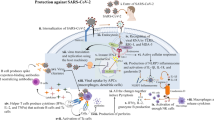
Abstract
Vaccines developed against SARS-CoV-2 have proven to be highly effective in preventing symptomatic infection. Similarly, prior infection with SARS-CoV-2 has been shown to provide substantial protection against reinfection. However, it has become apparent that the protection provided to an individual after either vaccination or infection wanes over time. Waning protection is driven by both waning immunity over time since vaccination or initial infection, and the evolution of new variants of SARS-CoV-2. Both antibody and T/B-cells levels have been investigated as potential correlates of protection post-vaccination or post-infection. The activity of antibodies and T/B-cells provide some potential insight into the underlying causes of waning protection. This review seeks to summarise what is currently known about the waning of protection provided by both vaccination and/or prior infection, as well as the current information on the respective antibody and T/B-cell responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The protection provided against SARS-CoV-2 wanes over time post-vaccination or post-initial infection. |
Protection against severe disease is more durable and takes longer to wane. |
Waning protection is driven by both waning immunity over time following vaccination or initial infection and the evolution of new variants of SARS-CoV-2. |
The evidence supports the hypothesis that protection is initially provided by neutralising antibodies with the more durable T-cell and B-cell responses, providing a large amount of the protection from severe infection. |
Future studies should investigate the impact of patient-specific variables, such as age, ethnicity, comorbidities, and concomitant medications, on the effectiveness of the vaccines, as well as prior infection. |
An established process is needed to evaluate the durability and protection provided by new vaccines designed with new variants so that they may be evaluated and rolled out in time for peaks in SARS-CoV-2-related burden. |
Introduction
Randomised placebo-controlled trials showed that the initial efficacy of vaccines in preventing symptomatic SARS-CoV-2 infection ranges from 66 to 95% [1,2,3,4,5,6,7,8,9]. However, this high efficacy wanes over time, resulting in substantive reductions in vaccine protection [10, 11]. Similarly, while prior infection with SARS-CoV-2 can provide a high level of protection (87%) from re-infection [12], this protection declines over time. Despite waning protection against mild to moderate SARS-CoV-2 infection, protection against severe infection appears to be more durable, for underlying reasons which are not yet fully understood [10].
Waning protection is driven by both waning immunity over time following vaccination or initial infection and the evolution of new variants of SARS-CoV-2. Immunity is not a singular state: a wide range of immune states now exist globally, including those who are infection and vaccination-naïve, those who are fully vaccinated with or without booster shots, those recovered from one or more prior infections, and those who have both been vaccinated and recovered from prior infection. This is quite different from the context in which COVID-19 vaccines were first introduced.
Waning immunity is compounded by the evolution of new SARS-CoV-2 variants with greater immune escape. The first available vaccines for SARS-CoV-2 were developed against the original D614 variant, but multiple new variants of concern (VOC) have since arisen and spread globally [13]. The Omicron variant emerged at the end of November 2021, and the current global epidemiology of SARS-CoV-2 is characterised by the continued rapid global spread of Omicron sub-lineages. Updated SARS-CoV-2 vaccines, which incorporate the spike protein of the variants Omicron BA.1, BA.4, and BA.5 and the SARS-CoV-2 original strain, have received regulatory approval [14, 15]. The World Health Organization (WHO) has to date named five of the genetically mutated SARS-CoV-2 viruses as VOC, the Alpha, Beta, Gamma, Delta and Omicron variants [13]. At the time of publication, Omicron and its various sub-lineages were the only currently recognised VOC to be circulating [13]. Each VOC is designated based on mutations compared to the ancestral strain, with varying levels of immune escape against both previous infection and vaccination.
The aim of this review was to assimilate the current knowledge on the waning of protection by both vaccination and/or prior infection, as well as antibody and T/B-cell responses. A comprehensive understanding of these characteristics is required to support future vaccine products and programme development. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Ethics approval was not required for this narrative review.
Waning Vaccine Efficacy and Effectiveness
Both the effectiveness and the efficacy of vaccines developed against SARS-CoV-2 have been extensively studied. Efficacy refers to the performance of the vaccines under ideal and controlled circumstances, for example clinical trials, compared with effectiveness, which measures vaccine performance under real-world conditions, such as observational trials. It is now well-established that the vaccine effectiveness of a primary series of SARS-CoV-2 vaccination wanes over time, resulting in an increased risk of breakthrough infection. The waning of vaccine efficacy and effectiveness have been assessed in mRNA vaccines (BNT162b2 [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] and mRNA-1273 [20, 25, 30, 31]), adenoviral vector vaccines (ChAdOx1-S [16, 20, 28, 32] and Ad26.COV2.S [20, 25, 27, 30]), and an inactivated virus vaccine (CoronaVac) [22]. Additionally, studies have reported combined results [33, 34].
Overall, every vaccine platform studied has shown reduced vaccine effectiveness against symptomatic infection over time [18, 27, 28]. Two systematic reviews and meta-analyses have summarised the rate at which protection against infection (both asymptomatic and symptomatic) waned in SARS-CoV-2 vaccines, regardless of vaccine platform (Table 1). Feikin et al.[10] found a 23% decrease in protection over 6 months post-vaccination, while Ssentongo et al.[11] found a decline in protection from 83 to 21% over 5 months. It was noted that the latter analysis relied on only two studies for the latest timepoint [11].
Despite substantial waning protection against symptomatic SARS-CoV-2 infection, most vaccines are still highly efficacious in preventing severe disease, hospitalisation, and death over time [16, 19, 20, 22, 30, 32, 33]. In the meta-analysis on severe infection by Feikin et al. [10], vaccine effectiveness declined by only 9.0% over the first 6 months post-full vaccination (Table 1) [10]. When limited to severe disease, Ssentongo et al. [11] found no evidence of decline at 5 months post-vaccination (Table 1).
Both meta-analyses represent studies in which multiple variants were circulating whilst the studies were performed, and that were conducted after the emergence of the Delta variant but before Omicron [10, 11].
In some studies, high-risk groups, including older people and persons with immunosuppression [16, 25, 35], have been observed to have faster rates of waning protection [16]. However, reports on this observation have not been consistent in their findings. For example, there have been some studies which have not found an impact of age on waning vaccine effectiveness [18, 29], and no statistically significant effect of age on the rate at which immunity waned was found in the meta-analysis by Feikin et al. [10].
The emergence and broad circulation of a potentially new VOC presents an additional variable which should be accounted for when discussing the durability of protection provided by vaccines, particularly in the case of the Omicron variant, where the virus-neutralizing activity of vaccine-induced antibodies is substantially lower compared to the earlier VOCs [18, 36]. Effectiveness as low as, − 2.7% (95% CI − 4.2 to − 1.2), 8.8% (95% CI 7.0–10.5) and 14.9% (95% CI 3.9–24.7) against Omicron at 25 + weeks post-full vaccination has been reported for the ChAdOx1, BNT162b2, and mRNA-1273 vaccines, respectively [37]. There is, however, still evidence that, despite a reduction in effectiveness against any Omicron infections, vaccination continues to provide substantial protection against severe infections. A test-negative case–control study has shown that the effectiveness against symptomatic Omicron infections of 3 doses of mRNA vaccine is 54% (95% CI 50.4, 57.3). Despite the reduced level of protection against symptomatic infection, the effectiveness against severe, critical, or fatal Omicron infections has remained remarkably high at 92.5% (95% CI 84.4, 96.3) [38].
Waning in Post-Infection Protection
Multiple systematic and pragmatic literature reviews have investigated the effectiveness of natural post-infection protection [12, 39,40,41,42,43]. Published meta-analyses show consistently high levels of protection (81.0–87.0%) provided by prior infection, even over 7 months post-initial infection (Table 2) [12, 41, 43]. One meta-analysis assessed the estimated protection provided by prior infection from either any reinfection or symptomatic reinfection and found similar levels of protection (~ 87.0%) [12]. None of the three meta-analyses reported an analysis by variant, which would be challenging to study, since variants of both the initial infection and the reinfection must be considered [12, 41, 43].
Five studies investigating the risk of reinfection over time found no statistically significant waning in protection from reinfection (Table 3), [28, 44,45,46,47] with the protection from reinfection at the final study follow-up estimated to range from 69.0 to 93.0%. The protection against reinfection at > 1 year was estimated to be 69.0% in the study with the longest follow-up [28]. The estimated protection against milder versus more severe infections was only compared in two studies, which produced contradictory results [45, 47].
Nordstrom et al. (2022) found the estimated protection against hospitalisation (which the study stated was not affected by limitations associated with selection bias) was lower than protection against any infection at both 3–6 months and ≥ 9 months, although a larger study should be performed to achieve a more accurate result, as hospitalisation events in this study were rare [45]. Sheehan et al. (2021) found that the estimated protection against symptomatic infection (71%) was higher than protection against any infection (60%) at 90–150 days post-infection, although statistical significance was not assessed [47]. This was not seen at either the 151–210- or ≥ 210-day timepoints, where estimated protection was ~ 90% against both types of infection [47].
Only one study, Hall et al. (2022), took place during a time period in which the Delta VOC predominated [28]. The original infections included in the study occurred prior to the emergence and spread of Delta, which could explain the substantial, but not statistically significant, reduction in protection (Table 3) [28]. The other studies all took place prior to the widespread prevalence and predominance of VOC [44,45,46,47].
The relative protection provided by natural immunity compared to vaccination has been assessed in a systematic review and meta-analysis [42], which categorised studies into randomised controlled trials (RCTs) (3 studies) [48,49,50] and observational studies (4 studies) [51,52,53,54]. In the RCTs, no statistically significant difference [overall RR of 0.59 (95% CI 0.04–8.28, P = 0.69)] between vaccination and natural immunity was found, while in the observational studies, natural immunity provided better protection [3.71 (95% CI 1.75–7.86; P = 0.0006)] against any infection [42].
Some preliminary clinical trial evidence casts doubt on the ability of prior infection with an earlier variant of SARS-CoV-2 to provide protection against a different, newer VOC [55]. A trial assessing the efficacy of NVX-CoV2373 against the B.1.351 variant found that prior infection with a pre-B.1.351 virus did not appear to reduce the risk of Covid-19 due to subsequent infection with B.1.351 variants among placebo recipients during the initial 2 months of follow-up [55]. Data from a test-negative, case–control study from Qatar found that the effectiveness of previous infection in preventing reinfection ranged from 90.2% (95% CI 60.2–97.6) against the alpha variant, 85.7% (95% CI 75.8–91.7) against the beta variant and 92.0% (95% CI 87.9–94.7) against the delta variant, while against the Omicron variants, protection was reduced to 56.0% (95% CI 50.6–60.9). Prior infection did provide robust protection against hospitalisation or death regardless of variant, including 87.8% protection against hospitalisation due to the Omicron variant [56]. A separate analysis found that prior infection and a median interval of 324 days prior to reinfection, provided 50.8% (45.4–55.7) protection against symptomatic infections and 71.6% (15.7–90.4) protection against severe, critical, or fatal infections with the Omicron variant [38].
Boosted and Hybrid Immunity
Several studies have demonstrated that a single booster dose restores protection to the levels seen soon after either full vaccination or recovery from SARS-CoV-2 infection, including against the Omicron variant [18, 29, 57, 58]. However, protection against symptomatic infection provided by current mRNA boosters, compared to no booster dose, was higher against Delta (93.5–97.0%) than Omicron (62.4–67.3%) [18, 37].
Heterologous booster doses, in which the booster vaccine is different to the original vaccine series, may in some cases provide superior protection to homologous boosters [37]. Vaccination with a primary two-dose series of ChAdOx1, followed by a booster dose of ChAdOx1, provided protection against Omicron of only 46.7% (95% CI 34.3–56.7) at 5–9 weeks post-booster vaccination versus 52.9% (95% CI 52.1–53.7) and 60.9% (95% CI 59.7–62.1) at the same timepoint post-booster with BNT162b2 and mRNA1273, respectively [37]. No benefit of a heterologous booster was observed when the two mRNA vaccines were given sequentially [37].
The advantages of a fourth booster dose of BNT162b2 against Omicron were investigated in a recent observational study in Israel [59]. While a two-fold increase in protection against confirmed SARS-CoV-2 infection was seen at 4 weeks post-booster vaccination, the effect had waned by 8 weeks. However, against severe cases of SARS-CoV-2, the fourth dose provided a four-fold increase in effectiveness at 6 weeks with no results provided for 8 weeks [59].
Hybrid immunity, in which people are either vaccinated after a prior SARS-CoV-2 infection or have a breakthrough infection after vaccination, is an increasingly common immune status [28]. The combination effect seems to provide greater protection than natural immunity on its own (> 90.0%), with no waning ≥ 1 year after infection or > 6 months after vaccination [28].
Relative protection provided by hybrid immunity versus natural immunity was investigated in a meta-analysis. In the three RCTs, no statistically significant difference between hybrid immunity and natural immunity was found, whereas in the four observational studies, hybrid immunity provided statistically significantly better protection against infection (risk ratio 1.94 95% CI (1.17–3.21), P = 0.01] [42].
Two additional observational studies supported the conclusion that hybrid immunity gives greater protection than natural immunity. One study found that one-dose hybrid immunity with either ChAdOx1, BNT162b2, or mRNA-1273, was associated with a 58.0% lower risk of SARS-CoV-2 reinfection than natural immunity for up to 2 months, with evidence of attenuation thereafter up to the 9-month follow-up. Two-dose hybrid immunity improved this further to a 66.0% lower risk of SARS-CoV-2 reinfection than natural immunity, with no statistically significant attenuation up to 9 months [45]. In the second study, the patients who had recovered from SARS-CoV-2 and received one or two doses of the BNT162b2 vaccine had a significantly lower risk of recurrent infection. Vaccine effectiveness in this previously infected population was estimated to be 82% (95% CI 80–84) in patients between 16 and 64 years old and 60% (95% CI 36–76) among those who were over 65 years old [60].
Antibody Dynamics Over Time
Levels of neutralising antibodies have been shown to correlate with protection from symptomatic infection [61, 62]. Understanding the antibody dynamics after initial SARS-CoV-2 infection and vaccination is crucial for estimating the potential levels of protection provided.
Post-Initial Infection
In a systematic review and meta-analysis of adaptive immunity and reinfection after recovery from SARS-CoV-2 over the first 6–8 months post-infection by Chivese et al., 90.0% of individuals had evidence of SARS-CoV-2 specific immunological memory [43].
Anti-receptor binding domain (RBD) immunoglobulins (Ig) IgM and IgA are the main contributors to neutralization in the early phase of SARS-CoV-2 infection, while anti-RBD IgG represents most of the neutralising activity in the late phase of infection and during convalescence [63,64,65].
Levels of neutralising antibodies decline over the first 4 months post-initial SARS-CoV-2 infection, especially for IgA and IgM, with less evidence of a substantial decline over the same time period for IgG [66]. Despite these initial declines in some antibodies, the neutralising antibody response after natural infection persists for up to 18 months, even following mild infection [63, 67].
However, the initial levels and durability of the neutralising antibody response depended upon the severity of the initial SARS-CoV-2 infection. Mild SARS-CoV-2 infections gave heterogeneous neutralising antibody titres and patients with asymptomatic SARS-CoV-2 had low titres or no measurable response at all [63, 68].
Antibodies elicited by initial SARS-CoV-2 variants show reduced activity against the RBD proteins of new variants of concern, which correlates with the reduction in protection provided by prior infection with an initial variant [67, 69]. One small study found that sera and plasma collected within 2 months of convalescence from mild or severe SARS-CoV-2 inhibited entry driven by the Omicron viral spike protein 80-fold less efficiently as compared with the B.1 spike (which is identical to the S protein of the Wuhan-Hu-1 isolate, except for the presence of mutation D614G), and 44-fold less efficiently compared with the Delta spike [69].
Post-Vaccination
Antibody dynamics post-vaccination depend on the vaccine used. The mRNA vaccines, BNT162b2 and mRNA-1273, produce a high peak neutralising antibody response which then rapidly declines within 6–8 months post-vaccination [70,71,72,73], while the adenoviral vector vaccines have a lower initial antibody response [74]. The inactivated virus vaccine, CoronaVac, also produces a lower initial antibody response than mRNA vaccines, and this level falls below the positive cut-off by 4 months post-vaccination [75, 76].
Multiple factors, including age and underlying conditions, can affect post-vaccination antibody levels and their longer-term dynamics [35]. The impact of age on post-vaccination levels have been seen to vary by vaccine with age over 50 years, being associated with lower IgG levels in people receiving BNT162b2 but not in those receiving mRNA-1273 [77]. The impact of various immunocompromised individuals and associated factors have been investigated in a prospective study. Seropositivity in participants with various immunocompromising conditions was statistically significantly lower than in healthcare workers [78]. Factors associated with poor seropositivity included age, greater immunosuppression, time since vaccination, anti-CD20 monoclonal antibody levels, and type of vaccination, with mRNA-1273 being superior to BNT162b2 or adenovirus vector vaccines [78].
Antibodies generated by the currently available vaccines, designed against the original D614 SARS-CoV-2 strain, produce antibodies with a substantially reduced recognition of, and activity against, new variants of concern, particularly Omicron [69, 79]. Sera and plasma collected mostly within 1 month post-vaccination with BNT162b2 has been seen to inhibit entry by the Omicron spike protein, with 34-fold lower efficiency than the B.1. spike, and with 12-fold lower efficiency than the Delta spike [69]. Furthermore, sera and plasma collected 1 month after heterologous vaccination with a first dose of ChAdOx1 and a second dose of BNT162b2 was 14-fold less efficient when compared with the B.1. spike, but only threefold less efficient relative to the Delta spike [69].
Booster doses of homologous and heterologous vaccines seem to be effective in recovering the neutralising antibody response in both fully vaccinated people and those with prior SARS-CoV-2 infections against variants of SARS-CoV-2, including the Omicron variant [58, 69, 80,81,82,83,84,85,86]. A recent meta-analysis concluded that heterologous immunisation was more effective than homologous immunisation in increasing antibody levels [87].
T-Cell and Memory B-Cell Response Over Time
Although the contribution of T-cells and memory B-cells to protection against SARS-CoV-2 is still to be fully established, there is good evidence to support important roles of CD4 and CD8 virus-specific T-cells, as well as memory B-cells, in their response to SARS-CoV-2 infection [43, 88,89,90,91,92,93,94]. It must be noted that there is currently a lack of data on the tissue-associated (lung, lymph node, mucosa) T- and B-cell memory in response to SARS-CoV-2.
Post-Initial Infection
A cellular response has been shown to occur without an antibody response, although the overall relationship between the two response types is not fully understood. The concept of “cellular sensitization without seroconversion” refers to people who have developed a virus-specific cellular response, while not exhibiting the presence of neutralizing antibodies post-infection with SARS-CoV-2 [93, 95,96,97,98]. However, a study in healthcare workers found that only 1.5% (16/1076) of seronegative individuals responded to a SARS-CoV-2-specific peptide pool, which argues against widespread generation of T-cell immunity in the absence of seroconversion [99].
A recent systematic literature review and meta-analysis has combined available studies reporting the prevalence of T-cells and memory B-cells after SARS-CoV-2 infection [43]. Synthesis of the four reported studies, with a total of 118 participants [100,101,102,103], showed the prevalence of CD4 + T-cells reduced slightly after 6–8 months to 91.7%, while the prevalence of CD8 + T cells fell significantly to 50% (Table 4) [43]. Two additional studies reported a prevalence of SARS-CoV-2-specific memory B-cells of 92.9% (95% CI 68.5–98.7) for anti-spike-RBD class-switched memory B-cells at 2–3 months post-recovery and 80.6% (95% CI 65.0–90.2) having RBD-specific memory B-cells at 4–5 months [43].
Durability up to 1 year post-infection has been demonstrated in some patients by the maintained prevalence and induction of virus-specific CD4 + and CD8 + T-cells, and memory B-cells [104,105,106].
The severity of the original SARS-CoV-2 infection can affect the cellular response; T-cell responses are significantly higher at 1-year post-in patients with severe infection compared to patients with milder infections [99, 104]. However, one study showed that, even when the magnitudes of both humoral and cellular immune responses were dependent on disease severity, asymptomatic to mild infection was still associated with a substantially reduced risk of reinfection ≥ 9 months [99].
Post-Vaccination
All available SARS-CoV-2 vaccines produce T-cell and B-cell responses, with differing responses depending on the vaccine used [107]. Both mRNA vaccines elicit a robust T-cell and B-cell response, although studies comparing the two show that the mRNA-1273 vaccine appears to produce a stronger T-cell and B-cell response than the BNT162b2 vaccine [108,109,110,111].
One study which directly compared mRNA vaccines and the adenoviral vector vaccine Ad26.COV2.S showed a similar magnitude of response [112]. However, other studies have indicated that the T-cell and B-cell responses of mRNA vaccines, especially mRNA-1273, are superior to adenoviral vector vaccines. One study analysed the T-cell responses in the mRNA vaccines, BNT162b2 and mRNA-1273, versus the adenoviral vector vaccine, Ad26.COV2.S. Superior bulk T-cell response and anti-spike cytotoxic T-cell response in recipients of mRNA-1273 or BNT162b2 was observed compared to recipients given Ad26.COV2.S [113]. Another study evaluated BNT162b2, mRNA-1273, Ad26.COV2.S, and NVX-CoV2373 vaccination-induced immune responses longitudinally for 6 months [114]. The magnitude of the CD4 + T cell responses was greatest with mRNA-1273, BNT162b2 and NVX-CoV2373 vaccination, which were equivalent, whereas Ad26.COV2.S-vaccinated subjects had the smallest response. Additionally, both mRNA and Ad26.COV2.S vaccines induced comparable acute and memory CD8 + T cell frequencies, with NVX-CoV2373 having the lowest response, which was in line with previous findings for a protein-based vaccine [114]. Finally, a study on the SARS-CoV-2–specific T-cell response elicited by the ChAdOx1 and BNT162b2 vaccines over a 3-month period indicated that the BNT162b2 vaccine caused the more durable response of the two comparators [115].
The inactivated vaccine, CoronaVac, has been shown to cause a response in CD4 + and CD8 + T-cells, and in memory B-cells, in a study of up to 8–10 weeks [76], but the studies identified in this review comparing inactivated vaccines to other vaccine platforms do not provide details regarding the relative levels of T-cells and B-cells [75, 116].
As discussed, the antibody response to all available vaccines shows signs of waning with time since vaccination. However, there is evidence that T-cell and B-cell immunity produced by vaccination is more durable than the antibody response in studies of up to 8 months [71, 114, 117,118,119,120].
Additionally, while the antibodies produced by current SARS-CoV-2 vaccines have been shown to have a greatly reduced neutralising effect against new variants, most significantly Omicron, this has not been the case for T-cell responses [121,122,123,124,125,126]. Effective T-cell responses have been shown against both the Delta and Omicron variants, which could partially explain why current vaccines still provide significant protection against severe infection, hospitalisation, and death, despite an observed fall in protection against infection [127, 128]. Retention of the T-cell response can likely be ascribed to the ability of vaccine-induced T-cells to recognise spike proteins regardless of variant, as evidenced in both mRNA [122, 129, 130] and adenovirus-based vaccine responses [131].
As well as T-cells, memory B-cells elicited by the currently available vaccines are able to recognise variants of concern up to, and including, Delta [124, 132]. Unfortunately, this ability has been reduced in the case of Omicron, with, in one study, recognition of the RBD being reduced to 42.0% compared to other variants [124].
Some evidence suggests that T-cell and B-cell responses may be impacted by the timing of vaccination. For instance, a longer dosing interval of the BNT162b2 vaccine can give rise to more typical helper T-cells and long-term memory T-cells, indicating greater promotion of immune memory and generation of antibodies [117, 133]. In a separate study, extending the dosing interval of the BNT162b2 vaccine also led to an increase in peak B-cells and a skew in the T-cells produced towards S-specified CD4 + T cells [134].
Correlates of Protection
There is an urgent need to establish correlates of protection against SARS-CoV-2 infection, as proxy measurements for vaccine effectiveness and duration of immunity against emerging variants, and to help in the development of new vaccines [135]. Neutralising antibodies have been considered the prime candidate as a correlate of protection against clinical infection [61, 62], but, with novel variants emerging, the extent to which these antibody levels still correlate to a good level of protection is diminished in those who have received vaccines based on wild-type SARS-CoV-2 [69, 79].
Emerging evidence shows that different components of the immune system are involved in protection against asymptomatic or mild SARS-CoV-2 infection compared with severe SARS-CoV-2 infection [107]. For example, studies note that protection is seen against severe disease in vaccinated people with a robust T-cell response despite reduced neutralising antibody levels. This may lead to a need to stratify correlates of protection by disease severity [135].
Many potential correlates of protection have yet to be assessed and further study will be required [136].
Conclusions
The protection provided against SARS-CoV-2 infection wanes with time from vaccination or prior infection. The protection provided by vaccination against symptomatic SARS-CoV-2 infection wanes over time, diminishing by a quarter to a third in 6 months. The protection from symptomatic reinfection provided by previous infection wanes at a slower rate, with only slight declines seen at 12 months. With both vaccination and prior infection, protection against symptomatic disease wanes more rapidly than protection against severe, critical, or fatal disease. Booster vaccinations have been shown to recover protection levels of primary vaccination series, and hybrid immunity may provide more robust protection than either vaccination or primary infection; however, in both cases, the protection provided still wanes over time.
The emergence of new VOC has reduced the levels of protection provided by vaccination and prior infection with an earlier variant. However, while protection against symptomatic infection/reinfection is greatly reduced, especially with the Omicron variant, protection against severe, critical, or fatal infection/reinfection remains robust.
The antibody and T/B-cell dynamics post-vaccination or reinfection provides some potential insights into understanding why protection from severe, critical, and fatal infection/reinfection are more robust against waning with time and new VOC. Antibody dynamics post-vaccination are dependent upon multiple factors, including the vaccine used, the number of doses, the presence of hybrid immunity, age and whether the individual is immunocompromised. However, in all cases, circulating antibody levels provided by vaccination are greatly reduced by 6–8 months post-vaccination. This aligns with the time period over which protection against symptomatic infection declines. Post-infection antibody dynamics show a slower decline than post-vaccination titres, which matches the longer-lasting protection seen. However, the initial antibody levels provided by an infection are heavily dependent upon the severity of the initial infection, implying that asymptomatic or mild infections may not provide robust protection. Antibodies elicited by currently available vaccines and prior infections with older variants are not as effective at neutralising new VOC, especially Omicron.
The T/B-cell response to both vaccination and prior infection are more long lasting than the antibody response. Additionally, T/B-cells elicited from current vaccinations and prior infections with older variants show a reduced but still robust ability to recognise new VOC, including Omicron.
The current evidence supports the hypothesis that the initial protection provided by SARS-CoV-2 vaccination or prior infection is initially provided by neutralising antibodies, with the more durable T-cell and B-cell responses providing a large amount of the protection from severe infection. Additionally, antibodies from both vaccines or prior infections seem to lose neutralising activity against new variants more rapidly. T-cells and B-cells provide more robust protection against severe, critical, and fatal infection/reinfection.
While a large amount of research has been performed on the topic of waning protection provided by vaccination and prior infection, many topics still require investigation. These include the impact of patient-specific variables, such as age, ethnicity, comorbidities, and concomitant medications, on the effectiveness of the vaccines, as well as prior infection. Other topics for further investigation should also include the definition of a standardised antibody test and the timepoint of testing. Such studies should be the focus of future investigations. An established process is needed to evaluate the durability and protection provided by new vaccines designed with new variants (e.g. Beta or Omicron) so that they may be evaluated and rolled out in time for peaks in SARS-CoV-2-related disease burden. To date, vaccine roll-out has been conducted in more of an ad hoc manner: it is recognised that high antibody levels are required to prevent breakthrough infection, especially when a new variant of concern arises, so that booster vaccinations are administered as antibody titres wane. Peak antibody levels are typically reached after three vaccine doses, then further doses boost levels back to this peak following waning. There is substantial debate on whether maintaining a saw-tooth level of antibody titres through repeated vaccinations, as is being done in Israel, is a sustainable public health strategy in the long term. Further research is required to develop vaccines that produce a more durable response or an immune response that is not variant-dependent.
Data availability
This manuscript has no associated data or the data will not be deposited. [Authors’ comment: There are no associated data available.].
References
Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. The Lancet [Internet]. 2021 [cited 2022 Jun 24];398:213–22. http://www.thelancet.com/article/S014067362101429X/fulltext.
al Kaabi N, Zhang Y, Xia S, Yang Y, al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 Infection in adults: a randomized clinical trial. JAMA [Internet]. 2021 [cited 2022 Jun 24];326:35–45. https://jamanetwork.com/journals/jama/fullarticle/2780562.
Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 covid-19 vaccine. N Engl J Med [Internet]. 2021;385:1172–83. https://doi.org/10.1056/NEJMoa2107659. (cited 2022 Jun 24).
Logunov DY, Dolzhikova I v., Shcheblyakov D v., Tukhvatulin AI, Zubkova O v., Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet [Internet]. 2021 [cited 2022 Jun 24];397:671–81. https://pubmed.ncbi.nlm.nih.gov/33545094/.
Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. N Engl J Med [Internet]. 2021;384:2187–201. https://doi.org/10.1056/NEJMoa2101544. (cited 2022 Jun 24).
Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. The Lancet [Internet]. 2021 [cited 2022 Jun 24];397:881–91. http://www.thelancet.com/article/S0140673621004323/fulltext.
Baden LR, el Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med [Internet]. 2021;384:403–16. https://doi.org/10.1056/NEJMoa2035389. (cited 2022 Jun 24).
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med [Internet]. 2020;383:2603–15. https://doi.org/10.1056/nejmoa2034577. (cited 2022 Jun 24).
Rotshild V, Hirsh-Raccah B, Miskin I, Muszkat M, Matok I. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Repo [Internet]. 2021 [cited 2022 Jun 24];11:1–9. https://www.nature.com/articles/s41598-021-02321-z.
Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. The Lancet. 2022;399:924–44.
Ssentongo P, Ssentongo AE, Voleti N, Groff D, Sun A, Ba DM, et al. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis. BMC Infect Dis [Internet]. 2022;22:1–12. https://doi.org/10.1186/s12879-022-07418-y. (cited 2022 May 25).
Mao Y, Wang W, Ma J, Wu S, Sun F. Reinfection rates among patients previously infected by SARS-CoV-2: Systematic review and meta-analysis. Chin Med J (Engl) [Internet]. 2022 [cited 2022 May 17];135:145–52. www.cmj.org.
World Health Organization. Tracking SARS-CoV-2 variants [cited 2022 Jul 2022] https://www.who.int/activities/tracking-SARS-CoV-2-variants. 2022. Accessed 25 July 2022
Ema-ecdc. ECDC-EMA statement on booster vaccination with Omicron adapted bivalent COVID-19 vaccines. [cited 2022 Oct 18]; www.ema.europa.eu/contact. Accessed 18 Oct 2022
EMA. Adapted vaccine targeting BA.4 and BA.5 Omicron variants and original SARS-CoV-2 recommended for approval | European Medicines Agency [Internet]. [cited 2022 Dec 14]. https://www.ema.europa.eu/en/news/adapted-vaccine-targeting-ba4-ba5-omicron-variants-original-sars-cov-2-recommended-approval. Accessed 14 Dec 2022
Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med [Internet]. 2022;386:340–50. https://doi.org/10.1056/NEJMoa2115481. (cited 2022 Mar 29).
Bedston S, Akbari A, Jarvis CI, Lowthian E, Torabi F, North L, et al. COVID-19 vaccine uptake, effectiveness, and waning in 82,959 health care workers: a national prospective cohort study in Wales. Vaccine [Internet]. 2022 [cited 2022 Mar 29];40:1180–9. https://pubmed.ncbi.nlm.nih.gov/35042645/.
Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA [Internet]. 2022 [cited 2022 Mar 29];327:639–51. https://jamanetwork.com/journals/jama/fullarticle/2788485.
Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med [Internet]. 2021;385:e83. https://doi.org/10.1056/NEJMoa2114114. (cited 2022 Mar 29).
Corrao G, Franchi M, Cereda D, Bortolan F, Zoli A, Leoni O, et al. Persistence of protection against SARS-CoV-2 clinical outcomes up to 9 months since vaccine completion: a retrospective observational analysis in Lombardy, Italy. Lancet Infect Dis [Internet]. 2022 [cited 2022 Mar 29];0. http://www.thelancet.com/article/S1473309921008136/fulltext.
Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, et al. Waning Immunity after the BNT162b2 Vaccine in Israel. N Engl J Med [Internet]. 2021;385:e85. https://doi.org/10.1056/nejmoa2114228.
Suah JL, Husin M, Keng PS, Hwa B, Thevananthan T, Low EV, et al. Waning COVID-19 vaccine effectiveness for BNT162b2 and CoronaVac in Malaysia: an observational study. Int J Infect Dis [Internet]. 2022 [cited 2022 Mar 30]; https://pubmed.ncbi.nlm.nih.gov/35331933/.
Israel A, Merzon E, Schäffer AA, Shenhar Y, Green I, Golan-Cohen A, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test-negative design study. BMJ [Internet]. 2021 [cited 2022 Mar 30];375. https://www.bmj.com/content/375/bmj-2021-067873.
Mizrahi B, Lotan R, Kalkstein N, Peretz A, Perez G, Ben-Tov A, et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun [Internet]. Nature Publishing Group; 2021 [cited 2022 Mar 30];12:1–5. https://www.nature.com/articles/s41467-021-26672-3.
Rosenberg ES, Dorabawila V, Easton D, Bauer UE, Kumar J, Hoen R, et al. Covid-19 vaccine effectiveness in New York State. N Engl J Med [Internet]. 2022 [cited 2022 Mar 30];386:116–27. https://pubmed.ncbi.nlm.nih.gov/34942067/.
Tenforde MW, Self WH, Adams K, Gaglani M, Ginde AA, McNeal T, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA [Internet]. 2021 [cited 2022 Mar 30];326:2043–54. https://jamanetwork.com/journals/jama/fullarticle/2786039.
Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions — United States, March–August 2021. MMWR Morb Mortal Wkly Rep [Internet]. 2021 [cited 2022 Mar 30];70:1337–43. https://www.cdc.gov/mmwr/volumes/70/wr/mm7038e1.htm.
Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after covid-19 vaccination and previous infection. New Engl J Med [Internet]. 2022. https://doi.org/10.1056/NEJMoa2118691. (cited 2022 Mar 30).
Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman LS, Ash N, et al. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N Engl J Med [Internet]. 2022 [cited 2022 Nov 3];386:2201–12. https://pubmed.ncbi.nlm.nih.gov/35613036/.
Zheutlin A, Ott M, Sun R, Zemlianskaia N, Meyer CS, Rubel M, et al. Durability of protection Post–Primary COVID-19 vaccination in the United States. Vaccines [Internet]. 2022 [cited 2022 Nov 3];10:1458. https://www.mdpi.com/2076-393X/10/9/1458/htm.
Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS, et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test-negative case-control study. BMJ [Internet]. 2021 [cited 2022 Mar 29];375. https://www.bmj.com/content/375/bmj-2021-068848.
Katikireddi SV, Cerqueira-Silva T, Vasileiou E, Robertson C, Amele S, Pan J, et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil. The Lancet [Internet]. 2022 [cited 2022 Mar 30];399:25–35. http://www.thelancet.com/article/S0140673621027549/fulltext.
Poukka E, Baum U, Palmu AA, Lehtonen TO, Salo H, Nohynek H, et al. Cohort study of Covid-19 vaccine effectiveness among healthcare workers in Finland, December 2020 - October 2021. Vaccine [Internet]. 2022 [cited 2022 Mar 30];40:701–5. https://pubmed.ncbi.nlm.nih.gov/34953607/.
Fowlkes A, Gaglani M, Groover K, Thiese MS, Tyner H, Ellingson K. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 Infection among frontline workers before and during B.1.617.2 (Delta) variant predominance—eight U.S. Locations, December 2020-August 2021. MMWR Morb Mortal Wkly Rep [Internet]. 2021 [cited 2022 Mar 30];70:1167–9. https://pubmed.ncbi.nlm.nih.gov/34437521/.
Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N Engl J Med [Internet]. 2021;385:e84. https://doi.org/10.1056/nejmoa2114583. (cited 2022 Apr 6).
Regev-Yochay G, Gonen T, Gilboa M, Mandelboim M, Indenbaum V, Amit S, et al. Efficacy of a fourth dose of covid-19 mRNA vaccine against omicron. N Engl J Med [Internet]. 2022 [cited 2022 Nov 3];386:1377–80. https://pubmed.ncbi.nlm.nih.gov/35297591/.
Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med [Internet]. 2022;386:1532–46. https://doi.org/10.1056/NEJMoa2119451. (cited 2022 May 13).
Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med [Internet]. 2022. https://doi.org/10.1056/NEJMoa2203965.
Pilz S, Theiler-Schwetz V, Trummer C, Krause R, Ioannidis JPA. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ Res. 2022;209:112911.
Sotoodeh Ghorbani S, Taherpour N, Bayat S, Ghajari H, Mohseni P, Hashemi Nazari SS. Epidemiologic characteristics of cases with reinfection, recurrence, and hospital readmission due to COVID-19: a systematic review and meta-analysis. J Med Virol [Internet]. 2022;94:44–53. https://doi.org/10.1002/jmv.27281. (cited 2022 May 17).
Petráš M. Highly Effective Naturally Acquired Protection Against COVID-19 Persists for at Least 1 Year: A Meta-Analysis. J Am Med Dir Assoc [Internet]. 2021 [cited 2022 May 17];22:2263–5. http://www.jamda.com/article/S1525861021007660/fulltext.
Shenai MB, Rahme R, Noorchashm H. Equivalency of protection from natural immunity in COVID-19 Recovered versus fully vaccinated persons: a systematic review and pooled analysis. 2021;
Chivese T, Matizanadzo JT, Musa OAH, Hindy G, Furuya-Kanamori L, Islam N, et al. The prevalence of adaptive immunity to COVID-19 and reinfection after recovery—a comprehensive systematic review and meta-analysis. Pathogen Glob Health. 2022. https://doi.org/10.1080/20477724.2022.2029301. (cited 2022 May 16).
Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. The Lancet [Internet]. 2021 [cited 2022 Mar 30];397:1204–12. http://www.thelancet.com/article/S0140673621005754/fulltext.
Nordström P, Ballin M, Nordström A. Risk of SARS-CoV-2 Reinfection and COVID-19 hospitalisation in individuals with natural- and hybrid immunity: a retrospective, total population cohort study in Sweden. SSRN Electron J [Internet]. 2022 [cited 2022 Mar 30]; https://papers.ssrn.com/abstract=4000584
Spicer KB, Glick C, Cavanaugh AM, Thoroughman D. Protective immunity after natural infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)—Kentucky, USA, 2020. Int J Infect Dis [Internet]. 2022 [cited 2022 Mar 30];114:21. https://pmc/articles/PMC8506664/.
Sheehan MM, Reddy AJ, Rothberg MB. Reinfection rates among patients who previously tested positive for coronavirus disease 2019: a retrospective cohort study. Clin Infect Dis [Internet]. 2021 [cited 2022 Mar 30];73:1882–6. https://pubmed.ncbi.nlm.nih.gov/33718968/
Fda. Vaccines and related biological products advisory committee December 10, 2020 Meeting Briefing Document- FDA. 2020. Accessed 26 May 2022
Fda, Cber. Vaccines and related biological products advisory committee December 17, 2020 Meeting Briefing Document -FDA. 2020. Accessed 26 May 2022
Biotech J. Vaccines and related biological products advisory committee February 26, 2021 Meeting Briefing Document- FDA. 2021. Accessed 26 May 2022
Lumley SF, Rodger G, Constantinides B, Sanderson N, Chau KK, Street TL, et al. An observational cohort study on the incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Infection and B.1.1.7 variant infection in healthcare workers by antibody and vaccination status. Clin Infect Dis [Internet]. 2022 [cited 2022 May 26];74:1208–19. https://academic.oup.com/cid/article/74/7/1208/6314286.
Satwik R, Satwik A, Katoch S, Saluja S. ChAdOx1 nCoV-19 effectiveness during an unprecedented surge in SARS COV-2 infections. Eur J Intern Med [Internet]. 2021 [cited 2022 May 26];93:112–3. http://www.ejinme.com/article/S0953620521002715/fulltext.
Shrestha NK, Burke PC, Nowacki AS, Terpeluk P, Gordon SM. Necessity of Coronavirus Disease 2019 (COVID-19) vaccination in persons who have already had COVID-19. Clin Infect Dis [Internet]. 2022 [cited 2022 Nov 3];75:e662–71. https://pubmed.ncbi.nlm.nih.gov/35028662/.
Gazit S, Shlezinger R, Perez G, Lotan R, Peretz A, Ben-Tov A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) naturally acquired immunity versus vaccine-induced immunity, reinfections versus breakthrough infections: a retrospective cohort study. Clin Infect Dis [Internet]. 2022 [cited 2022 Nov 3];75:e545–51. https://academic.oup.com/cid/article/75/1/e545/6563799.
Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med [Internet]. 2021;384:1899–909. https://doi.org/10.1056/NEJMoa2103055. (cited 2022 May 24).
Altarawneh HN, Chemaitelly H, Hasan MR, Ayoub HH, Qassim S, AlMukdad S, et al. Protection against the omicron variant from previous SARS-CoV-2 infection. N Engl J Med [Internet]. 2022;386:1288–90. https://doi.org/10.1056/nejmc2200133. (cited 2022 Aug 8).
Amanatidou E, Gkiouliava A, Pella E, Serafidi M, Tsilingiris D, Vallianou NG, et al. Breakthrough infections after COVID-19 vaccination: Insights, perspectives and challenges. Metabol Open [Internet]. 2022 [cited 2022 Apr 6];14:100180. http://pmc/articles/PMC8928742/.
Pajon R, Doria-Rose NA, Shen X, Schmidt SD, O’Dell S, McDanal C, et al. SARS-CoV-2 omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med. 2022;386:1088–91.
Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Amir O, Freedman L, et al. Protection by a fourth dose of BNT162b2 against omicron in Israel. Ne Engl J Med. 2022;386:1712–20. https://doi.org/10.1056/NEJMoa2201570. (cited 2022 May 13).
Hammerman A, Sergienko R, Friger M, Beckenstein T, Peretz A, Netzer D, et al. Effectiveness of the BNT162b2 vaccine after recovery from Covid-19. N Engld J Med [Internet]. 2022;386:1221–9. https://doi.org/10.1056/nejmoa2119497. (cited 2022 Jun 28).
Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med [Internet]. 2021 [cited 2022 Apr 6];27:1205–11. https://pubmed.ncbi.nlm.nih.gov/34002089/.
Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science [Internet]. 2022 [cited 2022 Apr 6];375:43–50. https://pubmed.ncbi.nlm.nih.gov/34812653/.
Yang Y, Yang M, Peng Y, Liang Y, Wei J, Xing L, et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat Microbiol [Internet]. 2022 [cited 2022 Apr 7];7:423–33. https://www.nature.com/articles/s41564-021-01051-2.
Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med [Internet]. 2021 [cited 2022 Apr 7];13. https://pubmed.ncbi.nlm.nih.gov/33288662/.
Gasser R, Cloutier M, Prévost J, Fink C, Ducas É, Ding S, et al. Major role of IgM in the neutralizing activity of convalescent plasma against SARS-CoV-2. Cell Rep. 2021;34:108790.
Crawford KHD, Dingens AS, Eguia R, Wolf CR, Wilcox N, Logue JK, et al. Dynamics of neutralizing antibody titers in the months after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis [Internet]. 2021 [cited 2022 Apr 6];223:197–205. https://academic.oup.com/jid/article/223/2/197/5916372.
Choe PG, Hong J, Park J, Chang E, Kang CK, Kim NJ, et al. Persistent antibody responses up to 18 months after mild severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis [Internet]. 2022. https://doi.org/10.1093/infdis/jiac099/6550287. (cited 2022 May 18).
Legros V, Denolly S, Vogrig M, Boson B, Siret E, Rigaill J, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol [Internet]. 2021 [cited 2022 May 18];18:318–27. https://www.nature.com/articles/s41423-020-00588-2.
Hoffmann M, Krüger N, Schulz S, Cossmann A, Rocha C, Kempf A, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185:447-456.e11.
Brunner WM, Freilich D, Victory J, Krupa N, Scribani MB, Jenkins P, et al. Comparison of antibody response durability of mRNA-1273, BNT162b2, and Ad26COV2.S SARS-CoV-2 vaccines in healthcare workers. Int J infect Dis. 2022. https://doi.org/10.1101/2022.01.14.22269297. (cited 2022 Apr 5).
Collier AY, Yu J, McMahan K, Liu J, Chandrashekar A, Maron JS, et al. Differential kinetics of immune responses elicited by covid-19 vaccines. N Engl J Med [Internet]. 2021;385:2010–2. https://doi.org/10.1056/NEJMc2115596. (cited 2022 May 23).
Pegu A, O’Connell S, Schmidt SD, O’Dell S, Talana CA, Lai L, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science. 2021;373(6561):1372–77. https://doi.org/10.1126/science.abj4176.
Falsey AR, Frenck RW, Walsh EE, Kitchin N, Absalon J, Gurtman A, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med [Internet]. 2021;385:1627–9. https://doi.org/10.1056/NEJMc2113468. (cited 2022 Apr 5).
Shrotri M, Navaratnam AMD, Nguyen V, Byrne T, Geismar C, Fragaszy E, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet [Internet]. 2021 [cited 2022 May 18];398:385. http://pmc/articles/PMC8285117/.
Kwok SLL, Cheng SMS, Leung JNS, Leung K, Lee CK, Peiris JSM, et al. Waning antibody levels after COVID-19 vaccination with mRNA Comirnaty and inactivated CoronaVac vaccines in blood donors, Hong Kong, April 2020 to October 2021. Eurosurveillance [Internet]. 2022 [cited 2022 Apr 4];27:1. http://pmc/articles/PMC8759113/.
Chen Y, Yin S, Tong X, Tao Y, Ni J, Pan J, et al. Dynamic SARS-CoV-2-specific B-cell and T-cell responses following immunization with an inactivated COVID-19 vaccine. Clin Microbiol Infect [Internet]. 2022;28:410–8. https://doi.org/10.1016/j.cmi.2021.10.006.
Keshavarz B, Richards NE, Workman LJ, Patel J, Muehling LM, Canderan G, et al. Trajectory of IgG to SARS-CoV-2 after vaccination With BNT162b2 or mRNA-1273 in an employee cohort and comparison with natural infection. Front Immunol. 2022;13:1144.
Haidar G, Agha M, Bilderback A, Lukanski A, Linstrum K, Troyan R, et al. Prospective evaluation of COVID-19 vaccine responses across a broad spectrum of immunocompromising conditions: the COVICS study. Clinical infectious diseases. Oxford University Press (OUP); 2022.
Jalkanen P, Kolehmainen P, Häkkinen HK, Huttunen M, Tähtinen PA, Lundberg R, et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun [Internet]. 2021 [cited 2022 Apr 6];12:1–11. https://www.nature.com/articles/s41467-021-24285-4.
Zhang R, Khong KW, Leung KY, Liu D, Fan Y, Lu L, et al. Antibody response of BNT162b2 and CoronaVac platforms in recovered individuals previously infected by COVID-19 against SARS-CoV-2 wild type and delta variant. Vaccines [Internet]. 2021 [cited 2022 Apr 4];9:1442. https://www.mdpi.com/2076-393X/9/12/1442/htm.
Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2021;602(7898):654–56. https://doi.org/10.1038/s41586-021-04387-1
Gruell H, Vanshylla K, Tober-Lau P, Hillus D, Schommers P, Lehmann C, et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med. 2022; 28(3):477–80. https://doi.org/10.1038/s41591-021-01676-0.
Schmidt F, Muecksch F, Weisblum Y, da Silva J, Bednarski E, Cho A, et al. Plasma neutralization of the SARS-CoV-2 omicron variant. N Engl J Med. 2022;386:599–601.
Garcia-Beltran WF, St. Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457-466.e4.
Pérez-Then E, Lucas C, Monteiro VS, Miric M, Brache V, Cochon L, et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med [Internet]. 2022 [cited 2022 Apr 6];28:481–5. https://www.nature.com/articles/s41591-022-01705-6.
Zhao Z, Cui T, Huang M, Liu S, Su X, Li G, et al. Heterologous boosting with third dose of coronavirus disease recombinant subunit vaccine increases neutralizing antibodies and T cell immunity against different severe acute respiratory syndrome coronavirus 2 variants. Emerg Microbes Infect. 2022;11:829–40. https://doi.org/10.1080/22221751.2022.2048969. (cited 2022 Apr 6).
Cheng H, Peng Z, Si S, Alifu X, Zhou H, Chi P, et al. Immunogenicity and safety of homologous and heterologous Prime–Boost immunization with COVID-19 vaccine: systematic review and meta-analysis. Vaccines [Internet]. 2022 [cited 2022 May 25];10:798. https://www.mdpi.com/2076-393X/10/5/798/htm.
Bertoletti A, le Bert N, Qui M, Tan AT. SARS-CoV-2-specific T cells in infection and vaccination. Cell Molecul Immunol [Internet]. 2021 [cited 2022 Apr 5];18:2307–12. https://www.nature.com/articles/s41423-021-00743-3.
Martinez-Sobrido L, Almazan Toral F, Faraz Ahmed S, Abdul Quadeer A, McKay MR. SARS-CoV-2 T cell responses elicited by COVID-19 vaccines or infection are expected to remain robust against omicron. Viruses [Internet]. 2022 [cited 2022 May 18];14:79. https://www.mdpi.com/1999-4915/14/1/79/htm.
Çölkesen F, Kurt EK, Vatansev H, Korkmaz C, Çölkesen F, Yücel F, et al. Memory B cells and serum immunoglobulins are associated with disease severity and mortality in patients with COVID-19. Postgrad Med J [Internet]. 2022 [cited 2022 May 18];0:postgradmedj-2021–140540. https://pmj.bmj.com/content/early/2022/01/18/postgradmedj-2021-140540.
Ciabattini A, Pastore G, Fiorino F, Polvere J, Lucchesi S, Pettini E, et al. Evidence of SARS-CoV-2-specific memory B cells six months after vaccination with the BNT162b2 mRNA vaccine. Front Immunol. 2021;12:3751.
Zuo J, Dowell AC, Pearce H, Verma K, Long HM, Begum J, et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nate Immunol [Internet]. 2021 [cited 2022 Apr 6];22:620–6. https://www.nature.com/articles/s41590-021-00902-8.
Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol [Internet]. 2022 [cited 2022 Apr 5];23:186–93. https://www.nature.com/articles/s41590-021-01122-w.
Adam L, Rosenbaum P, Quentric P, Parizot C, Bonduelle O, Guillou N, et al. CD8+PD-L1+CXCR3+ polyfunctional T cell abundances are associated with survival in critical SARS-CoV-2-infected patients. JCI Insight [Internet]. 2021 [cited 2022 Apr 5];6. https://pubmed.ncbi.nlm.nih.gov/34283810/.
da Silva Antunes R, Pallikkuth S, Williams E, Dawen Yu E, Mateus J, Quiambao L, et al. Differential T-Cell Reactivity to Endemic Coronaviruses and SARS-CoV-2 in Community and Health Care Workers. J Infect Dis [Internet]. 2021 [cited 2022 Apr 5];224:70–80. https://pubmed.ncbi.nlm.nih.gov/33822097/.
Nelde A, Bilich T, Heitmann JS, Maringer Y, Salih HR, Roerden M, et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat Immunol [Internet]. 2021 [cited 2022 Apr 5];22:74–85. https://pubmed.ncbi.nlm.nih.gov/32999467/.
Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin JB, Olsson A, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell [Internet]. 2020 [cited 2022 Apr 5];183:158–168.e14. https://pubmed.ncbi.nlm.nih.gov/32979941/
Gallais F, Velay A, Nazon C, Wendling MJ, Partisani M, Sibilia J, et al. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg Infect Dis [Internet]. 2021 [cited 2022 May 19];27:113. http://pmc/articles/PMC7774579/.
Havervall S, Ng H, Jernbom Falk A, Greilert-Norin N, Månberg A, Marking U, et al. Robust humoral and cellular immune responses and low risk for reinfection at least 8 months following asymptomatic to mild COVID-19. J Intern Med [Internet]. 2022;291:72–80. https://doi.org/10.1111/joim.13387. (cited 2022 May 19).
Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell [Internet]. 2020 [cited 2022 May 19];183:996–1012.e19. https://pubmed.ncbi.nlm.nih.gov/33010815/.
Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell [Internet]. 2020 [cited 2022 May 19];181:1489–1501.e15. https://pubmed.ncbi.nlm.nih.gov/32473127/.
Fendler A, Shepherd STC, Au L, Wilkinson KA, Wu M, Byrne F, et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nature Cancer [Internet]. Nature 2021 [cited 2022 May 19];2:1305–20. https://www.nature.com/articles/s43018-021-00274-w
Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science [Internet]. 2021 [cited 2022 May 19];371. https://pubmed.ncbi.nlm.nih.gov/33408181/.
Li Y, Wang X, Shen X-R, Geng R, Xie N, Han J-F, et al. A 1-year longitudinal study on COVID-19 convalescents reveals persistence of anti-SARS-CoV-2 humoral and cellular immunity. Emerg Microbes Infect. 2022;11:902–13. https://doi.org/10.1080/22221751.2022.2049984. (cited 2022 Apr 5).
Adamo S, Michler J, Zurbuchen Y, Cervia C, Taeschler P, Raeber ME, et al. Signature of long-lived memory CD8+ T cells in acute SARS-CoV-2 infection. Nature [Internet]. 2021 [cited 2022 Apr 6];602:148–55. https://www.nature.com/articles/s41586-021-04280-x.
Mak WA, Koeleman JGM, van der Vliet M, Keuren F, Ong DSY. SARS-CoV-2 antibody and T cell responses one year after COVID-19 and the booster effect of vaccination: a prospective cohort study. J Infect. 2022;84:171–8.
Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021 [cited 2022 Apr 6];21:475–84. https://www.nature.com/articles/s41577-021-00578-z.
Gallagher KME, Leick MB, Larson RC, Berger TR, Katsis K, Yam JY, et al. Differential T-cell immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in mRNA-1273– and BNT162b2-vaccinated individuals. Clin Infect Dis Internet. 2022. https://doi.org/10.1093/cid/ciac201/6547848. (cited 2022 May 20).
Markewitz R, Pauli D, Dargvainiene J, Steinhagen K, Engel S, Herbst V, et al. The temporal course of T- and B-cell responses to vaccination with BNT162b2 and mRNA-1273. Clin Microbiol Infect. 2022;28:701–9.
Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature [Internet]. 2021 [cited 2022 May 19];595:572–7. https://www.nature.com/articles/s41586-021-03653-6.
Woldemeskel BA, Garliss CC, Blankson JN. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J Clin Invest [Internet]. 2021. https://doi.org/10.1172/JCI149335DS1. (cited 2022 May 20).
Ukey R, Bruiners N, Mishra H, Mishra PK, McCloskey D, Onyuka A, et al. Dichotomy between the humoral and cellular responses elicited by mRNA and adenoviral vector vaccines against SARS-CoV-2. BMC Med [Internet]. 2022;20:1–7. https://doi.org/10.1186/s12916-022-02252-0. (cited 2022 May 20).
Naranbhai V, Garcia-Beltran WF, Chang CC, Berrios Mairena C, Thierauf JC, Kirkpatrick G, et al. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 vaccines. J Infect Dis [Internet]. 2022 [cited 2022 May 19];225:1141–50. https://academic.oup.com/jid/article/225/7/1141/6458467.
Zhang Z, Mateus J, Coelho CH, Dan JM, Moderbacher CR, Gálvez RI, et al. Humoral and cellular immune memory to four COVID-19 vaccines. bioRxiv [Internet]. 2022. https://doi.org/10.1101/2022.03.18.484953v1. (cited 2022 May 20).
Kim JY, Lim SY, Park S, Kwon JS, Bae S, Park JY, et al. Immune responses to the ChAdOx1 nCoV-19 and BNT162b2 vaccines and to natural coronavirus disease 2019 infections over a 3-month period. J Infect Dis [Internet]. 2022 [cited 2022 May 20];225:777–84. https://academic.oup.com/jid/article/225/5/777/6440288.
Wen Lim W, Mak L, Leung GM, Cowling BJ, Peiris M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. The Lancet. 2021. https://doi.org/10.1038/s41591-021-01377-8. (cited 2022 May 20).
Zhuang C, Liu X, Chen Q, Sun Y, Su Y, Huang S, et al. Protection Duration of COVID-19 vaccines: waning effectiveness and future perspective. Front Microbiol [Internet]. 2022 [cited 2022 Apr 4];13. http://pmc/articles/PMC8902038/.
Ameratunga R, Woon ST, Lea E, Steele R, Lehnert K, Leung E, et al. The (apparent) antibody paradox in COVID-19. Expert Rev Clin Immunol. 2022;18:335–45. https://doi.org/10.1080/1744666X.2022.2044797. (cited 2022 May 20).
Mateus J, Dan JM, Zhang Z, Moderbacher CR, Lammers M, Goodwin B, et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. https://doi.org/10.1126/science.abj9853 (cited 2022 May 20).
Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science [Internet]. 2021 [cited 2022 May 23];374. https://pubmed.ncbi.nlm.nih.gov/34648302/.
Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022 603:7901 [Internet]. 2022 [cited 2022 Apr 4];603:488–92. https://www.nature.com/articles/s41586-022-04460-3.
Tarke A, Sidney J, Methot N, Yu ED, Zhang Y, Dan JM, et al. Impact of SARS-CoV-2 variants on the total CD4 + and CD8 + T cell reactivity in infected or vaccinated individuals. Cell Rep Med [Internet]. 2021 [cited 2022 Apr 4];2. https://pubmed.ncbi.nlm.nih.gov/34230917/.
Riou C, Keeton R, Moyo-Gwete T, Hermanus T, Kgagudi P, Baguma R, et al. Escape from recognition of SARS-CoV-2 variant spike epitopes but overall preservation of T cell immunity. Sci Transl Med [Internet]. 2022;14:eabj6824. https://doi.org/10.1126/scitranslmed.abj6824. (cited 2022 Apr 4).
Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185:847-859.e11.
Jung MK, Jeong SD, Noh JY, Kim D-U, Jung S, Song JY, et al. BNT162b2-induced memory T cells respond to the Omicron variant with preserved polyfunctionality. Nat Microbiol. 2022 [Internet]. 2022 [cited 2022 May 19];1–9. https://www.nature.com/articles/s41564-022-01123-x.
Ahmed SF, Quadeer AA, McKay MR. SARS-CoV-2 T cell responses elicited by COVID-19 vaccines or infection are expected to remain robust against omicron. Viruses [Internet]. 2022 [cited 2022 May 19];14:79. https://www.mdpi.com/1999-4915/14/1/79/htm.
GeurtsvanKessel CH, Geers D, Schmitz KS, Mykytyn AZ, Lamers MM, Bogers S, et al. Divergent SARS CoV-2 Omicron-reactive T- and B cell responses in COVID-19 vaccine recipients. Sci Immunol [Internet]. 2022 [cited 2022 Apr 4];7. http://pmc/articles/PMC8939771/.
Naranbhai V, Nathan A, Kaseke C, Berrios C, Khatri A, Choi S, et al. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals. Cell. 2022;185:1041-1051.e6.
Geers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol [Internet]. 2021 [cited 2022 Apr 4];6. https://pubmed.ncbi.nlm.nih.gov/34035118/.
Reynolds CJ, Pade C, Gibbons JM, Butler DK, Otter AD, Menacho K, et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science [Internet]. 2021 [cited 2022 Apr 4];372:1418–23. http://pmc/articles/PMC8168614/.
Alter G, Yu J, Liu J, Chandrashekar A, Borducchi EN, Tostanoski LH, et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature [Internet]. 2021 [cited 2022 Apr 4];596:268. http://pmc/articles/PMC8357629/.
Sokal A, Barba-Spaeth G, Fernández I, Broketa M, Azzaoui I, de La Selle A, et al. mRNA vaccination of naive and COVID-19-recovered individuals elicits potent memory B cells that recognize SARS-CoV-2 variants. Immunity [Internet]. 2021 [cited 2022 May 23];54:2893–2907.e5. http://www.cell.com/article/S1074761321003964/fulltext.
Mahase E. Covid-19: Longer interval between Pfizer doses results in higher antibody levels, research finds. 2021 [cited 2022 May 24]; http://www.bmj.com/.
Payne RP, Longet S, Austin JA, Skelly DT, Dejnirattisai W, Adele S, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell [Internet]. 2021 [cited 2022 Apr 4];184:5699. http://pmc/articles/PMC8519781/.
Abdool Karim SSA. Vaccines and SARS-CoV-2 variants: the urgent need for a correlate of protection. The Lancet [Internet]. 2021 [cited 2022 Apr 6];397:1263–4. http://www.thelancet.com/article/S0140673621004682/fulltext.
Chmielewska AM, Czarnota A, Bieńkowska-Szewczyk K, Grzyb K. Immune response against SARS-CoV-2 variants: the role of neutralization assays. npj Vaccines [Internet]. 2021 [cited 2022 Apr 6];6:1–8. https://www.nature.com/articles/s41541-021-00404-6.
Acknowledgements
We are grateful to Lorenzo Bertizzolo for his help in the development of the review.
Funding
This work was supported by Sanofi who also funded the journal’s Rapid Service Fee.
Medical Writing/Editorial Assistance
We are grateful to Lorenzo Bertizzolo for his help with the initial development of the review. Lorenzo Bertizzolo is an employee of Sanofi and may own shares or stock options.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Author Contributions
Nick Pooley, Salim S. Abdool Karim, Behazine Combadiere, Eng Eong Ooi, Rebecca C Harris, Clotilde El Guerche Seblain, Masoume Kisomi and Nabila Shaikh interpreted the data and drafted the manuscript.
Disclosures
Nick Pooley and Masoume Kisomi received consulting fees from Nestle Health Sciences. Behazine Combadiere is a member of Sanofi and Osivax advisory boards and notes participation to conferences on behalf of Sanofi, Pfizer and GSK. Salim Abdool Karim is a member of Sanofi’s medical advisory board on Covid-19 vaccines. Eng Eong Ooi is a member of Sanofi’s COVID-19 vaccine advisory board. Rebecca C. Harris, Clotilde El Guerche Seblain and Nabila Shaikh are employees of Sanofi and may own shares or stock options.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors. Ethics approval was not required for this narrative review.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pooley, N., Abdool Karim, S.S., Combadière, B. et al. Durability of Vaccine-Induced and Natural Immunity Against COVID-19: A Narrative Review. Infect Dis Ther 12, 367–387 (2023). https://doi.org/10.1007/s40121-022-00753-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00753-2