Abstract
Introduction
Clostridioides difficile infection (CDI) is a recognized global threat especially for vulnerable populations. It is of particular concern to healthcare providers as it is found in both hospital and community settings, with severe courses, frequent recurrence, high mortality and substantial financial impact on the healthcare system. The CDI burden in Germany has been described and compared by analysing data from four different public databases.
Methods
Data on hospital burden of CDI have been extracted, compared, and discussed from four public databases for the years 2010–2019. Hospital days due to CDI were compared to established vaccine preventable diseases, such as influenza and herpes zoster, and also to CDI hospitalisations in the United States (US).
Results
All four databases reported comparable incidences and trends. Beginning in 2010, population-based hospitalised CDI incidence increased to a peak of > 137/100,000 in 2013. Then, incidence declined to 81/100,000 in 2019. Hospitalised patients with CDI were predominantly > 50 years of age. The population-based incidence of severe CDI was between 1.4 and 8.4/100,000 per year. Recurrence rates were between 5.9 to 6.5%. More than 1,000 CDI deaths occurred each year, with a peak of 2,666 deaths in 2015. Cumulative CDI patient days (PD) were between 204,596 and 355,466 each year, which exceeded cumulated PD for influenza and herpes zoster in most years, though year-to-year differences were observed. Finally, hospitalized CDI incidence was higher in Germany than in the US, where the disease is well recognized as a public health threat.
Conclusions
All four public sources documented a decline in CDI cases since 2013, but the disease burden remains substantial and warrants continued attention as a severe public health challenge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
CDI poses a significant health risk globally. CDI surveillance at national and regional level is a powerful tool to improve the understanding of CDI epidemiology and to support disease prevention and control. |
In Germany, four public surveillance systems publish their results independently utilizing different methodologies. |
What was learned from the study? |
This is the first analysis providing a comprehensive overview of data from these sources and describing the CDI burden on a national level over a 10-year period. |
The four systems yielded similar population-based incidences and trends over time, indirectly confirming their validity. |
The documented burden of CDI on the German healthcare system is large, with high numbers of hospitalizations, severe cases, recurrences, and deaths. |
No public surveillance for community-treated CDI exists in Germany, and it is likely that CDI in Germany is still underreported. |
Introduction
Clostridioides difficile (formerly Clostridium difficile, C. difficile) is a ubiquitous, anaerobic, Gram-positive spore-forming bacterium. About 5–10% of healthy persons are C. difficile carriers, but, under certain conditions, toxigenic strains can lead to C. difficile infection (CDI). Two clostridial toxins primarily mediate pathogenicity: toxin A and toxin B [1]. Of note, some strains such as ribotype 027 (RT027) may express an additional toxin (CDT, binary toxin, gene: cdtAB) which may lead to more severe courses of disease. RT027 has also been the cause for rising CDI incidence in several countries, including Germany [2]. Multidrug resistance in RT027 and other nosocomial strains is common, and might have facilitated its spread [3]. In contrast, between 2015 and 2017, new guidelines for infection prevention and hygiene in the hospital setting have been implemented in Germany and new treatment options became available. These measures can cause a reduction of CDI incidence [4,5,6,7].
Clinical symptoms of CDI range from mild, self-limiting diarrhoea to fulminant colitis, and can include pseudomembranous colitis, toxic megacolon, bowel perforation, sepsis, and multiple organ dysfunction syndrome [1]. Known risk factors for CDI include antibiotic exposure, previous hospitalisation, defined underlying diseases, and advanced age [8]. In developed countries, C. difficile is considered the main infectious cause of antibiotic-associated diarrhoea [1].
CDI poses a significant health risk for vulnerable populations and is of particular concern to healthcare providers due to severe disease outcomes, frequent recurrence, high mortality, and associated financial impact on the healthcare system. CDI surveillance at national and regional level is therefore required to improve our understanding of CDI epidemiology and to support efficient allocation of healthcare resources for disease prevention and control [9].
In Germany, three different nation- or statewide public surveillance systems and one database provide information on hospitalised CDI cases (Table 1). These individual surveillance systems publish their results separately, and, until today, no comparisons between them are available. In this analysis, data from these sources are compared and discussed to provide physicians and healthcare decision-makers with a comprehensive overview of the overall burden of CDI in German hospitals. To our knowledge, this is the first study describing the burden of CDI on a nationwide level in Germany.
Overall hospitalizations, population-based incidences, disease severity, recurrence rates and mortalities are described over a period of 10 years. In addition, CDI burden is compared to those of herpes zoster and influenza, two other infectious diseases that have a high prevalence in the elderly population. To put the German burden into a global perspective, it is compared to data from the Emerging Infections Program (EIP) of the United States Centers for Disease Control and Prevention (CDC); C. difficile has been classified as an urgent public health threat in the United States (US) [10].
Methods
Data Sources
No ethical approval was required for this study as data were extracted from public sources. Its collection was conducted as part of the routine surveillance and health data monitoring in accordance with German and European law and regulations. All public databases were queried during June/July 2021 for the period from 2010 to 2019. Data from the CDC EIP were available only from 2012 to 2019. The COVID-19 pandemic and associated public health interventions led to a dramatic decrease in the number of reported cases for most infectious diseases in Germany in and after 2020. Regarding CDI, the difference between expected and reported case numbers was over 30% and it remains unclear to what degree this reduction reflects a true decline or is the result of fewer diagnoses due to social distancing and inadequate reporting [11]. To obtain a representative evaluation, data from 2020 and later were therefore not included in our analysis.
CDI Diagnosis in Germany
All CDI cases recorded in the four public databases were identified in the context of routine clinical work-up following recommendations in clinical guidelines. According to the recent ECCMID guideline, there is no unique and simple way to obtain a diagnosis for CDI. The combination of clinical signs and a two-step testing algorithm remains the recommended gold standard [12]. The German clinical guidelines on gastrointestinal infections recommend prompt testing in all patients that present with diarrhoea or elevated risk for CDI. The recommended first step of the testing algorithm is an immunoblot to directly detect glutamate dehydrogenase in the stool sample. A positive result should then be confirmed by testing for toxins A and B [13].
Robert Koch-Institute (RKI)
Nationwide mandatory reporting is limited to severe hospitalised CDI cases based on the German Federal Infection Protection Act. Physicians report cases to the RKI’s infection surveillance database. Severe CDI is defined as:
-
The patient is admitted to a medical facility for treatment of a community-onset CDI,
-
The patient is admitted to an intensive care unit for treatment of CDI or its complications,
-
Surgical intervention, e.g. colectomy, is performed due to megacolon, perforation or refractory colitis; or
-
The patient dies within 30 days of being diagnosed with CDI and the infection is considered a direct cause of death or a contributing cause of death.
In addition, all reported cases must be laboratory-confirmed.
The first criterion was modified in 2016: previously, only a hospital re-admission due to recurrent CDI was reported. The data from the RKI online database Survstat@RKI [14] were queried stratified by patient age, and the respective RKI annual reports [15] were consulted for the number of CDI-associated deaths.
Landesuntersuchungsanstalt (LUA) Saxony
Hospitalised and non-hospitalised CDI cases in Saxony (approximately 4.1 million inhabitants in 2019) must be reported by the attending physician to the public health offices, as mandated in the federal state’s Infection Protection Act. Subsequently, they are reviewed to differentiate between severe and mild or moderate symptomatic cases using a short physician questionnaire, and the data are then transferred to the State of Saxony's Health and Veterinary Research Institute. All cases must be laboratory-confirmed. CDI cases and related deaths were extracted from the yearly reports published on the LUA homepage [16].
Clostridioides Difficile-Associated Disease Krankenhaus-Infektions-Surveillance-System (CDAD-KISS)
CDAD-KISS is a voluntary surveillance program that included 572 German hospitals in 2019, covering approximately 43% of all patient-days in Germany. All hospitalised CDI cases are recorded. A laboratory confirmation is not explicitly required for the reporting, but CDAD cases must fulfil one or more of three criteria:
-
Diarrhoea or toxic megacolon and either culture-based detection of toxigenic C. difficile or detection of its toxins and/or toxin genes (e.g. through ELISA or PCR) in a stool sample;
-
Endoscopic detection of pseudomembranous colitis;
-
Histopathologic detection of CDI (even without diarrhoea) by endoscopy, colectomy or autopsy.
A CDAD case is defined as recurrent if symptoms disappeared for more than 1 week and a new CDAD case occurs within 8 weeks after symptom resolution. The definition for a severe case in CDAD-KISS is identical to that used by RKI. Data on total (2010–2019), severe (2016–2019) and recurrent cases (2019) were extracted from the yearly reports published on the CDAD-KISS homepage [17].
Gesundheitsberichterstattung des Bundes (GBE-Bund)
The diagnoses of all discharged inpatients are recorded by the German Federal Statistical Office in an annual survey in the GBE-Bund database. It contains administrative claims based on International Classification of Disease, Tenth Revision (ICD-10) coded discharge diagnoses. The number of hospitalised patients with a main diagnosis of CDI, their average length of stay, and the number of CDI-related deaths are accessible online; secondary diagnoses or ICD-10 subcategories can be obtained through request to the German Federal Statistical Office [18]. Our queries included main and secondary CDI-related ICD-10 codes A04.7 (enterocolitis due to C. difficile) and U69.40! (recurrent C. difficile infection; only available 2016–2019) stratified by age. To investigate severe cases, the following subcategories were requested for 2016–2019 A04.71 (“without megacolon, with organ complication”), A04.72 (“with megacolon, without organ complication”) or A04.73 (“with megacolon, with organ complication”). Similar queries were conducted for patients with seasonal and zoonotic/pandemic influenza (ICD-10 J09 and J10) and herpes zoster (ICD-10 B02). GBE-Bund also provides cause of death statistics by collecting mandatory data from official death certificates.
Emerging Infections Program (EIP)
The EIP of the United States Centers for Disease Control and Prevention (CDC) is a population- and laboratory-based CDI surveillance system that records hospitalised and non-hospitalised cases. In 2019, EIP covered 35 counties in 10 states with a total of > 12 million inhabitants. Hospitalisation status is determined by medical records review for hospitalisation within 7 days of laboratory diagnosis. Annual reports of CDI cases and incidences are available for 2012–2019 on the EIP homepage [19]. The number of total US hospital admissions (2016–2019) was taken from the CDC Healthcare-associated Infections (HAI) progress report data tables published on the CDC HAI homepage [20].
Data Evaluation
RKI
To allow comparison between the different data sources, population-based hospitalised severe CDI incidence (number of CDI hospitalised severe cases/100,000 population per year) was calculated. To describe the demographic characteristics of CDI, cases were aggregated into age groups in 5-year increments between the ages 50 and ≥ 85 years. Overall and age-specific incidence was calculated for each age cohort obtained from the German Federal Statistical Office [23].
LUA
Population-based CDI incidence (number of CDI cases/100,000 population per year) was calculated using the average annual German population of Saxony for the respective years [23].
CDAD-KISS
Since not all hospitals participate in CDAD-KISS, to derive estimates of the population-based hospitalized CDI incidence, the reported CDI cases were extrapolated nationwide. As the total number of patients treated in CDAD-KISS hospitals is reported yearly (i.e. 2017: 9,490,100), the annual CDAD-KISS market share was calculated based on GBE-Bund total German hospital admissions (i.e. 2017: 19,952,735). Nationwide CDI case numbers were extrapolated using the CDAD-KISS market share (i.e. 2017: 47.6%) [23].
GBE-Bund
Population-based hospitalised CDI incidence was calculated using the average annual German population [23]. To determine the CDI recurrence rate, the number of primary CDI cases was calculated as the sum of main and secondary diagnoses A04.7 per year minus the number of recurrences that are coded as secondary diagnoses (U69.40!) in the same year. Case aggregation into age groups and calculation of age-specific incidence were conducted as described for RKI data. For mortality, the official German cause of death statistics based on physician-issued death certificates and the ICD code A04.7 as primary cause of death was counted. Deaths with secondary diagnosis of CDI are not recorded in the death statistics. The mortality statistics include nosocomial and community-acquired CDI cases.
EIP
For calculation of population-based hospitalised CDI incidence, the yearly population estimates from the United States Census Bureau were used [24]. The US CDI hospitalisation rate is available in the EIP 2017 and 2019 reports, Tables 4 [19] and A1, respectively [25]. Aggregation over all CDI cases and age groups results in a proportion of 51% of CDI cases hospitalised in 2017 and of 50% in 2019. The 2018 report directly states a proportion of 47% of treated CDI cases that either required hospitalisation for their CDI or were already hospitalised at the time of their CDI diagnosis. To extrapolate the proportion of hospitalised CDI from 2012–2016, the average of 49% was used.
Results
CDI Case Numbers and Incidences
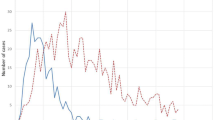
In 2010, GBE-Bund recorded 86,510 hospitalised CDI cases (main and secondary diagnoses A04.7). Case numbers increased to > 100,000 hospitalised cases per year 2012–2015, reaching a peak in 2013 with 110,961 cases. Starting in 2016, the case numbers steadily declined to 67,247 in 2019. The GBE-Bund population-based hospitalised CDI incidence increased from 105.8 cases/100,000 in 2010 to a peak at 137.6/100,000 in 2013. From 2015, population-based hospitalised CDI incidence steadily declined to 80.9/100,000 in 2019 (Fig. 1). Similar trends were observed in CDAD-KISS and LUA (Fig. 1). As RKI data include severe cases only, reported cases and incidence were 30- to 200-fold lower. However, in contrast to the other surveillance systems, the population-based incidence of severe cases in RKI continued to increase until 2018. The population-based hospitalised CDI incidence for 2019 from GBE-Bund, stratified by age groups, increased with advancing age (Table 2).
Trends of hospitalised CDI cases/100.0000 population/year from the three German surveillance systems RKIa, LUAb, CDAD-KISS, the German GBE-Bund database and the US CDC Emerging Infections Program. CDI Clostridioides difficile infection; CDAD-KISS Hospital Infection Surveillance System; GBE-Bund Information System of the Federal Health Monitoring; LUA Saxon State Health and Veterinary Research Institute Saxony; RKI Robert Koch-Institute, US CDC EIP US Centers for Disease Control and Prevention Emerging Infections Program, aincludes severe cases only, balso includes non-hospitalized cases
Differences in population-based hospitalised CDI incidences between individual Federal States did not show any particular trends, and therefore could not be attributed to factors such as age distribution or rural versus urban areas (data not shown).
Recurrence and Severity
Data on CDI recurrence are available in GBE-Bund from 2016 onwards and in CDAD-KISS from 2019. In 2019, GBE-Bund recorded 63,571 primary hospitalised CDI cases and 3,676 recurrent cases, resulting in a recurrence rate of 5.8%. This was similar to CDAD-KISS, which reported 26,403 hospitalised CDI cases and 1,600 (6.0%) recurrent cases in 2019. For 2016–2018, the numbers of primary hospitalised CDI cases and recurrent cases (%) were 90,384 and 5,289 (5.9%), 83,748 and 5,449 (6.5%) and 76,254 and 4,866 (6.4%), respectively. The population-based recurrent CDI incidence in 2019 increased with age (Table 2), while the recurrence rate was stable over age groups (data not shown).
Table 3 shows the incidence of severe hospitalised CDI for 2016–2019. In all four reporting systems, severe hospitalised cases accounted for only a small proportion of hospitalised CDI cases. There was no clear increase or decrease over time. Population-based incidence of severe hospitalised CDI in CDAD-KISS was twice as high as for RKI and LUA. GBE-Bund incidence was lowest. Age-stratification of RKI severe hospitalised CDI cases for 2019 increased with advanced age (Table 2).
Mortality
Data on CDI mortality were analysed from GBE-Bund. Parallel to the incidence of CDI, the number of CDI-related deaths increased from 1,131 in 2010 to a peak of 2,666 cases in 2015. Subsequently the number fell to 1,006 cases in 2019 (Fig. 2). However, CDI death trends were more pronounced than those of CDI cases (approx. 136% increase of deaths vs. approx. 14% increase of cases, then 62% vs. 37% decrease, respectively).
The majority of CDI-related deaths occurred among patients of advanced age (Fig. 2). In 2019, 959 of the 1,006 CDI-related deaths (> 95%) were among patients ≥ 65 years, and more than 80% were among patients ≥ 75 years.
Hospital Burden
To better quantify the overall burden on the German healthcare system, the annual number of patient-days (PD) required to treat CDI in hospitals were calculated. For this analysis only main CDI diagnoses were considered (i.e. the reason why a patient was admitted to hospital) to avoid the potential bias that hospitalization itself is a risk for acquiring CDI. Based on GBE-Bund main A04.7 diagnosis from 2019, 20,257 patients were treated in German hospitals with an average length of stay of 10.1 days, resulting in overall 204,596 PD.
To put this burden in a broader context, it was compared to herpes zoster and influenza, which are two other infectious diseases that mainly affect the elderly. CDI PD exceeded those for influenza (ICD-10 J09 and J10) and herpes zoster (ICD-10 B02) in all years 2010–2017 (Fig. 3). CDI cases decreased from 2015 while zoster cases steadily increased over the whole reporting period. In 2019, zoster surpassed CDI PD. Influenza cases fluctuated more and, only during the strong influenza seasons 2017/18 and 2018/19, influenza PD exceeded those for CDI.
Among CDI hospitalisations, the average length of hospitalisation decreased from 11.5 to 10.1 days between 2010 and 2019, while for zoster, length decreased from 8.7 to 8.1 days. For influenza, no clear trend was observed, and the average length of hospitalisation varied over the years between 5.2 and 10.1 days.
Comparison to US CDI Incidence
Next, GBE-Bund population-based incidence of hospitalised CDI was compared to the US EIP surveillance system for the years 2012–2019. The incidence of hospitalised CDI in Germany ranged from 98–138 hospitalised cases per 100,000 population per year, which was higher than in the US (58–73/100,000). Both countries showed similar trends with a slight and steady decrease from 2015 onwards (Fig. 1).
In addition, the population-based incidence of overall hospital admission was compared. The incidence of overall admissions was 2.5-fold higher in Germany (2018: 23,893 admission/100,000 population per year) compared to the US (2018: 10,238 admissions/100,000 population per year). Also in 2019, average length of hospitalisation for CDI was much shorter in US with 5.4 days [26] compared to 10.1 in Germany.
Discussion
The analysis of four CDI surveillance systems comprehensively assessed the hospital burden of CDI in Germany. Countrywide reporting occurs through the administrative claims database of the hospitals (GBE-BUND) and the mandatory reporting of severe cases by the RKI. This is complemented by two partial reporting systems: the voluntary reporting in a subset of hospitals (CDAD-KISS) and the mandatory reporting of hospitalised and non-hospitalised cases in the State of Saxony (LUA).
The four reporting systems are independent and utilize different methodologies for CDI surveillance. LUA is the most comprehensive, as it is the only system that includes both hospitalised and non-hospitalised CDI cases. CDAD-KISS and GBE-Bund concentrate on hospitalized cases but neither of them requires laboratory confirmation. Lastly, the RKI describes only a small proportion of the disease as it focuses on severe hospitalized cases only.
Despite these differences in the individual surveillance systems, LUA, CDAD-KISS, and GBE-Bund yielded similar population-based incidences and trends over time. This indicates a general validity of the reported data without individual bias in one of the systems. Until 2016, there were more than 100,000 hospitalised CDI cases reported each year. Since then, the number has declined. Three new guidelines have been issued by the Commission for Hospital Hygiene and Infection Prevention in 2015 and 2016, updating prevention measures of proper hand hygiene, isolation of infected patients and capacity planning of hospital hygiene staff [5,6,7]. In addition, efforts of antimicrobial stewardship have been intensified in the same period [27,28,29]. These are likely important drivers of the observed reduction of CDI incidences in the surveillance datasets.
Nevertheless, there might be general bias affecting all three systems, like incomplete stool sampling and testing leading to underdiagnosis of the true CDI incidence in Germany. Previous studies suggested that underdiagnosis of CDI is likely. A recent study conducted in the Muenster/Coesfeld area showed that only 34% of hospitalised patients with new onset diarrhoea had a stool specimen collected and tested for CDI [30]. Similar results have been obtained for the US [31]. In addition, among patients with stool samples collected, a substantial proportion are not tested for CDI due to lack of clinical suspicion, or have suboptimal laboratory testing, leading to further underestimation of disease. The EUCLID study estimated that 24.5% of CDI cases in Germany are missed due to these factors [32]. For example, an initially undetected increase of transmission and spread of the hypervirulent C. difficile strain RT027 after 2010 was identified only by literature review [33].
Also, no public surveillance for community-treated CDI exists in Germany and the burden of disease in the outpatient setting cannot therefore be quantified. LUA surveillance reports community-treated CDI cases together with hospitalized cases. Overall LUA cases are very similar to the other surveillance system and, therefore, reporting of non-hospitalised CDI cases appears to be low. It may be concluded that CDI can be regarded as a nosocomial infection, but C. difficile is increasingly being recognized as a cause of community-onset diarrhoea [1, 34]. Therefore, it is more likely that CDI in Germany is highly underreported in the community due to lack of clinical awareness and testing. Implementation of a national surveillance system of community-treated CDI could help to fully describe the burden of CDI and to raise physicians’ awareness.
The RKI reports severe hospitalised CDI only. RKI population-based incidence of severe hospitalised CDI increased from 2015 onwards. This is likely related to the broadening of case definition that became effective in 2016. LUA reported a similar incidence of severe hospitalised CDI as RKI surveillance. This is not surprising as the LUA cases are routinely forwarded to the RKI by the local Saxony health departments. GBE-Bund reported a lower incidence of severe hospitalised CDI than RKI and LUA: It is likely that severe diagnoses are only coded if they are relevant for hospital revenue. CDAD-KISS reported the highest incidence of severe hospitalised CDI. A possible explanation could be the overrepresentation of larger hospitals within the voluntary KISS reporting system. In 2019, the market share of all German hospitalisation days in the 572 CDAD-KISS hospitals was 43%. This indicates that participating hospitals are mainly large clinics as there are approximately 1900 hospitals in total in Germany [21]. Such hospitals have the resources to support isolation rooms, in-house laboratory facilities and specialised infection control personnel dedicated to and well trained in infection surveillance, and may therefore treat more severe cases compared to smaller institutions. Like with overall CDI cases, underreporting of severe CDI by the RKI may be common. In Munich and Nuremberg between 2013–2016, there were 4.6- and 2.3-fold more CDI deaths reported on death certificates compared to deaths among severe hospitalised CDI cases in the RKI surveillance [35]. Similar results were obtained from a study in Frankfurt [36].
The data from GBE-Bund and CDAD-KISS report comparable rates of recurrence of 5.8–6.5% for 2016–2019. In contrast, a health claims study analysing German data reported higher rates of 18.2% for first recurrence and up to 30% for subsequent recurrences in 2012 [37]. Other international studies confirm these higher rates [1, 38]. There may be several reasons for the discrepancy. The health claims study contained hospitalised and non-hospitalised CDI patients, while the surveillance systems include hospitalised patients only and therefore miss a substantial proportion of recurrences. Moreover, the health claims analysis was conducted in 2012 when CDI incidences peaked. In contrast, the recurrence data collection in the public surveillance started in 2016. Another reason could be the update of the CDI clinical treatment guideline in 2017 [4]. In this update, metronidazole was replaced by vancomycin as the drug of choice to treat initial CDI, and vancomycin is associated with superior clinical cure rates and lower recurrence rates.
A substantial number of deaths are associated with hospitalised CDI in Germany. Hospitalised CDI cases and deaths showed parallel trends in 2010–2019 but the increase 2010–2013 as well as the decrease in 2015–2019 was steeper for mortality. A similar trend was observed in the epidemiology of the hypervirulent subtype RT027 in Germany that is associated with increased morbidity and mortality [29]. The spread of RT027, subsequent intensified antibiotic stewardship efforts, the new clinical treatment guideline and the curbing of infection transmission in healthcare facilities can explain observed mortality trends. As with CDI cases, there may be significant underreporting of CDI-associated deaths in Germany. Mortality data are obtained from death certificates which are prone to underreporting of specific diseases especially in the elderly population [39]. Also, the GBE-Bund surveillance system only counts deaths where CDI can be assumed to be the causative factor for death. Deaths with secondary diagnosis of CDI are not recorded in the death statistics and are therefore most likely underreported.
The surveillance data show that advanced age is a risk factor for a more severe disease, recurrent infection or death among hospitalised CDI cases. This is also described extensively in the literature [40]. Therefore, any new preventative measures targeted to reduce CDI burden will have to take this into account. Similarly, the assessment of new therapeutic or preventive interventions by health economic models will need to consider the increased risk associated with advanced age. The age-stratified data presented in this work could be a valuable source in any such analyses.
The overall burden of disease of hospitalised CDI on the German healthcare system is large. We showed that 204,596–355,466 PD are associated with hospitalised CDI annually. To put these estimates into further context, this yearly hospital burden of CDI was compared to those of influenza and herpes zoster. This study showed that, in most years, CDI hospitalisations resulted in more hospitalization days than influenza or zoster. Impressively, even in 2017, when influenza vaccine effectiveness was suboptimal [41], the resulting high influenza hospital burden in 2018 was only as high as the yearly burden of CDI in 2011–2015. This analysis clearly shows that CDI represents a substantial healthcare burden that requires attention from physicians, the public and healthcare stakeholders.
Finally, the incidence of hospitalised CDI in Germany was compared to that in the US EIP surveillance system. Differences exist in reporting methodologies, as the EIP conducts active population-based surveillance and ascertains all laboratory-confirmed CDI cases within the surveillance area, whereas GBE-Bund represents administrative ICD-10 data. Even though a certain degree of variance between EIP and GBE-Bund can be assumed, studies showed the usefulness and comparability of these sources [42]. Compared to EIP, the incidence of hospitalised CDI was higher in Germany. Furthermore, the incidence of overall hospital admission per 100,000 population was 2.5 times higher in Germany than in the US. Also, on average, patients stay 5.4 days for CDI in hospital in the US [26] compared to 10.1 days in Germany. The prolonged hospital exposure of the German population may be one reason for the increased population-based CDI incidence, as hospitalisation is a well-known risk factor for CDI. The US incidence of CDI treated exclusively in the outpatient setting is estimated to be the same as that treated in hospitals [43]. For Germany, no surveillance exists for non-hospitalized CDI, hampering a complete comparison between the two countries. However, the higher incidence of hospitalised CDI in Germany impressively shows the high burden of CDI on the healthcare system and patients’ lives. In the US, the CDC has classified CDI as an “urgent threat” that requires immediate and consequent action by all healthcare stakeholders [10]. In Germany, CDI continues to be underestimated as a public health problem.
This study analysed data from public surveillance systems which have individual limitations due to their structure and the design of case definitions. Limitations are highlighted in the Discussion, and can result in individual under- or overestimation of the overall CDI burden. Besides highlighting the high burden of CDI on healthcare systems, this work has described in detail each public surveillance system established in Germany, and discussed individual limitations and strengths in order to provide physicians and healthcare decision-makers with a comprehensive and recent overview, and to allow them an accurate interpretation of the publicly available data.
Conclusions
The four public surveillance systems provide comprehensive information on the yearly burden of CDI in German hospitals on a national level. Despite different surveillance methodologies utilized, population-based incidences and trends over time are similar, indicating a general validity of the reported data. Albeit numbers of hospitalised CDI cases have been declining since 2016, our analysis shows that the burden of CDI on the healthcare system remains at a high level. New therapeutic or preventive interventions are required to further address this public health problem. The data of the four systems can be valuable for health economic modelling studies to assess such new approaches in future. In addition, the establishment of a surveillance system to measure the burden in the outpatient setting is required to fully assess the impact of CDI disease.
References
Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2:16020.
Valiente E, Cairns MD, Wren BW. The Clostridium difficile PCR ribotype 027 lineage: a pathogen on the move. Clin Microbiol Infect. 2014;20(5):396–404.
Vernon JJ, Wilcox MH, Freeman J. Effect of fluoroquinolone resistance mutation Thr-82→Ile on Clostridioides difficile fitness. J Antimicrob Chemother. 2019;74(4):877–84.
McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1–48.
Empfehlung zum Kapazitätsumfang für die Betreuung von Krankenhäusern und anderen medizinischen Einrichtungen durch Krankenhaushygieniker/innen. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2016;59(9):1183–8.
Händehygiene in Einrichtungen des Gesundheitswesens : Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut (RKI). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2016;59(9):1189–220.
Ruscher C. Infektionspravention im Rahmen der Pflege und Behandlung von Patienten mit ubertragbaren Krankheiten. Empfehlung der Kommission fur Krankenhaushygiene und Infektionspravention (KRINKO) beim Robert Koch-Institut. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58(10):1151–70.
Davies K, Lawrence J, Berry C, Davis G, Yu H, Cai B, et al. Risk factors for primary Clostridium difficile infection; results from the observational study of risk factors for Clostridium difficile infection in hospitalized patients with infective diarrhea (ORCHID). Front Public Health. 2020;8:293.
Kuijper EJ, Coignard B, Tull P, Difficile ESGfC, States EUM, European Centre for Disease P, et al. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12(Suppl 6):2–18.
Solomon SL, Oliver KB, Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States: stepping back from the brink. Am Fam Physician. 2014;89(12):938–41.
Robert Koch-Institut. Epidemiologisches Bulletin. 18. Februar 2021/Nr. 7. https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2021/Ausgaben/07_21.pdf?__blob=publicationFile.
Guery B, Barbut F, Tschudin-Sutter S. Diagnostic and therapy of severe Clostridioides difficile infections in the ICU. Curr Opin Crit Care. 2020;26(5):450–8.
Hagel S, Epple HJ, Feurle GE, Kern WV, Lynen Jansen P, Malfertheiner P, et al. S2k-guideline gastrointestinal infectious diseases and Whipple’s disease. Z Gastroenterol. 2015;53(5):418–59.
Robert Koch-Institut. SurvStat@RKI. Abfrage der Meldedaten nach Infektionsschutzgesetz (IfSG) über das Web. 2023. https://www.rki.de/DE/Content/Infekt/SurvStat/survstat_node.html . Accessed June/July 2021.
Robert Koch-Institut. Infektionsepidemiologisches Jahrbuch 2001–2020. 2023. https://www.rki.de/DE/Content/Infekt/Jahrbuch/jahrbuch_node.html. Accessed June/July 2021.
Landesuntersuchungsanstalt Sachsen. Jahresberichte. 2023. https://www.lua.sachsen.de/lua-jahresberichte-4103.html. Accessed June/July 2021.
Nationales Referenzzentrum für Surveillance von nosokomialen Infektionen. CDAD-KISS. 2023. https://www.nrz-hygiene.de/KISS-Modul/KISS/CDAD. Accessed June/July 2021.
Das Informationssystem der Gesundheitsberichterstattung des Bundes. 2023. https://www.gbe-bund.de/gbe/pkg_isgbe5.prc_isgbe?p_uid=gast&p_aid=61436483&p_sprache=D. Accessed June/July 2021.
Centers for Disease Control and Prevention. Healthcare-Associated Infections-Community Interface (HAIC). Clostridioides difficile Infection (CDI) Tracking. 2023. https://www.cdc.gov/hai/eip/cdiff-tracking.html. Accessed June/July 2021.
Centers for Disease Control and Prevention. Data Archive. National and State HAI Progress Reports or SIR Reports. 2023. https://www.cdc.gov/hai/data/archive/archive.html. Accessed June/July 2021.
statista. Anzahl der Krankenhäuser in Deutschland in den Jahren 2000 bis 2019. 2023. https://de.statista.com/statistik/daten/studie/2617/umfrage/anzahl-der-krankenhaeuser-in-deutschland-seit-2000/. Accessed June/July 2021.
Fallpauschalenbezogene Krankenhausstatistik. 2021. In: Krankenhaus-Report 2021 Versorgungsketten - Der Patient im Mittelpunkt [Internet]. 2023; Springer, Berlin, Heidelberg, pp. 443–74. https://link.springer.com/book/10.1007%2F978-3-662-62708-2. Accessed June/July 2021.
Gesundheitsberichterstattung des Bundes. Bevölkerung im Jahresdurchschnitt. Tabelle (gestaltbar). Gliederungsmerkmale: Jahre, Region, Alter, Geschlecht, Nationalität (Grundlage Zensus 2011). 2023. https://www.gbe-bund.de/gbe/pkg_isgbe5.prc_menu_olap?p_uid=gast&p_aid=53766444&p_sprache=D&p_help=2&p_indnr=5&p_indsp=&p_ityp=H&p_fid=. Accessed June/July 2021.
United States Census Bureau. ACS Demographic and Housing Estimates. 2023. https://data.census.gov/cedsci/table?q=United%20States&g=0100000US&tid=ACSDP1Y2018.DP05&vintage=2017&layer=state&cid=DP05_0001E. Accessed June/July 2021.
Centers for Disease Control and Prevention. Emerging Infections Program, Healthcare-Associated Infections – Community Interface Surveillance Report, Clostridioides difficile infection (CDI), 20192022. 2023. https://www.cdc.gov/hai/eip/pdf/cdiff/2019-CDI-Report-H.pdf. Accessed June/July 2021.
Solanki D, Kichloo A, El-Amir Z, Dahiya DS, Singh J, Wani F, et al. Clostridium difficile infection hospitalizations in the United States: insights from the 2017 national inpatient sample. Gastroenterology Res. 2021;14(2):87–95.
Sweileh WM. Bibliometric analysis of peer-reviewed literature on antimicrobial stewardship from 1990 to 2019. Glob Health. 2021;17(1):1.
Mielke M. Die Rolle der Infektionsprävention bei der Eindämmung der Antibiotikaresistenzentwicklung. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2018;61(5):553–61.
Abdrabou AMM, Ul Habib Bajwa Z, Halfmann A, Mellmann A, Nimmesgern A, Margardt L, et al. Molecular epidemiology and antimicrobial resistance of Clostridioides difficile in Germany 2014–2019. Int J Med Microbiol. 2021;311(4):151507.
Effelsberg N, Buchholz M, Kampmeier S, Lucke A, Schwierzeck V, Angulo FJ, et al. Frequency of diarrhea, stool specimen collection and testing, and detection of Clostridioides difficile infection among hospitalized adults in the Muenster/Coesfeld area, Germany. Curr Microbiol. 2022;80(1):37.
Angulo F, Pena S, Carrico R, Stephen F, Joann Z, Gonzalez E, et al. Frequency of testing for Clostridioides difficile in adults hospitalized with diarrhea in Louisville Kentucky. Infect Control Hosp Epidemiol. 2020;41(S1):s444-s.
Davies KA, Longshaw CM, Davis GL, Bouza E, Barbut F, Barna Z, et al. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect Dis. 2014;14(12):1208–19.
Marujo V, Arvand M. The largely unnoticed spread of Clostridioides difficile PCR ribotype 027 in Germany after 2010. Infect Prev Pract. 2020;2(4): 100102.
Guh AY, Mu Y, Winston LG, Johnston H, Olson D, Farley MM, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382(14):1320–30.
Gleich S, Schaffer A, Mai CH, Schick S, Hirl B. Clostridium difficile-assoziierte Todesfälle 2013–2016 in München und Nürnberg. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2017;60(10):1067–74.
Heudorf U, Berres M, Dogan O, Steul KS. Legal obligation to notify severe Clostridiodes difficile infections. Data from Frankfurt am Main, Germany. Overview and Discussion. Gesundheitswesen. 2021.
Lübbert C, Zimmermann L, Borchert J, Horner B, Mutters R, Rodloff AC. Epidemiology and recurrence rates of Clostridium difficile infections in Germany: a secondary data analysis. Infect Dis Ther. 2016;5(4):545–54.
Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(Suppl 6):21–7.
Gleich S, Schmidt S, Wohlrab D. COVID-19- und influenzaassoziierte Sterbefälle in München ab März 2020 – eine standardisierte Auswertung von Todesbescheinigungen. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2021;64(9):1125–35.
Bednarska A, Bursa D, Podlasin R, Paciorek M, Skrzat-Klapaczyńska A, Porowski D, et al. Advanced age and increased CRP concentration are independent risk factors associated with Clostridioides difficile infection mortality. Sci Rep. 2020;10(1):14681.
Kissling E, Rondy M, Team I-MI-Ms. Early 2016/17 vaccine effectiveness estimates against influenza A(H3N2): I-MOVE multicentre case control studies at primary care and hospital levels in Europe. Eurosurveillance. 2017;22(7):30464.
Olsen MA, Young-Xu Y, Stwalley D, Kelly CP, Gerding DN, Saeed MJ, et al. The burden of Clostridium difficile infection: estimates of the incidence of CDI from U.S. Administrative databases. BMC Infect Dis. 2016;16:177.
Centers for Disease Control and Prevention. 2017 Annual Report for the Emerging Infections Program for Clostridioides difficile infection. 2022. https://www.cdc.gov/hai/eip/Annual-CDI-Report-2017.html. Accessed June/July 2021.
Acknowledgements
Funding
This study was sponsored by Pfizer and non-Pfizer authors except Friederike Maechler were funded for manuscript development. The journal's rapid service fee was also funded by Pfizer.
Medical Writing
The authors acknowledge Karolin Eberle, Senior Medical Writer at AMS Advanced Medical Services GmbH, for preparing the manuscript and for providing editorial assistance, funded by Pfizer.
Author Contributions
Gordon Brestrich developed the study concept and design. Alexander Mellmann provided expert opinion, input on study concept and design, edited the initial draft and reviewed and approved the final manuscript. Friederike Maechler, Thomas Weinke, Stefan Hagel, Frederick J. Angulo, Andreas Leischker, Holly Yu, Fabian K. Berger, Nadia Miranovic, Sophie-Susann Merbecks, Lutz von Müller, Christoph Lübbert, Phillip A. Reuken and Jennifer C. Moïsi, provided expert opinion, edited the initial draft and reviewed and approved the final manuscript. Christian Brösamle performed the data analysis, provided medical writing services and editorial support.
Disclosures
Stefan Hagel reports grants from the Federal Ministry of Education and Research (BMBF), lecture fees from Pfizer, MSD, InfectoPharm, Advanz, Tillotts and Philips, support for attending meetings from Pfizer and Advanz. Christoph Lübbert reports lecture and consulting fees from Tillots. Phillip A. Reuken reports lecture or consulting fees from Pfizer, CSL Behring, BMS and Dr. Schwabe Pharma. Fabian K. Berger reports consultant fees from Pfizer, MSD and research support from Tillots. Thomas Weinke reports lecture or consulting fees from BioNTech, Falk Foundation, GSK, MSD, Pfizer, Roche, Sanofi Pasteur and Seqirus. Gordon Brestrich, Frederick J. Angulo, Christian Brösamle, Stefan Hagel, Andreas Leischker, Friederike Maechler, Sophie-Susann Merbecks, Nadia Minarovic, Jennifer C. Moïsi, Lutz von Mueller Holly Yu and Alexander Mellman declare that they have no competing interests.
Compliance with Ethics Guidelines
No ethical approval was required for this study. Data were extracted from public sources. Its collection is conducted as part of the routine surveillance and health data monitoring in accordance with German and European law and regulations.
Data Availability
The datasets generated during and/or analysed during the current study are available in different public repositories and can be assessed using the references 10–21 of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Brestrich, G., Angulo, F.J., Berger, F.K. et al. Epidemiology of Clostridioides difficile Infections in Germany, 2010–2019: A Review from Four Public Databases. Infect Dis Ther 12, 1057–1072 (2023). https://doi.org/10.1007/s40121-023-00785-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00785-2