs are associated with an array of adverse outcomes, including prolonged hospital stays, disabling long-term sequelae, excess mortality, additional increases in AMR, and high financial burdens for healthcare systems and patients [9].
In Middle Eastern countries, multidrug-resistant (MDR) pathogens are particularly problematic because of the presence of several predisposing elements: inappropriate use of antibiotics in humans and animals; absent or inadequate infection control measures; and significant, ongoing population movement [2]. In line with these factors, available surveillance data show broad dissemination of ESBL and carbapenemase-producing GNB in Middle Eastern hospitals. In tandem with the growing prevalence of MDR GNB, effective treatment options are dwindling. Subsequently, knowledge of resistance patterns among the commonly isolated Gram-negative pathogens in the local setting and consideration of individual patient characteristics are critical for making informed antimicrobial treatment decisions [1].
Characterizing the epidemiology of Gram-negative pathogens is thus important, both to assess the prevalence of MDR and to extend knowledge of the mechanisms being used to develop resistance [3]; however, because of inadequate surveillance programs, data describing GNB infections and their associated MDR patterns are often limited and disjointed. Therefore, this literature review was undertaken to consolidate and summarize the available data related to HAIs due to GNB among pediatric patients (< 18 years of age) across Middle Eastern countries, including the prevalence and incidence of pediatric infections due to GNB of concern in Middle Eastern countries and antibiotic susceptibility profiles and MDR rates of these GNB. Additionally, the review aimed to describe epidemiologic trends by age, country/region, and hospital setting [e.g., intensive care unit (ICU), NICU, and PICU] as well as the relationship of GNB to outcomes in the pediatric population, such as mortality, infectious complications, and length of hospital stay.
Methods
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
PubMed and Embase database searches were conducted on 19 October 2021, using the search terms [(Egypt OR Lebanon OR Iraq OR Jordan OR Saudi Arabia OR KSA OR Kuwait OR UAE OR United Arab Emirates OR Qatar OR Bahrain OR Oman) AND (prevalence OR epidemiology OR surveillance OR incidence) AND (enterobacteriaceae OR budviciaceae OR erwiniaceae OR hafniaceae OR morganellaceae OR pectobacteriaceae OR yersiniaceae OR citrobacter OR edwardsiella OR enterobacter OR hafnia OR kluyvera OR morganella OR pantoea OR proteus OR providencia OR raoultella OR serratia OR yersinia OR kingella OR elizabethkingia OR chryseobacter* OR acinetobacter OR campylobacter OR enterobacteria* OR “escherichia coli” OR “e coli” OR “e. coli” OR haemophilus influenzae OR klebsiella OR pseudomonas OR salmonell* OR shigell*) AND (hospital* OR nosocom* OR inpatient OR in-patient OR (surg* AND infect*))]. The PubMed search was limited to English-language articles published during the preceding 10 years that were indexed for the 0- to 18-year age group; review articles were excluded. The Embase search was limited to articles, articles in press, conference abstracts, and letters in the Embase database, with publication years 2011–2021 and including any age group from newborn (0–1 month) through to adolescent (13–17 years). Publications returned by the searches were reviewed by the authors to identify articles reporting original data related to GNB infections; articles that did not report data specific to GNB, hospital-acquired infections, pediatric age groups, and the countries of interest were excluded. In addition to the articles identified by database literature searching, articles identified by hand searches could also be included.
Results
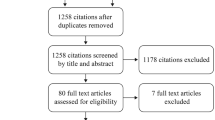
In total, 220 unique publications were retrieved by the PubMed and Embase database searches, of which 49 described studies that met criteria for inclusion in the review: 18 studies set in Egypt (including 1 study set in both Egypt and Saudi Arabia), 27 studies set in Gulf Cooperation Council (GCC) countries, and 4 studies set in Levant countries (Figs. 1 and 2). An additional study set in Egypt was provided from author files. Details of the studies including the study period, type of infection, prevalence or incidence of infections, number of isolates, type of GNB pathogens, and AMR percentages are listed in Supplementary Tables S1–S4.
Egypt
A total of 19 of the publications included in the review described studies set in Egypt (including 1 study set across Egypt and Saudi Arabia): 18 articles were identified by the database literature searches [8, 10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] and an additional article was provided from author files [27] (Supplementary Table S1). Studies were classified according to patient setting: NICUs (n = 9) [8, 10,11,12,13,14,15,16,17], PICUs (n = 3) [18, 19, 27], patients of mixed age (n = 3) [20,21,22], and special populations (n = 4) [23,24,25,26]. Across all settings in studies that reported the prevalence of GNB pathogens, Klebsiella spp./K. pneumoniae (up to 96% of GNB) and Escherichia coli (up to 53% of GNB) were frequently among the most common Gram-negative isolates [8, 10,11,12,13,14,15,16, 18, 19, 23,24,25]. AMR was assessed in 17 studies [10,11,12,13,14, 16,17,18,19,20,21,22,23,24,25,26,27], including 6 that assessed carbapenem resistance [11, 12, 17, 19, 20, 25], with rates as high as 86% for the latter being reported [25]. Seven studies assessed MDR pathogens, with rates ranging from 0–100% across all GNB isolates [13, 14, 16, 18, 19, 24, 25].
Studies Set in NICUs
Among the 9 studies from Egypt set in NICUs (Supplementary Table S1) [8, 10,11,12,13,14,15,16,17], late-onset sepsis (LOS) was the focus of 5 studies [10,11,12,13,14]. GNB were prominent, accounting for 93% of nosocomial LOS episodes in preterm neonates [10] and 45–69% of confirmed LOS cases in neonates [11,12,13,14]. Klebsiella spp./K. pneumoniae were the most prominent GNB in LOS. Where frequency was reported among all pathogens, Klebsiella spp. caused between 20% and 59% of LOS cases [10, 11, 14]. Where frequency was reported among GNB pathogens, K. pneumoniae isolates accounted for 36% of GNB LOS cases [12].
Carbapenem resistance was common among GNB isolates, ranging from about 30–70% [13, 14]. Among 23 K. pneumoniae isolates [22 from LOS and 1 from early-onset sepsis (EOS)], 44% were resistant to imipenem and 57% to meropenem, and 52% harbored both blaOXA-48 and blaNDM-1 genes [11]. Among 158 GNB isolates from LOS, 58 (37%) were carbapenem resistant [12]; neonates with carbapenem-resistant GNB infections had significantly higher rates of mortality and infectious complications and significantly longer durations of mechanical ventilation and hospital stay. Two studies reported susceptibility in combined EOS (approximately 35%) and LOS isolates (approximately 65%). Susceptibility to imipenem and meropenem ranged from 38–80% across GNB species in one study [13], and resistance to imipenem was 30% among all GNB isolates in the other study [14].
Three studies in NICUs assessed all HAIs in admitted neonates [8, 15, 16]. Overall, HAI rates ranged from 7.4/1000 patient-days to 17.5/1000 patient-days. In the 3 studies, HAIs were predominantly BSIs (41–91% of all HAIs) and pneumonia (8–53% of all HAIs) [8, 15, 16]. Among HAIs, GNB prevalence ranged from 54–73%. Klebsiella spp. was the most commonly isolated GNB, accounting for 42–65% of GNB isolates. Although none of the three NICU articles reported specific antibiotic susceptibility data, one study indicated that no MDR GNB isolates were detected [16]. One study focused on A. baumannii HAIs in neonates [17]. Among 124 neonatal cases, 73% were caused by carbapenem-resistant A. baumannii (CRAB) isolates. Mechanical ventilation and previous carbapenem or aminoglycoside use were independent risk factors for CRAB infections and for mortality. Among all A. baumannii isolates, 76–100% were resistant to the other tested antibiotics, with the exception of tigecycline (10–12% resistant).
Studies Set in PICUs
Three of the included studies from Egypt were based in PICUs [18, 19, 27]. GNB were a common cause of PICU-acquired infections in all three studies, ranging from 20–83% of total infections in PICU patients or episodes [18, 19, 27]. One study reported that PICU-acquired infections occurred in 15.6% of overall PICU admissions [27]. Among GNB pathogens, the most common isolates were Klebsiella spp. (32–37%), Acinetobacter spp. (22–38%), P. aeruginosa (0–30%), and E. coli (6–13%) [18, 19, 27]. Two studies reported the prevalence of MDR among GNB isolates, which ranged from 81–100% [18, 19]. Where reported, GNB susceptibility was highest to polymyxin (90–100%) [18]. Susceptibility to carbapenems varied by pathogen and study, with E. coli being the least susceptible (0–5%) and Klebsiella spp. (17–20%) and Acinetobacter spp. (15–43%) showing higher sensitivity to carbapenems [18, 27].
Studies Set in Hospital Populations of Mixed Age
Three studies from Egypt described patients of all ages but reported certain data specific to pediatric patients [20,21,22]. In one study, K. pneumoniae accounted for 56% among patients aged < 10 years who developed HAIs. Among a total of eight K. pneumoniae isolates from sputum, urine, and wound samples in patients aged < 18 years, resistance was high to all tested antibiotics, including ciprofloxacin (75%), amikacin (63%), and carbapenems (75% were resistant to imipenem and/or meropenem). Genotyping detected the blaOXA-48 gene in three isolates and blaVIM in two isolates, whereas the blaKPC gene was not detected [20]. Another study investigated DA-HAIs in adult ICUs (median age [range], 56 years [10–93 years]), PICUs (median age [range], 6 months [1 month to 12 years]), and a NICU (median age [range], 5 days [1–52 days]), rates of which were 7.1, 26.8, and 22.8 per 1000 device days, respectively. Among the 72 bacterial isolates from all age groups (60/72 from VAP), 97% were GNB, most commonly A. baumannii (36%), K. pneumoniae (29%), and P. aeruginosa (22%) [21]. A third multicenter study in Egypt and Saudi Arabia assessed the prevalence of P. aeruginosa in burn and wound exudate, blood, urine, endotracheal aspirates from patients with VAP, and sputum. The percentage of P. aeruginosa in samples from patients aged 12–20 years was 25% [22].
Studies Set in Special Populations (Pediatric Patients with Cancer)
Four studies from Egypt assessed GNB HAIs in pediatric patients with cancer [23,24,25,26]. Two studies conducted in children’s cancer hospitals characterized isolates from hospital-acquired BSI episodes [23, 24]. Among all BSI isolates, the most common GNB were E. coli (18–28%), K. pneumoniae (6–12%), and Acinetobacter spp. (6%). In both studies, high levels of resistance to fluoroquinolones were observed in E. coli isolates (85–96%). MDR was reported for 21% among all GNB reported in one study [24].
Another study in a children’s cancer hospital assessed GNB isolates from various clinical specimens. Similar to the previous studies, E. coli (46% of GNB isolates) and Klebsiella spp. (38% of GNB isolates) were the most commonly identified bacteria [25]. The rate of MDR was high in both Enterobacterales (94%) and nonfermentative isolates (86%). Resistance to ≥ 1 carbapenem was also substantial (66% and 86%, respectively), with blaOXA-48 and blaNDM being the most commonly identified genes among carbapenemase-producing isolates (prevalence of 53% and 25%, respectively). A study of patients of all ages with cancer hospitalized in a tertiary care center found greater genetic variability and higher susceptibility to antimicrobials in A. baumannii isolated from patients < 18 years compared with adults [26].
Gulf Cooperation Council Countries
A total of 27 studies from GCC countries were included in this review: 12 studies in Saudi Arabia (including 1 study in hospitals across Saudi Arabia, Kuwait, and the UAE; Supplementary Table S2) [28,29,30,31,32,33,34,35,36,37,38,39], 5 in Kuwait [40,41,42,43,44], 3 each in Oman [45,46,47] and Qatar [48,49,50], and 4 in the UAE [51,52,53,54] (Supplementary Table S3). No applicable studies conducted in Bahrain were identified in the literature search. Across studies set in Saudi Arabia that reported data related to GNB prevalence, Klebsiella spp./K. pneumoniae and E. coli were consistently among the most frequently isolated GNB pathogens [28, 29, 33, 36, 38, 39]. AMR was assessed in 12 studies [28,29,30,31,32,33,34,35,36,37,38,39], including 8 that looked at carbapenem resistance, reporting rates as high as 100% [29, 31,32,33,34,35,36, 38]. Two of the smaller studies reported that all isolates were susceptible to carbapenem [34, 38]. Five studies assessed MDR, with rates ranging from 10–100% across GNB isolates [32,33,34,35, 38]. Among the other GCC countries, Klebsiella spp./K. pneumoniae and E. coli were again the most frequently reported GNB infections [40, 46, 48, 51,52,53,54]. AMR was assessed in 15 studies [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54], including 9 that looked at carbapenem resistance, with rates as high as 78% [41,42,43,44, 48, 50, 51, 53], although rates of 100% susceptibility to carbapenem antibiotics were also reported across different GNB isolates [53]. Three studies reported MDR, with up to 100% showing MDR across all bacterial isolates [40, 44, 50].
Kingdom of Saudi Arabia
Three of the studies from Saudi Arabia reported data from NICUs [28,29,30], two of which investigated LOS [28, 29]. Among neonates admitted to a tertiary hospital NICU, the most prevalent GNB isolates causing LOS were Klebsiella spp. (18%), P. aeruginosa (10%), E. coli (9%), and Enterobacter (9%). Antimicrobial susceptibilities were reported for combined EOS and LOS isolates (EOS, 11%; LOS, 89%). Susceptibility of the four most common GNB was highest to carbapenems (91–100%), piperacillin/tazobactam (78–100%), and aminoglycosides (62–100%). Susceptibility of A. baumannii isolates was similar for aminoglycosides (67%) but lower for the carbapenems (67%) and piperacillin/tazobactam (50%) [29]. Among neonates admitted to NICUs in Saudi Arabia, Kuwait, and the UAE, 45% of LOS cases were caused by GNB, with Klebsiella spp. (23%), E. coli (5%), Acinetobacter (5%), and Pseudomonas spp. (4%) being most common. Among these pathogens, Pseudomonas was associated with the highest case fatality rate (51.4%). Across the six GNB families reported, 0–6.1% of isolates were resistant both to third-generation cephalosporins and aminoglycosides [28]. Another study was conducted during an outbreak of ESBL K. pneumoniae in the NICU unit [30]. During a 3-month period from April to July 2008, 3.4% of admitted neonates developed BSI or pneumonia caused by ESBL K. pneumoniae and 6.8% of neonates became colonized, corresponding to an infection/colonization incidence of 5.3 per 1000 patient-days. Treatment with vancomycin was significantly associated with increased risk of infection/colonization (incidence density ratio, 4.22; 95% CI 1.47–5.15).
Two studies included only pediatric patients [31, 32]. During a 1-year period in 2016–2017, 19 patients < 18 years of age who tested positive for carbapenem-resistant Enterobacteriaceae (CRE) infection ≥ 24 h after hospital admission were compared with matched controls infected with carbapenem-sensitive Enterobacteriaceae [31]. Most of the CRE identified were K. pneumoniae (47%) and E. coli (32%). Although antimicrobial susceptibilities were not reported, prior use of antibiotics and recent exposure to carbapenem were both significantly more common in patients with CRE isolates. The other study focused on Acinetobacter spp. HAIs in children aged < 12 years [32]. Among these patients, 34% were MDR, with MDR Acinetobacter more frequently isolated than susceptible Acinetobacter in VAP, CAUTI, burn wounds, and soft tissue. MDR Acinetobacter were > 70% resistant to all classes of antimicrobials tested except colistin and tigecycline (0%). In contrast, the susceptible HAI isolates were < 5% resistant to the tested antimicrobials, whereas the colonizing isolate group varied between 19% and 43% resistant and included a single colistin-resistant isolate.
Five Saudi Arabia studies carried out in a range of age groups reported some data specific to pediatric patients [33,34,35,36,37]. Two studies assessed the prevalence of ESBL-producing GNB in samples of urine, wound swab, blood, and sputum [33, 34]. In patients ≤ 19 years of age treated at a referral hospital, 30% of GNB were ESBL-producing. Across all age-groups, most ESBL-producing E. coli and K. pneumoniae isolates were susceptible to carbapenems (≥ 98%) and tigecyline (81–100%) [33]. The second study specifically assessed ESBL-producing E. coli. Among these isolates from patients ≤ 18 years of age (43% from HAIs), 100% were MDR [34]. Across age groups, all ESBL-producing E. coli isolates were susceptible to carbapenems and all but one were susceptible to tigecycline. Genotyping revealed a high prevalence of blaCTX-M (95%) and blaTEM (84%) genes. A study set in four tertiary care hospitals isolated 120 Enterobacteriaceae among 864 specimens (mainly urine, blood, pus, and sputum; nosocomial acquisition not confirmed), which included 12 isolates from pediatric patients [35]. Among these isolates, two were carbapenem resistant and one harbored triple-gene resistance (KPC/NDM-1/OXA-48). A second study, which characterized GNB in a range of specimens from ICU patients in a single hospital, reported high prevalence of MDR in GNB isolated from infants and children (73% and 80%, respectively) [36]. A 6-year study (2013–2019) carried out in a single hospital characterized Chryseobacterium/Elizabethkingia spp. infections and colonizations among patients of all ages [37]. Over the study period, 27 patients were infected or colonized with Chryseobacterium/Elizabethkingia spp, 16 of whom were aged ≤ 12 years. In total, 11 of the pediatric episodes were identified as infection (8 VAP, 1 CLABSI, 1 septic shock, 1 bacteremia) and 5 as colonization. Antimicrobial susceptibility was reported for all isolates, with the highest rates for fluoroquinolones (> 70%), cotrimoxazole (100%), and piperacillin/tazobactam (approximately 81%), and < 15% against all other antimicrobials tested.
High-risk pediatric populations were the focus of two Saudi Arabian studies [38, 39]. The prevalence of CAUTI in postoperative patients < 14 years of age admitted to a pediatric coronary ICU during a 1-year period was 7%. Among these UTIs, 72% were caused by GNB, most commonly Klebsiella spp. (38% of GNB) and E. coli (29% of GNB). Among all Enterobacterales UTI isolates, 41% were ESBL-producing and 12% were classified as MDR [38]. Catheter-related BSIs in eight pediatric patients with tunneled catheters for home parenteral nutrition were assessed over a 7-year period [39]. Of the 112 BSI pathogens isolated, the most common GNB were K. pneumoniae (5.4%) and E. coli (3.6%).
Kuwait
Among the five studies set in Kuwait, two reported HAI data from NICU or PICU settings [40, 41]. LOS in the neonatal unit of a major tertiary hospital was analyzed over a 5-year period (2005–2009) [40]. Overall, 949 LOS infections were identified, corresponding to an overall incidence of 16.9 episodes per 1000 live births. GNB were isolated in 43% of cases, with the most common isolates being Klebsiella spp. (44% of GNB), Enterobacter spp. (15%), E. coli (13%), Acinetobacter spp. (11%), and Pseudomonas spp. (9%). Klebsiella spp. was the major bacterial pathogen isolated from meningitis (46%) and was also a prominent bloodstream isolate in neonates with necrotizing enterocolitis (21%). There was no difference in case fatality rates between GNB and overall infections (11% and 12%, respectively). However, a higher percentage of Pseudomonas spp. infections resulted in death (18%). Overall, 13% of GNB isolates were resistant to both cephalosporins and gentamicin. Among individual GNB pathogens, resistance to both cephalosporins and gentamicin was highest in E. coli (21%) and Klebsiella spp. (19%). DA-HAI rates (CLABSI, CAUTI, and VAP) in two PICUs and one NICU across two hospitals in Kuwait City were determined [41]. CLABSI rate in the NICU was 15.3 per 1000 device-days, whereas rates of CAUTI and VAP across the PICUs and NICU were ≤ 1.4 per 1000 device-days. Although the prevalence of individual pathogens was not reported, carbapenem (imipenem or meropenem) resistance across GNB isolates from all DA-HAIs in all settings (i.e., ICUs, PICUs, and NICUs) ranged from 0% in E. coli to 29%, 63%, and 78% in K. pneumoniae, P. aeruginosa, and A. baumannii, respectively.
Three studies investigated CRE in mixed-age settings and reported at least some pediatric-specific data [42,43,44]. Among Enterobacterales isolates from patients of all ages across several Kuwaiti hospitals, 8% were CRE [42]. A total of 21 CRE were positive for blaNDM-1, including E. coli from a rectal swab of an infant with pneumonia and K. pneumoniae from a urine sample of an infant with atypical uremic syndrome. Both isolates were susceptible to amikacin, colistin, and tigecycline and resistant to most of the other antimicrobials tested. In a study conducted across public hospitals, < 1% of Enterobacterales isolates in patients of all ages were blaOXA-48–positive CRE [43]. The CRE isolates included three from patients aged 2–36 months with isolates obtained from recurrent UTI (K. pneumoniae), meningitis (E. coli), and pneumonia (K. pneumoniae). The K. pneumoniae isolates co-harbored blaSHV gene (−11 and −1, respectively) and the E. coli isolate co-harbored blaCTX-M-14 gene. Carbapenem-nonsusceptible K. pneumoniae isolated from a central venous pressure tip in a 4-month-old infant was phenotypically MBL positive and possessed resistance genes for VIM-4 carbapenemase and multiple beta-lactamases (SHV-11, CTX-M-15, and TEM-1) [44]. Antimicrobial susceptibility testing (AST) showed susceptibility only to colistin and tigecycline.
Oman
Of the studies conducted in Oman, one characterized pathogens causing LOS in NICU patients [45]. During the 8-year study period, LOS was confirmed in 125 neonates (prevalence among NICU admissions, 4%), with 42% of cases caused by GNB. K. pneumoniae was the most prevalent GNB, causing 17% of all LOS. The 11 deaths were associated with GNB LOS, with Klebsiella spp. being most frequently implicated (7 deaths). Another study assessed culture isolates from wound infections in hospitalized patients [46]. Among 32 swabs collected from pediatric patients ≤ 19 years of age, 26 isolates were cultured, including 7 GNB (K. pneumoniae, n = 3; P. aeruginosa, n = 2; and Stenotrophomonas maltophilia and Proteus mirabilis, n = 1 each). Although AMR data specific to the pediatric isolates were limited, it could be deduced that none of the K. pneumoniae isolates were ESBL-producing or MDR, the P. aeruginosa isolates were resistant to several antibiotics but susceptible to amikacin and ciprofloxacin, S. maltophilia was susceptible only to ceftazidime and trimethoprim-sulfonamide, and P. mirabilis was susceptible to all except nitrofurantoin.
In a study of 100 patients admitted to a pediatric cardiac ICU for > 48 h, 28% returned positive bacterial cultures (types/sites not specified) [47]. Pseudomonas spp., ESBL Klebsiella, and Stenotrophomonas were the most common isolated pathogens.
Qatar
Of the three studies in Qatar, two included patients of all ages but reported pediatric-specific data [48, 49]. In hospitalized patients ≤ 14 years of age with acute bacterial meningitis caused by community-acquired infections (CAIs) or HAIs, 33% of cases were due to GNB [48]. Although the GNB isolates were not all categorized as HAI and CAI, it could be determined that HAI isolates included Enterobacter aerogenes and P. aeruginosa, and potentially included E. coli, K. pneumoniae, H. influenzae, and Acinetobacter lwoffii. The overall prevalence of MDR P. aeruginosa isolates was 8% (205/2533) among routine specimens collected from patients of all ages across several Qatari hospitals [49]. Most MDR isolates (95%) were hospital acquired, and resistance was lowest to colistin (3%). Among the 12 isolates from pediatric patients < 14 years of age, 4 were mucoid MDR P. aeruginosa. A case study report described a patient of 6 years of age with hemophagocytic lymphohistiocytosis who was hospitalized in Doha and later died due to multiorgan failure associated with highly drug-resistant bacteria [50]. GNB isolated during later stages of the disease course included Elizabethkingia meningoseptica and extensively resistant, carbapenemase-producing E. coli [both from central venous catheters (CVCs)], and pandrug-resistant K. pneumoniae (from peripheral blood). All 3 isolates harbored multiple beta-lactam resistance genes; several aminoglycoside, quinolone, and macrolide resistance genes were also detected in the Enterobacteria.
United Arab Emirates
All included publications from the UAE were congress abstracts, and thus, provided relatively limited data specifically related to GNB HAIs [51,52,53,54]. Three of the studies assessed neonatal sepsis/BSI [51,52,53]. The most recent study (January 2013 to December 2015) reported the etiology of sepsis in 123 infants (69% LOS, 31% EOS) [51]. GNB were identified in 29% of cases, most commonly K. pneumoniae (66% of GNB cases). Overall, 19% of sepsis was caused by ESBL K. pneumoniae and E. coli, the majority of which were sensitive to meropenem. In an earlier study (January 2011 to June 2013) conducted in 51 neonates aged < 7 days with culture-confirmed sepsis (EOS and LOS combined), E. coli accounted for 17% of isolates [52]. A third study carried out before 2012 cultured bacteria from 5% of submitted neonatal blood samples (EOS/LOS or HAI not differentiated) [53]. GNB accounted for 61% of cultured isolates, most commonly E coli, Klebsiella spp., and Pseudomonas spp. All E. coli and Klebsiella isolates were susceptible to carbapenems and tigecycline, and all Pseudomonas isolates were susceptible to carbapenems, gentamicin, and amikacin (tigecycline not tested). An additional UAE study assessed infections in pediatric patients < 13 years of age with leukemia [54]. Among 27 pathogens isolated from 46 patients during febrile neutropenic episodes, 60% were GNB, most commonly E. coli (20%, of GNB isolates), K. pneumoniae (12%), and P. aeruginosa (12%).
Levant Countries
Four studies from Levant countries were included in the review: two studies conducted in Jordan [55, 56] and two in Lebanon [57, 58] (Supplementary Table S4). No studies from Iraq met the inclusion criteria. Among studies that reported the prevalence of GNB pathogens, Klebsiella spp./K. pneumoniae (up to 40% of UTIs in infants) and E. coli (up to 79% of UTIs in children < 18 years of age) were the most common GNB-causing infections [56,57,58]. All four studies reported AMR, with two reporting carbapenem resistance [55,56,57,58].
Jordan
Both studies from Jordan were conducted in NICUs [55, 56]. One study characterized a total of 68 isolates of pathogens causing sepsis in the NICU of a hospital in northern Jordan [55]. GNB were isolated from 73% of LOS episodes, most commonly A. baumannii (42% of GNB isolates), K. pneumoniae (36%), and E. coli (8%). AMR was reported across EOS and LOS isolates. Among 18 A. baumannii isolates, all were MDR, 17% were susceptible to tigecycline, and 100% were susceptible to colistin. Among 15 K. pneumoniae isolates, 80% were ESBL producing and 13% were KPC producing. All four E. coli isolates were ESBL producing. Sepsis caused by A. baumannii and KPC-producing K. pneumoniae was significantly associated with higher mortality (75% versus 26%, P = 0.001). The other study investigated UTIs in infants who were admitted to a single NICU at < 3 months of age [56]. Overall, 31 out of 1075 infants (3%) had a UTI during their NICU stay, and 5% developed a UTI ≥ 7 days after discharge. The most common pathogens isolated from NICU and ex-NICU UTIs were K. pneumoniae (41% and 42%, respectively) and E. coli (31% and 28%, respectively). K. pneumoniae isolates from ex-NICU UTIs showed a tendency toward higher AMR compared with K. pneumoniae isolated from UTIs that developed during the NICU stay (NICU, 40–82% resistance; ex-NICU, 69–100% resistance).
Lebanon
In one of the studies from Lebanon, DA-HAIs were investigated in PICU patients ≤ 20 years of age in a tertiary care hospital in Beirut [57]. GNB were isolated from 32 of 59 DA-HAIs: 8 CLABSI episodes, 8 CAUTI episodes, and 16 VAP episodes. The most common GNB were K. pneumoniae (17% of all DA-HAIs), Pseudomonas spp. (12%), E. coli (10%), and Enterobacter spp. (7%). In total, 80% of K. pneumoniae isolates and 67% of E. coli isolates were ESBL producing, whereas 33% P. aeruginosa isolates were classified as MDR. Another study conducted in Beirut analyzed the case files of patients < 18 years of age who were hospitalized with UTIs during 2001–2011 [58]. A total of 675 UTI cases met inclusion criteria, 79% of which were caused by E. coli, 8% by Klebsiella spp., 2% by P. aeruginosa, and 2% by Enterobacter spp. Among the E. coli and Klebsiella spp. isolates, 16% were ESBL producing. Over the study period, the percentage of UTIs caused by ESBL-producing bacteria increased from 8% (2001) to 25% (2011). Although healthcare-associated and community-acquired UTIs were not differentiated, several healthcare-related factors were significantly more common in patients with UTIs caused by ESBL-producing pathogens compared with those with UTIs caused by non-ESBL E. coli and Klebsiella spp. These factors included previous hospitalization, previous surgery, placement of a urinary catheter, placement of a CVC or arterial line, chemotherapy, and previous antibiotic use. Previous antibiotic use was also identified as an independent risk factor for ESBL-producing E. coli and Klebsiella spp.
Discussion
This literature review was conducted to summarize the available data related to HAI with GNB among pediatric patients in Middle Eastern countries. The available data indicate that GNB are prominent causes of HAIs among pediatric patients in Middle Eastern countries, though the prevalence of the different Gram-negative pathogens and resistance patterns varied geographically across pediatric patient populations and institutions.
In total, 13 of the 50 included studies characterized GNB pathogens in neonatal LOS [10,11,12,13,14, 28, 29, 40, 45, 51,52,53, 55]. GNB were prominent LOS pathogens, making up 42–73% of LOS isolates (from CAI and HAI combined) across studies [11,12,13,14, 28, 29, 40, 45, 55], and where specifically reported, 93% of HAI LOS isolates [10]. Most of the studies that assessed neonatal LOS did not distinguish between HAIs and CAIs. However, in a study that provided this information, healthcare-associated LOS accounted for 68% of confirmed LOS cases [13]. K. pneumoniae/Klebsiella spp. was consistently the most common GNB isolated from neonatal LOS, with prevalence varying from 17–58% among all culture-confirmed LOS cases where reported [11, 14, 28, 29, 45].
Across studies in Egypt set in NICUs, K. pneumoniae accounted for 36% of GNB isolates [12], and carbapenem resistance ranged from approximately 30–70% [13, 14]. Similarly, studies reporting data from PICU settings reported that Klebsiella spp. was the most commonly isolated GNB pathogen (32–37% of GNB), and up to 43% of GNB pathogens were susceptible to carbapenems [18, 19, 27]. Furthermore, studies reporting data on pediatric patients with cancer indicated that E. coli was the most common GNB isolate [23,24,25]. Among neonates admitted to NICUs in Saudi Arabia, Kuwait, and the UAE, 45% of LOS cases were caused by GNB, with Klebsiella spp. (23%) being the most common pathogen, and with 0–6% of GNB isolates being resistant both to third-generation cephalosporins and aminoglycosides [28]. A study from a PICU in Saudi Arabia similarly reported K. pneumoniae (47%) as the most commonly identified pathogen, followed by E. coli (32%), among the CRE cases [31]. Several studies assessed all HAIs in NICU or PICU settings and all observed a high prevalence of GNB, which accounted for 54–83% of infections [8, 15, 16, 19, 27]. Again, Klebsiella spp. were consistently the most common GNB isolates, accounting for 32–65% of GNB. GNB isolates were prominent in device-associated infections, making up 54% of DA-HAI in a PICU study; 97% of DA-HAI in a study set across combined NICU, PICU, and ICU settings; and 72% of CAUTI in pediatric coronary ICU patients [21, 38, 57]. Frequently isolated GNB among DA-HAI included Klebsiella spp./K. pneumoniae, Pseudomonas spp./P. aeruginosa, E. coli, and A. baumannii [21, 38, 41, 57]. Klebsiella spp. still represented the most common pathogen causing UTIs in this population [38].
Most of the studies included in the literature review reported on the antimicrobial susceptibility of isolated GNB. ESBL-producing isolates accounted for 12–30% across all isolates studied, including up to about 80% of K. pneumoniae isolates and 100% of E. coli isolates [19, 33, 38, 51, 55, 57, 58]. Considerable rates of MDR were reported in some studies [14, 18, 24, 32, 34, 36, 55], including 100% of ESBL-producing E. coli isolates from HAIs in patients ≤ 18 years of age [25, 34] and of 15 episodes of neonatal LOS caused by A. baumannii [55]. Carbapenem resistance was frequently reported [11, 12, 17, 20, 23, 25, 31, 35], with rates of up to 86%, including 72–73% of A. baumannii isolates and up to 75% of K. pneumoniae isolates [17, 20, 23]. While considerable rates of MDR were reported across some studies, A. baumannii isolates were more prominent in some instances [36, 55].
Results from this review are broadly similar to other reviews that also reported high rates of GNB causing HAIs in PICUs/NICUs, as well as high rates of MDR [59,60,61]. A literature review of bloodstream infections in Chinese neonates reported that Klebsiella spp. (42%) and E. coli (35%) were the most commonly isolated GNB [59]; both of these pathogens were highly resistant to ampicillin and cefazolin and showed varying resistance rates to third-generation cephalosporins. A systematic review of GNB in PICUs and NICUs in Latin America reported higher overall infection rates than those in more developed countries (5–37% versus 6–15%) [61]. Cultures were common in almost all countries for Klebsiella spp., P. aeruginosa, and E. coli; resistance rates to third-generation cephalosporins were 64–75%, 23–24%, and 19–40%, respectively.
Although molecular data were relatively limited, there are notable similarities between this review and a study conducted in 13 tertiary care hospitals in Saudi Arabia in 2018, where K. pneumoniae was the predominant GNB pathogen among the 440 identified CRE isolates [62]. The data from the study were not included in this review article since no pediatric-specific results were provided. However, similar to the data reviewed here, blaOXA-48 and blaNDM-1 were the most commonly identified resistance genes (84% of isolates harbored ≥ 1 of these genes), blaVIM was rare (< 1% of isolates), and blaIMP1 was not identified. In contrast, the study did not identify blaKPC, whereas this gene was identified in a small number of pediatric isolates in two of the studies reviewed here [25, 35]. Although genotyping was infrequently reported, blaOXA-48 and blaNDM-1 were the most commonly identified genotypes [25, 35, 62]. For studies reporting AMR where malignancies may or may not be identified, it should be noted that patients with cancer receive empiric broad-spectrum antibiotics in multiple courses and are at higher risk of developing AMR [63, 64].
The global burden of AMR is highest among low- and middle-income countries (LMICs) [65]. Globally in 2019, E. coli, Staphylococcus aureus, K. pneumoniae, S. pneumoniae, A. baumannii, and P. aeruginosa were responsible for 929,000 out of 1.27 million deaths attributable to AMR, as well as 3.57 million out of 4.95 million deaths associated with AMR. Of these reported deaths, the most common pathogens observed in North Africa and the Middle East were S. aureus followed by E. coli. In 2019, the burden across North Africa and the Middle East was < 50 deaths per 100,000 associated with AMR. Methicillin-resistant S. aureus was shown to have highest resistance (between 60% and 80%) in North African/Middle Eastern countries, such as Iraq and Kuwait.
The clinical impact of carbapenem resistance has become a public health problem in terms of increased mortality, longer hospital stays, and higher costs [66, 67]. The pediatric population is of particular concern, as it is a naturally vulnerable population in which risks of infection vary depending on immunological maturity, the presence of comorbidities, and the use of invasive medical devices. To monitor the spread and resistance of GNB, the adoption of local and national surveillance programs across healthcare facilities to examine the trends and health and economic outcomes associated with MDR infections has been advocated. Antimicrobial stewardship, which refers to the careful and responsible management of antimicrobial use, is a vital component of an integrated health system approach. In a systematic review of studies that assessed antimicrobial stewardship programs (ASPs) in Middle Eastern countries, all studies that described the effects of ASPs reported marked improvements in antimicrobial use, resistance rates, and patient outcomes [68]. Although the review showed that the introduction of ASPs was helping to advance healthcare systems in the Middle East, it also emphasized the region’s scarcity of data on ASPs.
In tandem with AMR surveillance programs and ICP practices, antimicrobial stewardship has the complementary aims of optimizing antimicrobial use and controlling AMR [69]. For instance, the common use of antibiotic drugs without a prescription highlights the importance of ASPs in the hospital and community settings. Antibiotic dispensing without a prescription poses a threat to public health as it leads to excessive antibiotic consumption. In a review of antimicrobial control at country, hospital, and program levels in Asia Pacific, more than half of survey respondents from India, the Philippines, Saudi Arabia, Sri Lanka, Taiwan, Thailand, and Vietnam reported the ability to obtain antibiotics without a prescription [70]. Across the Middle East, prevalence of antibiotic misuse by self-medication was reported as high as 85% in Syria [71]. Similar rates of antibiotic self-medication were also reported for Lebanon (32–42%), Jordan (32–62%), Iran (up to 58%), United Arab Emirates (56%), Tunisia (20%), Yemen (60%), and Sudan (74% including misuse of either antibiotics or antimalarials). The roles of healthcare professionals, and their responsibilities to engage in effective communication and continuing education, are thus essential to promote the appropriate use of antimicrobials. The importance of relevant training and clear guidelines regarding the management of MDR bacteria was highlighted in a study that surveyed Saudi Arabia hospital healthcare workers. Overall, only 56% of the survey participants recognized carbapenem was highly recommended for treating infection related to ESBL-producing bacteria, 39% recognized the institution had a written policy regarding ESBL spread, and 43% agreed that ESBL-producing bacteria require isolation in the hospital [72].
A lack of sufficient data in some countries may cause gaps in the knowledge pertaining to GNB infections and AMR among pediatric patients in the Middle East. The gap may be more prevalent in low- and low-to-middle-income countries, where the number of laboratories capable of evaluating AMR is limited due to cost, infrastructure, or other considerations [73]. Limited data availability may also be attributable to inaccurate laboratory assessments of community-acquired antimicrobial-resistant infections, where analyses were performed without accurate clinical details or at inappropriate timing after collection of blood cultures [73]. Further complicating the issue, obtaining accurate data may be affected by trends in GNB and AMR constantly changing over time; a study conducted in Istanbul, Turkey, assessing data from 2012 through 2015 highlighted the notable change in AMR patterns among GNB isolates over only a 4-year period, emphasizing the need for timely regional surveillance data to inform effective infection control measures [74]. Therefore, sustained national and local surveillance efforts are required to track resistance in different settings over time, detect new resistance types, and reveal the underlying determinants of resistance in different microorganisms. Moreover, gaps in policy between evidence-based guidelines and regional clinical surveillance to monitor changes in AMR patterns have also been identified [73]. The gaps in knowledge identified herein may help guide future investigators in planning and carrying out research studies.
Another consideration to note is that some countries in the region are facing issues with immigrants, poverty, and poor living conditions. These issues greatly facilitate the dissemination of resistance in all environments. For instance, the impact on AMR rates from expatriate workers in GCC countries is highlighted by a 2-year study conducted in Kuwait evaluating antibiotic resistance among food handlers, where a majority of workers (86%) were non-Arab [75]. Moreover, countries with war-wounded civilians such as Syria and Iraq have become a major source of MDR bacteria that have spread not only to neighboring countries, but also globally [76, 77]. The protracted Syrian conflict has led to one of the largest global refugee crises, with 5.6 million refugees mainly fleeing to neighboring countries such as Turkey, Lebanon, and Jordan [78]. Evidence from countries affected by military conflict has shown that conflict promotes inappropriate surveillance of antimicrobial use and resistance, which therefore encourages conditions for AMR to develop within refugees and spread throughout host countries [78]. For instance, high rates of AMR have been detected in Syrian refugees, and drug-resistant M. tuberculosis was isolated from a refugee in Lebanon [78]. Furthermore, carriage of potential pathogens and AMR was identified in Syrian asylum seekers in Italy, whereas high prevalence of methicillin-resistant S. aureus was found among refugees in Finland [78]. Similar evidence was reported in Lebanon, where MDR bacteria were isolated from wastewater in Syrian refugee camps, with 53% of E. coli isolates testing positive for resistant genes [78].
This literature review has several limitations. More than half (31/50) of the included studies were from Saudi Arabia or Egypt; only 3 or fewer studies per country were included from Oman, Qatar, Lebanon, and Jordan; and no studies from Iraq or Bahrain were identified that met the inclusion criteria. Some Middle Eastern countries were not included because of concerns over data availability and robustness in those countries. Other limitations include the heterogeneity of data due to variable study designs; lack of standardized AST, analyzing, and reporting; small sample sizes in many of the studies reviewed; and many studies lacking relevant information such as minimum inhibitory concentrations. However, in studies where AST was performed according to corresponding international standards, the rate of resistance was substantial. In addition, many lab-based surveillance systems are not linked to patient diagnoses or outcomes, and there are frequent shortages of diagnostics and reagents. Some of the reports discussed herein reflected data from a single center, while others reported data from multicenter or multiregional studies. Additionally, the included studies used widely variable definitions related to HAIs, AMR, and early-onset versus late-onset period infections. In general, data collected from single-center studies are more likely to report elevated resistance rates than network studies. In some studies, HAIs were defined as infections first identified ≥ 24 h after hospital admission [31], whereas a timeframe of ≥ 48 h after admission was used in others [8, 10, 14, 16], with infections manifesting ≥ 48 h after admission or within 30 days after discharge being the more widely accepted standard [79]. Furthermore, several of the included articles did not specifically define HAIs. Additionally, the definition of MDR also varied considerably among the included studies, with resistance to ≥ 1, ≥ 2, or ≥ 3 antimicrobial classes being used to define MDR [13, 14, 38]. Finally, many of the studies that assessed neonatal LOS did not differentiate between HAIs and CAIs. Although a study that provided this information reported a prevalence of 68% for hospital-acquired LOS among confirmed LOS cases [13], this percentage could vary considerably among studies.
Conclusions
Despite the lack of representation of some countries, our review of the literature indicates that AMR GNB are a prominent cause of pediatric HAIs in the Middle East, highlighting an urgent need for increased adoption of infection control practices and ASPs across pediatric healthcare settings. Furthermore, the awareness of prevalence and resistant patterns is essential for guideline development to minimize the risk of MDR GNB infections in pediatric patients. The information presented in this review and our proposed solutions to address knowledge gaps, including sustained national and local surveillance efforts, may help guide future investigators in planning studies appropriately.
References
Kaye KS, Pogue JM. Infections caused by resistant Gram-negative bacteria: epidemiology and management. Pharmacotherapy. 2015;35:949–62.
Dandachi I, Chaddad A, Hanna J, Matta J, Daoud Z. Understanding the epidemiology of multi-drug resistant gram-negative bacilli in the Middle East using a One Health Approach. Front Microbiol. 2019;10:1941 (Epub).
World Health Organization. Global action plan on antimicrobial resistance. Geneva, Switzerland, 2015. https://www.who.int/publications/i/item/9789241509763. Accessed 29 Aug 2022.
Breijyeh Z, Jubeh B, Karaman R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25:1340 (Epub).
World Health Organization. WHO publishes list of bacteria for which new antibiotics are urgently needed. News release. February 27, 2017. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. Accessed 29 Aug 2022.
Centers for Disease Control and Prevention. Gram-negative bacteria infections in healthcare settings. January 17, 2011. https://www.cdc.gov/hai/organisms/gram-negative-bacteria.html. Accessed 29 Aug 2022.
Mehrad B, Clark NM, Zhanel GG, Lynch JP 3rd. Antimicrobial resistance in hospital-acquired Gram-negative bacterial infections. Chest. 2015;147:1413–21.
Gadallah MA, Aboul Fotouh AM, Habil IS, Imam SS, Wassef G. Surveillance of health care-associated infections in a tertiary hospital neonatal intensive care unit in Egypt: 1-year follow-up. Am J Infect Control. 2014;42:1207–11.
World Health Organization. Report on the burden of endemic health care-associated infection worldwide. Geneva, Switzerland, 2011. https://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.pdf. Accessed 29 Aug 2022.
Abd-Elgawad M, Eldegla H, Khashaba M, Nasef N. Oropharyngeal administration of mother’s milk prior to gavage feeding in preterm infants: a pilot randomized control trial. JPEN J Parenter Enteral Nutr. 2020;44:92–104.
Ghaith DM, Zafer MM, Said HM, et al. Genetic diversity of carbapenem-resistant Klebsiella pneumoniae causing neonatal sepsis in intensive care unit, Cairo, Egypt. Eur J Clin Microbiol Infect Dis. 2020;39:583–91.
Nour I, Eldegla HE, Nasef N, et al. Risk factors and clinical outcomes for carbapenem-resistant Gram-negative late-onset sepsis in a neonatal intensive care unit. J Hosp Infect. 2017;97:52–8.
Awad HA, Mohamed MH, Badran NF, Mohsen M, Abd-Elrhman AS. Multidrug-resistant organisms in neonatal sepsis in two tertiary neonatal ICUs, Egypt. J Egypt Public Health Assoc. 2016;91:31–8.
Shehab El-Din EM, El-Sokkary MM, Bassiouny MR, Hassan R. Epidemiology of neonatal sepsis and implicated pathogens: a study from Egypt. Biomed Res Int. 2015;2015: 509484.
El-Feky EA, Saleh DA, El-Kholy J, et al. Use of personal digital assistants to detect healthcare-associated infections in a neonatal intensive care unit in Egypt. J Infect Dev Ctries. 2016;10:1250–7.
Abdel-Wahab F, Ghoneim M, Khashaba M, El-Gilany AH, Abdel-Hady D. Nosocomial infection surveillance in an Egyptian neonatal intensive care unit. J Hosp Infect. 2013;83:196–9.
Sultan AM, Seliem WA. Identifying risk factors for healthcare-associated infections caused by carbapenem-resistant Acinetobacter baumannii in a neonatal intensive care unit. Sultan Qaboos Univ Med J. 2018;18:e75–80.
Labib JR, Ibrahim SK, Salem MR, Youssef MRL, Meligy B. Infection with gram-negative bacteria among children in a tertiary pediatric hospital in Egypt. Am J Infect Control. 2018;46:798–801.
El-Nawawy A, Ashraf GA, Antonios MAM, Meheissen MA, El-Alfy MMR. Incidence of multidrug-resistant organism among children admitted to pediatric intensive care unit in a developing country. Microb Drug Resist. 2018;24:1198–206.
Khairy RMM, Mahmoud MS, Shady RR, Esmail MAM. Multidrug-resistant Klebsiella pneumoniae in hospital-acquired infections: concomitant analysis of antimicrobial resistant strains. Int J Clin Pract. 2020;74: e13463.
El-Kholy A, Saied T, Gaber M, et al. Device-associated nosocomial infection rates in intensive care units at Cairo University hospitals: first step toward initiating surveillance programs in a resource-limited country. Am J Infect Control. 2012;40:e216–20.
Mansour SA, Eldaly O, Jiman-Fatani A, Mohamed ML, Ibrahim EM. Epidemiological characterization of P. aeruginosa isolates of intensive care units in Egypt and Saudi Arabia. East Mediterr Health J. 2013;19:71–80.
Elseady NSM, Khamis N, AbdelGhani S, et al. Antibiotic sensitivity/resistance pattern of hospital acquired blood stream infection in children cancer patients: a retrospective study. Int J Clin Pract. 2021;75: e14617.
Youssef A, Hafez H, Madney Y, et al. Incidence, risk factors, and outcome of blood stream infections during the first 100 days post-pediatric allogeneic and autologous hematopoietic stem cell transplantations. Pediatr Transpl. 2020;24: e13610.
Kamel NA, El-Tayeb WN, El-Ansary MR, Mansour MT, Aboshanab KM. Phenotypic screening and molecular characterization of carbapenemase-producing gram-negative bacilli recovered from febrile neutropenic pediatric cancer patients in Egypt. PLoS ONE. 2018;13: e0202119.
El Far MY, El-Mahallawy HA, Attia AS. Tracing the dissemination of the international clones of multidrug-resistant Acinetobacter baumannii among cancer patients in Egypt using the PCR-based open reading frame typing (POT) method. J Glob Antimicrob Resist. 2019;19:210–5.
El-Sahrigy SAF, Shouman MG, Ibrahim HM, et al. Prevalence and anti-microbial susceptibility of hospital acquired infections in two pediatric intensive care units in Egypt. Open Access Maced J Med Sci. 2019;7:1744–9.
Hammoud MS, Al-Taiar A, Al-Abdi SY, et al. Late-onset neonatal sepsis in Arab states in the Gulf region: two-year prospective study. Int J Infect Dis. 2017;55:125–30.
Al-Matary A, Heena H, AlSarheed AS, et al. Characteristics of neonatal sepsis at a tertiary care hospital in Saudi Arabia. J Infect Public Health. 2019;12:666–72.
Somily AM, Alsubaie SS, BinSaeed AA, et al. Extended-spectrum β-lactamase-producing Klebsiella pneumoniae in the neonatal intensive care unit: does vancomycin play a role? Am J Infect Control. 2014;42:277–82.
Alzomor OA, Alfawaz TS, Abu-Shaheen A, Alshehri MA, Al SD. A matched case-control study to assess the carbapenem-resistant Enterobacteriaceae infections among hospitalized children at King Fahad Medical City, Riyadh, Saudi Arabia. Saudi Med J. 2019;40:1105–10.
Balkhy HH, Bawazeer MS, Kattan RF, et al. Epidemiology of Acinetobacter spp.-associated healthcare infections and colonization among children at a tertiary-care hospital in Saudi Arabia: a 6-year retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2012;31:2645–51.
Al-Garni SM, Ghonaim MM, Ahmed MMM, Al-Ghamdi AS, Ganai FA. Risk factors and molecular features of extended-spectrum beta-lactamase producing bacteria at southwest of Saudi Arabia. Saudi Med J. 2018;39:1186–94.
Yasir M, Ajlan AM, Shakil S, et al. Molecular characterization, antimicrobial resistance and clinico-bioinformatics approaches to address the problem of extended-spectrum β-lactamase-producing Escherichia coli in western Saudi Arabia. Sci Rep. 2018;8:14847.
Khan MA, Mohamed AM, Faiz A, Ahmad J. Enterobacterial infection in Saudi Arabia: first record of Klebsiella pneumoniae with triple carbapenemase genes resistance. J Infect Dev Ctries. 2019;13:334–41.
Ibrahim ME. High antimicrobial resistant rates among gram-negative pathogens in intensive care units. A retrospective study at a tertiary care hospital in southwest Saudi Arabia. Saudi Med J. 2018;39:1035–43.
Alyami AM, Kaabia NM, AlQasim MA, et al. Chryseobacterium/Elizabethkingia species infections in Saudi Arabia. Saudi Med J. 2020;41:309–13.
Kabbani MS, Ismail SR, Fatima A, et al. Urinary tract infection in children after cardiac surgery: incidence, causes, risk factors and outcomes in a single-center study. J Infect Public Health. 2016;9:600–10.
Al-Tawil ES, Almuhareb AM, Amin HM. Catheter-related blood stream infection in patients receiving long-term home parenteral nutrition: tertiary care hospital experience in Saudi Arabia. Saudi J Gastroenterol. 2016;22:304–8.
Hammoud MS, Al-Taiar A, Thalib L, et al. Incidence, aetiology and resistance of late-onset neonatal sepsis: a five-year prospective study. J Paediatr Child Health. 2012;48:604–9.
Al-Mousa HH, Omar AA, Rosenthal VD, et al. Device-associated infection rates, bacterial resistance, length of stay, and mortality in Kuwait: International Nosocomial Infection Consortium findings. Am J Infect Control. 2016;44:444–9.
Jamal WY, Albert MJ, Rotimi VO. High prevalence of New Delhi metallo-β-lactamase-1 (NDM-1) producers among carbapenem-resistant Enterobacteriaceae in Kuwait. PLoS ONE. 2016;11: e0152638.
Jamal WY, Albert MJ, Khodakhast F, Poirel L, Rotimi VO. Emergence of new sequence type OXA-48 carbapenemase-producing Enterobacteriaceae in Kuwait. Microb Drug Resist. 2015;21:329–34.
Jamal W, Rotimi VO, Albert MJ, et al. High prevalence of VIM-4 and NDM-1 metallo-β-lactamase among carbapenem-resistant Enterobacteriaceae. J Med Microbiol. 2013;62:1239–44.
Abdellatif M, Al-Khabori M, Ur Rahman A, et al. Outcome of late-onset neonatal sepsis at a tertiary hospital in Oman. Oman Med J. 2019;34:302–7.
Al Habsi THA, Al-Lamki RNA, Mabruk M. Antibiotic susceptibility pattern of bacterial isolates from wound infections among patients attending a tertiary care hospital in Oman. Biomed Pharmacol J. 2020;13:2069–80.
Al Riyami Z, Al GM. Bacterial infections and antimicrobial use in pediatric cardiac intensive care unit at Royal Hospital [abstract]. Oman Med J. 2018;33:58.
Khan FY, Abu-Khattab M, Almaslamani EA, et al. Acute bacterial meningitis in Qatar: a hospital-based study from 2009 to 2013. Biomed Res Int. 2017;2017:2975610.
Sid Ahmed MA, Hassan AAI, Abu Jarir S, et al. Emergence of multidrug- and pandrug-resistant Pseudomonas aeruginosa from five hospitals in Qatar. Infect Prev Pract. 2019;1: 100027.
Hasan MR, Sundaram MS, Sundararaju S, et al. Unusual accumulation of a wide array of antimicrobial resistance mechanisms in a patient with cytomegalovirus-associated hemophagocytic lymphohistiocytosis: a case report. BMC Infect Dis. 2020;20:237.
Khan A. Pattern of neonatal sepsis in Dubai hospital [abstract]. J Matern-Fetal Neonatal Med. 2016;29:153.
Bystricka A, Khan J, Abu Asbeh J. Identification of bacterial pathogens and their antimicrobial susceptibility of early onset neonatal sepsis [abstract 133]. J Matern-Fetal Neonatal Med. 2014;27:172–3.
Dash N, Al Zarouni M, Panigrahi D. Bacteriological profile and susceptibility pattern of neonatal blood stream infections [abstract]. Int J Infect Dis. 2012;16:e215–6.
Hamici S. Infection pattern and outcome among febrile neutropenic children with acute lymphoblastic leukemia in the hematology oncology department of Dubai hospital in the past 2 years [abstract]. Eur J Pediatr. 2016;175:1763.
Yusef D, Shalakhti T, Awad S, Algharaibeh H, Khasawneh W. Clinical characteristics and epidemiology of sepsis in the neonatal intensive care unit in the era of multi-drug resistant organisms: a retrospective review. Pediatr Neonatol. 2018;59:35–41.
Khassawneh MY, Khriesat WM, Saqan RM, Hayajneh WA. Resistant bacteria cause urinary tract infection in graduates of neonatal unit. J Pediatr Infect Dis. 2013;8:87–91.
Ismail A, El-Hage-Sleiman AK, Majdalani M, et al. Device-associated infections in the pediatric intensive care unit at the American University of Beirut Medical Center. J Infect Dev Ctries. 2016;10:554–62.
Hanna-Wakim RH, Ghanem ST, El Helou MW, et al. Epidemiology and characteristics of urinary tract infections in children and adolescents. Front Cell Infect Microbiol. 2015;5:45 (Epub).
Wang J, Zhang H, Yan J, Zhang T. Literature review on the distribution characteristics and antimicrobial resistance of bacterial pathogens in neonatal sepsis. J Matern Fetal Neonatal Med. 2022;35:861–70.
Tzialla C, Borghesi A, Pozzi M, Stronati M. Neonatal infections due to multi-resistant strains: epidemiology, current treatment, emerging therapeutic approaches and prevention. Clin Chim Acta. 2015;451:71–7.
Berezin EN, Solórzano F. Gram-negative infections in pediatric and neonatal intensive care units of Latin America. J Infect Dev Ctries. 2014;8:942–53.
Al-Abdely H, AlHababi R, Dada HM, et al. Molecular characterization of carbapenem-resistant Enterobacterales in thirteen tertiary care hospitals in Saudi Arabia. Ann Saudi Med. 2021;41:63–70.
Worku M, Belay G, Tigabu A. Bacterial profile and antimicrobial susceptibility patterns in cancer patients. PLoS ONE. 2022;17: e0266919.
Nanayakkara AK, Boucher HW, Fowler VG Jr, et al. Antibiotic resistance in the patient with cancer: escalating challenges and paths forward. CA Cancer J Clin. 2021;71:488–504.
Antimicrobial RC. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–55.
Kousouli E, Zarkotou O, Polimeri K, Themeli-Digalaki K, Pournaras S. Impact of bloodstream infections caused by carbapenem-resistant Gram-negative pathogens on ICU costs, mortality and length of stay. Infect Prev Pract. 2019;1: 100020.
Zhou R, Fang X, Zhang J, et al. Impact of carbapenem resistance on mortality in patients infected with Enterobacteriaceae: a systematic review and meta-analysis. BMJ Open. 2021;11: e054971.
Ababneh MA, Nasser SA, Rababa’h AM. A systematic review of Antimicrobial Stewardship Program implementation in Middle Eastern countries. Int J Infect Dis. 2021;105:746–52.
World Health Organization. Antimicrobial Stewardship Programmes in health-care facilities in low- and middle-income countries. A practical toolkit. Geneva, 2019. https://apps.who.int/iris/handle/10665/329404. Accessed 29 Aug 2022.
Lee TH, Lye DC, Chung DR, et al. Antimicrobial stewardship capacity and manpower needs in the Asia Pacific. J Glob Antimicrob Resist. 2021;24:387–94.
Khalifeh MM, Moore ND, Salameh PR. Self-medication misuse in the Middle East: a systematic literature review. Pharmacol Res Perspect. 2017;5:e00323.
Aldrazi FA, Rabaan AA, Alsuliman SA, et al. ESBL expression and antibiotic resistance patterns in a hospital in Saudi Arabia: do healthcare staff have the whole picture? J Infect Public Health. 2020;13:759–66.
Le Doare K, Bielicki J, Heath PT, Sharland M. Systematic review of antibiotic resistance rates among gram-negative bacteria in children with sepsis in resource-limited countries. J Pediatr Infect Dis Soc. 2015;4:11–20.
Durdu B, Kritsotakis EI, Lee ACK, et al. Temporal trends and patterns in antimicrobial-resistant gram-negative bacteria implicated in intensive care unit-acquired infections: a cohort-based surveillance study in Istanbul, Turkey. J Glob Antimicrob Resist. 2018;14:190–6.
Moghnia OH, Rotimi VO, Al-Sweih NA. Monitoring antibiotic resistance profiles of faecal isolates of Enterobacteriaceae and the prevalence of carbapenem-resistant isolates among food handlers in Kuwait. J Glob Antimicrob Resist. 2021;25:370–6.
Yaacoub S, Truppa C, Pedersen TI, Abdo H, Rossi R. Antibiotic resistance among bacteria isolated from war-wounded patients at the Weapon Traumatology Training Center of the International Committee of the Red Cross from 2016 to 2019: a secondary analysis of WHONET surveillance data. BMC Infect Dis. 2022;22:1–11.
Jakovljevic M, Al Ahdab S, Jurisevic M, Mouselli S. Antibiotic resistance in Syria: a local problem turns into a global threat. Front Public Health. 2018;6:212.
Osman M, Rafei R, Ismail MB, et al. Antimicrobial resistance in the protracted Syrian conflict: halting a war in the war. Future Microbiol. 2021;16:825–45.
Alothman A, Al Thaqafi A, Al Ansary A, et al. Prevalence of infections and antimicrobial use in the acute-care hospital setting in the Middle East: results from the first point-prevalence survey in the region. Int J Infect Dis. 2020;101:249–58.
Acknowledgements
Funding
This study was sponsored by Pfizer Inc. Additionally, Pfizer Inc funded all manuscript submission fees.
Medical Writing and/or Editorial Assistance
The authors would like to thank Ayman Kurdi, formerly an employee of Pfizer Inc, for his thoughtful insights and early review of the manuscript. Editorial/medical writing support was provided by Erin O’Keefe, PhD, Kandyss Najjar, PhD, and Philippa Jack, PhD, of ICON (Blue Bell, PA) and was funded by Pfizer Inc.
Author Contributions
Conceptualization: Mohammad Alghounaim, Ghassan Dbaibo, Ashraf Hassanien Methodology: Mohammad Alghounaim, Ghassan Dbaibo, Ashraf Hassanien. Formal analysis and investigation: Ghassan Dbaibo. Writing—review and editing: Mona Al Dabbagh, Mohammad Alghounaim, Rana H. Almaghrabi, Ghassan Dbaibo, Ghassan Ghatasheh, Hanan M. Ibrahim, Mohamed Abdel Aziz, Ashraf Hassanien, Naglaa Mohamed. Funding acquisition: Ashraf Hassanien. Resources: Ghassan Dbaibo. Supervision: Ghassan Dbaibo.
Disclosures
Mohamed Abdel Aziz, Ashraf Hassanien, and Naglaa Mohamed are employees of Pfizer Inc and may hold stock or stock options. Ghassan Dbaibo has received institution grants and honoraria for presentations and advisory boards from Pfizer Inc, MSD, Sanofi Pasteur, and Abbott Laboratories. Mona Al Dabbagh has received honoraria for presentations from MSD and Sanofi Pasteur. Rana H. Almaghrabi, Ghassan Ghatasheh, and Hanan M. Ibrahim declare that they have no competing interests.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Al Dabbagh, M., Alghounaim, M., Almaghrabi, R.H. et al. A Narrative Review of Healthcare-Associated Gram-Negative Infections Among Pediatric Patients in Middle Eastern Countries. Infect Dis Ther 12, 1217–1235 (2023). https://doi.org/10.1007/s40121-023-00799-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00799-w