Abstract
Introduction
Non-invasive tests (NITs) have been alternative methods of liver biopsy for the cross-sectional assessment of liver fibrosis in patients with chronic hepatitis B (CHB). However, there are limited data on the longitudinal association between NITs and histological changes of liver fibrosis. This study aimed to evaluate whether NITs can be used to assess liver fibrosis regression (LFR) during anti-HBV treatment.
Methods
This retrospective study included 337 patients with CHB who underwent contemporaneous NITs, such as liver stiffness measurement (LSM), the aspartate aminotransferase to platelet ratio index (APRI), the fibrosis index based on four factors (FIB-4), and the γ-glutamyl transpeptidase to platelet ratio (GPR), and liver biopsy at baseline and followed by a repeated liver biopsy and NITs assessment. The LFR was defined as fibrosis regression by at least one stage assessed by METAVIR scoring system.
Results
The median interval between the two paired liver biopsy assessment was 31 months (IQR 24–45). At the first liver biopsy, the fibrosis stage was F2 in 159 (47.2%), F3 in 68 (20.2%), and F4 in 110 (32.6%) patients. At the second liver biopsy, the number of patients with fibrosis stages F0–1, F2, F3, and F4 was 102 (30.3%), 106 (31.5%), 63 (18.7%), and 66 (19.6%), respectively. At follow-up liver biopsy, 169 patients (50.1%) had LFR, 128 patients (38.0%) had no change in fibrosis stage, and 40 patients (11.9%) had liver fibrosis progression on histology. A decrease in liver stiffness measurement (LSM) by 25% is the optimal cutoff for predicting LFR. Patients with a 25% or larger decrease in LSM value had more LFR than those with a less than 25% decrease in LSM value (78.1% vs 22.9%, p < 0.001).
Conclusion
LSM might be used to monitor regression of liver fibrosis during antiviral treatment using nucleos(t)ide analogues (NUCs) in patients with CHB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
For patients with CHB, anti-HBV therapy using NUCs was associated with a decrease in LSM value, and LSM might be used to monitor regression of liver fibrosis during anti-HBV therapy. |
Using repeated liver biopsy as the gold standard, we found that a 25% decrease in LSM value is associated with regression of liver fibrosis on histology. |
Patients with a 25% or larger decrease in LSM value had more liver fibrosis regression than those with a less than 25% decrease in LSM value (78.1% vs 22.9%, p < 0.001). |
Serum-based NITs of liver fibrosis including APRI, FIB-4, and GPR did not appear to be useful in detecting liver fibrosis regression during anti-HBV treatment. |
Introduction
In recent decades, chronic hepatitis B virus (HBV) infection remains a global public health problem. Patients with chronic HBV infection have a significantly increased risk of progression to hepatic fibrosis, cirrhosis, liver failure, and hepatocellular carcinoma (HCC) [1]. According to the European Association for the Study of the Liver (EASL) 2017 clinical practice guidelines on the management of HBV infection, the induction of long-term suppression of HBV replication is the main endpoint of current anti-HBV treatment strategies, including peg-interferon (Peg-IFN) and nucleos(t)ide analogues (NUCs) [1]. Regression of liver fibrosis and cirrhosis can be regarded as a further endpoint of long-term antiviral therapy in patients with chronic hepatitis B (CHB) who have established liver fibrosis or cirrhosis, because it reduces the risk of liver decompensation, and HCC [1].
Currently, there are two main treatment options for patients with CHB: treatment with NUCs or with Peg-IFN. The main advantage of Peg-IFN is the potential for immune-mediated control of HBV infection with a chance of HBsAg loss [1]. The main disadvantages of Peg-IFN treatment are the high variability of response and its unfavorable safety profile making a significant number of patients ineligible or unwilling for this type of treatment [1]. NUCs can significantly inhibit viral replication and alleviate liver injury, although most patients require long-term or even life-long therapy. In addition, the standard duration of Peg-IFN therapy is 48 weeks. By contrast, long-term (at least 3 years) or even life-long therapy is recommended for patients with CHB to inhibit HBV replication [1].
Indeed, until 2001, liver fibrosis was considered to be irreversible [2]. However, in the last two decades, it has been reported that long-term suppression of HBV using NUCs can lead to liver fibrosis regression (LFR) [3, 4]. Therefore, in addition to estimation of the degree of liver fibrosis before starting anti-HBV therapy, it is important to monitor the dynamic changes in the degree of liver fibrosis after treatment, as mortality associated with CHB mainly results from the development of liver fibrosis and the subsequent occurrence of cirrhosis, liver decompensation, and HCC [5].
Liver biopsy has been considered the gold standard for determining liver fibrosis stage. However, because the procedure of liver biopsy is invasive and associated with a risk of life-threatening complications, repeated liver biopsy is rarely performed [6, 7]. In contrast to liver biopsy, non-invasive tests (NITs) of liver fibrosis are safe and reproducible, and are potential alternative methods for monitoring the dynamic changes in the degree of liver fibrosis [8]. Imaging-based NITs of liver fibrosis, most commonly transient elastography (TE) and magnetic resonance elastography (MRE), measure liver stiffness, which correlates with liver fibrosis severity [9]. The aspartate aminotransferase to platelet ratio index (APRI), the fibrosis index based on four factors (FIB-4), and the γ-glutamyl transpeptidase to platelet ratio (GPR) are the most commonly studied serum-based NITs of liver fibrosis in patients with CHB [10, 11].
Although several longitudinal investigations reported that the clinical utility of the dynamic changes in liver stiffness measurement (LSM) can predict LFR in patients with CHB and long-term suppression of HBV replication using NUCs, paired liver biopsy data were not available, and they did not compare the changes in NITs of liver fibrosis with the histological changes of liver fibrosis on repeated liver biopsies [4, 12]. In this study, we retrospectively collected data from patients with repeated liver biopsies and NITs assessment. Utilizing the cohort, we evaluated the association between the dynamic changes in NITs of liver fibrosis and LFR on repeated liver biopsies. We aimed to answer whether NITs can be used to assess the dynamic changes in the degree of liver fibrosis after treatment using NUCs in patients with CHB.
Methods
Patients
This retrospective study included 337 patients with CHB who underwent paired liver biopsy and NITs of liver fibrosis at two points between 2013 and 2020 in the Department of Liver Disease, Shanghai Public Health Clinical Center, Shanghai, China. The inclusion criteria were (1) HBsAg positive for more than 6 months; (2) NITs of liver fibrosis were measured contemporaneously with their baseline liver biopsy, and had a subsequent liver biopsy and contemporaneous NITs assessment; (3) initially significant hepatic fibrosis which mean METAVIR fibrosis score of at least F2 on liver histology; (4) detectable serum HBV DNA before anti-HBV treatment; (5) anti-HBV therapy using NUCs. The exclusion criteria were (1) underlying chronic liver disease other than CHB including chronic hepatitis C (CHC), hepatitis D, non-alcoholic fatty liver disease (NAFLD), autoimmune liver disease, hemochromatosis, Wilson’s disease, and cholestatic or vascular liver disease; (2) coinfection with human immunodeficiency virus (HIV); (3) alcohol consumption of 20 g/day or higher for more than 5 years; (4) unreliable LSM value or LSM failure (no valid shots); (5) lack of one or more lab value which is necessary for the study.
The study was approved by the Clinical Research Ethics Committee of Shanghai Public Health Clinical Center (No. 2022-Y051-01). Although this is a retrospective study, all patients provided verbal consent that their clinical data might be used for further medical study. The procedure was documented in the files we submitted to the Clinical Research Ethics Committee. The procedures were in accordance with the ethical standards of the Helsinki Declaration (1964, amended most recently in 2008) of the World Medical Association.
Indication for Liver Biopsy After Treatment
Marcellin et al. [3] found that long-term suppression of HBV can lead to regression of fibrosis and cirrhosis in 51% of patients with chronic HBV infection. Although there is no guideline to recommend repeated liver biopsy after long-term anti-HBV treatment, monitoring response to treatment and evaluating fibrosis regression are of irreplaceable importance for patients with CHB. In fact, some patients are eager to know if they have regression of fibrosis and cirrhosis after long-term NUCs therapy. In this study, the second liver biopsy were performed according to the wishes of patients after a long-term anti-HBV therapy of at least 2 years. Therefore, in this study, the liver biopsy interval is variable rather than a specific duration.
Liver Histologic Evaluation
All patients underwent a baseline liver biopsy, followed by a second liver biopsy for assessment of the histological changes of liver fibrosis. The median interval between the two paired assessment was 31 months (IQR 24–45). The biopsy specimens were fixed with 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin [13]. A minimum of 15 mm of liver tissue with at least six portal tracts was considered suitable for liver histopathological analysis [13]. The METAVIR scoring system was used to assess the stage of liver fibrosis and the grade of necroinflammatory activity [14]. The stage of liver fibrosis is evaluated on a 5-point scale (F0 means no fibrosis, F1 means portal fibrosis without septa, F2 means few septa, F3 means numerous septa without cirrhosis, F4 means cirrhosis) [14]. The grade of liver necroinflammatory activity is assessed on a 4-point scale (A0 means no necroinflammatory activity, A1 means mild necroinflammatory activity, A2 means moderate necroinflammatory activity, A3 means severe necroinflammatory activity) [14]. Significant liver fibrosis means a METAVIR fibrosis score of at least F2 and significant hepatic necroinflammation means a METAVIR necroinflammatory activity score of at least A2.
Liver Stiffness Measurement
Liver stiffness measurement using FibroScan was performed following the standard of practice and the company’s instruction [8]. Briefly, the tip of the probe is placed in contact with the intercostal skin using coupling gel in the 9th to 11th intercostal space. The operator locates a liver portion at least 6 cm deep and free of large vascular structures, and then presses the probe button to start the measurements [15]. The software determines whether each measurement is successful or not. The final result of a TE session can be regarded as valid if the following criteria are fulfilled [8]: (1) the number of valid shots is at least 10, (2) a success rate (the ratio of valid shots to the total number of shots) above 60%, and (3) an interquartile range (IQR) less than 30% of the median LSM value (IQR/M ≤ 0.30%). LSM scores are expressed as kilopascals (kPa).
Variables Extracted from Medical Records
The age, sex, HBV serological markers, HBV DNA levels, routine blood results including platelet, serum biochemical parameters including ALT, AST, and GGT, the results of liver biopsy, and NITs were extracted from the electronic medical records.
Serum-Based NITs of Liver Fibrosis
The APRI, FIB-4, and GPR were evaluated for comparison with LSM [10].
-
1.
APRI = [AST (IU/L)/ULN]/platelet (109/L) × 100 [10]
-
2.
FIB-4 = [age (years) × AST (IU/L)]/{platelet (109/L) × [ALT (IU/L)]1/2} [10]
-
3.
GPR = [γ-GT (IU/L)/ULN]/platelet (109/L) × 100 [10]
Definitions
LFR is defined as at least one stage improvement from baseline histologic assessment to follow-up histologic assessment. NITs 1 means the NITs value synchronized with the first liver biopsy. NITs 2 means the NITs value synchronized with the second liver biopsy. The decrease rate of NITs value = (NITs1 − NITs2)/NIT1 × 100%. For example, the decrease rate of LSM value = (LSM1 − LSM2)/LSM1 × 100%.
Statistical Analysis
Variables were expressed as mean ± SD or median (IQR) for numerical variables and as frequency and percentage for categorical variables. The Student’s t test was used for comparisons between normal distribution continuous variables, the Mann–Whitney U test was used for comparisons between non-normal distribution continuous variables, and the chi-squared test was used for comparisons between categorical variables [15]. Receiver operating characteristic (ROC) curves and the areas under the ROC curves (AUROC) were used to evaluate the performances of NITs for the prediction of LFR. The comparison of AUROCs was performed using the DeLong test [16]. A two-sided p < 0.05 was considered to be statistically significant. All statistical analysis were performed using SPSS V 0.19.0 (SPSS Inc., IL, and USA), and Graphpad Prism 8.0 (GraphPad Software Inc., La Jolla, and CA).
Results
Characteristics of Study Population
Patient characteristics are presented in Table 1. The median interval between the first and the second liver biopsy was 31 months (IQR 24–45). Patients were aged 35 years (IQR 30–42) at the first liver biopsy, and 210 patients (62.3%) were male. Serum HBV DNA was detectable in all patients, and HBeAg was detected in 242 patients (71.8%) before starting anti-HBV treatment. At baseline, the median HBV DNA was 4.93 log10 IU/ml (IQR 2.93–6.69), the median HBsAg was 3.50 log10 IU/ml (IQR 3.15–4.02), and the median ALT was 63 IU/L (IQR 33–202). NUCs used for antiviral treatment included lamivudine (17 patients, 5%), adefovir (10 patients, 3%), entecavir (235 patients, 69.7%), and tenofovir (75 patients, 22.3%). The selection of NUCs was made after considering the availability of national insurance coverage, drug prices, patient demand, and the decision of the clinician. This is a retrospective study including patients between 2013 and 2020; a small number of patients (27 patients, 8%) chose second-line drugs because of their cheap prices.
Changes in NITs of Liver Fibrosis and Histological Liver Fibrosis Stage
Changes in NITs of liver fibrosis and histological liver fibrosis stage are presented in Table 1. At the first liver biopsy, the fibrosis stage was F2 in 159 (47.2%), F3 in 68 (20.2%), and F4 in 110 (32.6%) patients. At the second liver biopsy, the number of patients with fibrosis stages F0–1, F2, F3, and F4 was 102 (30.3%), 106 (31.5%), 63 (18.7%), and 66 (19.6%), respectively. At follow-up liver biopsy, 169 patients (50.1%) had LFR, 128 patients (38.0%) had no change in fibrosis stage, and 40 patients (11.9%) had fibrosis progression on histology. The median baseline LSM value of the patients was 10.0 kPa. However, it decreased significantly to 7.8 kPa after antiviral treatment (p < 0.001). The median baseline APRI, FIB-4, and GPR values of the patients were 0.97, 1.66, and 0.64, respectively. However, they decreased significantly to 0.56, 1.18, and 0.38, respectively, after antiviral treatment (all p < 0.001).
Comparison of Patients with and Without LFR
Comparison of patients with and without LFR at baseline is shown in Table 2. The median time between liver biopsies did not vary between patients with and without LFR (32 vs 29 months, p = 0.234). Patients with LFR had significantly higher age (median, 37 vs 34 years, p = 0.047) and LSM value (median, 11.3 vs 9.0 kPa, p = 0.026) compared to those without LFR. No significant differences were found in baseline characteristics between patients with and without LFR in sex, HBeAg positive rate, HBV DNA, HBsAg, platelet, ALT, AST, γ-GT, APRI, FIB-4, and GPR (p > 0.05).
Variables Associated with LFR
Variables associated with LFR are shown in Table 3. Univariate analysis showed that age (p = 0.044) and the decrease rate of LSM value (p < 0.001) and GPR (p = 0.047) were associated with LFR. Figure 1 shows that the decrease rate of LSM value (41.3% vs − 2.9%, p < 0.001) and the decrease rate of GPR (26.9% vs − 15.0%, p = 0.016) were significantly higher in patients with LFR compared with those without LFR (Fig. 1a, c). Variables which were significant in univariate analysis were entered into a multivariate analysis; the latter showed that only the decrease rate of LSM value was associated with LFR [OR (95% CI) 1.023 (1.016–1.029), p < 0.001] (Table 3). Changes in APRI, FIB-4, or GPR are not associated with LFR on multivariate analysis (all p > 0.05).
Changes in NITs of liver fibrosis between patients with and without LFR. NITs, non-invasive tests; LFR, liver fibrosis regression. The decrease rate of LSM value (41.3% vs − 2.9%, p < 0.001) and the decrease rate of GPR (26.9% vs − 15.0%, p = 0.016) were significantly higher in patients with LFR compared with those without LFR (a, c). No significant differences were found in the decrease rate of APRI (p = 0.076) and FIB-4 (p = 0.096) between patients with LFR and those without LFR (b, d)
Clinical Utility of a Decrease in LSM in Predicting LFR
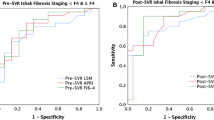
Using paired liver biopsies as the gold standard, we compared the performances of LSM value and serum-based NITs of liver fibrosis for the prediction of LFR (Table 4 and Fig. 2). The AUROC of the decrease rate of LSM value is significantly higher than that of APRI, FIB-4, and GPR for the prediction of LFR (0.78, 0.56, 0.55, and 0.57, respectively; all p < 0.001). Maximizing the Youden index (sensitivity + specificity − 1), a decrease in LSM value by 25% is the optimal cutoff value for the prediction of LFR (the corresponding sensitivity, specificity, positive predictive value, and negative predictive value were 68%, 81%, 78%, and 71%, respectively). In this study, of 337 patients with significant fibrosis at baseline, 146 (43.3%) had a 25% or larger decrease in LSM value during anti-HBV therapy. Patients with a 25% or larger decrease in LSM value had more LFR than those with a less than 25% decrease in LSM value (78.1% vs 22.9%, p < 0.001).
Discussion
Because the severity of liver fibrosis is one of the key prognostic factors in patients with CHB, serial assessment of the severity of liver fibrosis is of irreplaceable importance before, during, and after antiviral treatment [17]. Liver biopsy is still the gold standard for the diagnosis of liver fibrosis and cirrhosis, and frequently performed as a baseline assessment of liver fibrosis or cirrhosis before antiviral therapy in patients with CHB. However, the poor compliance and safety concerns have precluded liver biopsy for serial follow-up of the severity of liver fibrosis [6, 18]. In the real world, liver biopsy is rarely performed to monitor the dynamic changes of the severity of liver fibrosis during anti-HBV therapy, except in a few clinical trials that investigated LFR during anti-HBV treatment [3, 19]. Therefore, NITs of liver fibrosis to assess LFR would be highly useful to help clinicians reevaluating prognosis, treatment, and follow-up strategy in patients with CHB. Regression in liver fibrosis may indicate the possibility of reducing the frequency of screening for HCC.
TE is a rapid, non-invasive, and reproducible tool for assessing the degree of liver fibrosis by LSM [20]. In previous studies, the utility of TE for the evaluation of the dynamic changes in liver fibrosis during and after antiviral treatment has been reported in patients with CHC [21, 22]. Although Enomoto et al. reported that the LSM value of patients with CHB significantly decreased from 11.2 to 7.8 kPa during 12 months of treatment of entecavir, paired liver biopsies were lacking for demonstrating the reversal of liver fibrosis [23]. For patients with CHB, the association between the changes in LSM and the histological changes of liver fibrosis remains unclear. In this study, using a longitudinal cohort of 337 patients with CHB who had repeated liver biopsies and LSM measurements, we demonstrated that a 25% decrease in LSM was associated with histologic LFR. We suggested that LSM might be used to monitor regression of liver fibrosis during antiviral treatment using NUCs in patients with CHB.
Currently, serum-based NITs of liver fibrosis such as APRI, FIB-4, and GPR are available for the diagnosis of liver fibrosis and cirrhosis. A large study of 4731 patients with HCV infection has reported that anti-HCV therapy induced long-term regression of fibrosis based on FIB-4 scores [24]. Another study reported that the APRI may be a useful marker for longitudinal evaluation of the progression of liver disease in patients with HIV-HCV coinfection [25]. Although APRI or FIB-4 scores have been reported as the replacement of liver biopsy for longitudinal evaluation of the dynamic changes of liver fibrosis in patients with HCV infection or HIV-HCV coinfection [24, 25], the ability of serum-based NITs to predict LFR has not been investigated in patients with CHB. According to our results, serum-based NITs of liver fibrosis including APRI, FIB-4, and GPR did not appear to be useful in detecting LFR during anti-HBV treatment when compared with LSM. This can be explained by the confounding effects of extrahepatic conditions during long-term anti-HBV therapy, and the superior performance of LSM in predicting liver fibrosis [9, 26]. Consistent with our results, Kim et al. also reported that APRI and FIB-4 scores are not suitable for use in patients with CHB for assessment of hepatic fibrosis, especially in gauging improvements in liver fibrosis following therapy [27]. Therefore, different from patients with HCV infection or HIV-HCV coinfection, when utilizing APRI or FIB-4 one needs to be aware of their limitations in patients with HBV for longitudinal evaluation of the dynamic changes of liver fibrosis.
In this retrospective cohort study of 337 patients with CHB, 48 patients (14.2%) had NAFLD. In 2020, we assessed the accuracy of FibroScan and found that it provides high value for the diagnosis of liver fibrosis and cirrhosis in patients with concomitant CHB and NAFLD, compared to APRI and FIB-4 [28].
Although this study provides a rigorous evaluation of the association between longitudinal changes in NITs of liver fibrosis and liver histology among patients with CHB, several limitations exist. As a result of the retrospective design, patients were not randomized. Furthermore, this was a single-center study. Therefore, prospective, multicenter, large-scale, randomized studies with long-term follow-up will be needed to validate our conclusions.
Conclusions
For patients with CHB, anti-HBV therapy using NUCs was associated with a decrease in LSM value, and LSM might be used to monitor regression of liver fibrosis during anti-HBV therapy. Using repeated liver biopsy as the gold standard, we found that a 25% decrease in LSM value is associated with regression of liver fibrosis on histology. However, it is worth noting that the ALT level effects LSM value and when the ALT decreases early with HBV treatment that this can effect and overestimate changes in LSM as well as APRI and FIB-4.
References
EASL. Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;2017(67):370–98.
Bonis PA, Friedman SL, Kaplan MM. Is liver fibrosis reversible? N Engl J Med. 2001;344:452–4.
Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–75.
Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–93.
Wei H, Song B. Elastography for longitudinal assessment of liver fibrosis after antiviral therapy: a review. J Clin Transl Hepatol. 2020;8:445–53.
Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–57.
Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–8.
EASL-ALEH Clinical Practice Guidelines. Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–64.
Li Q, Chen L, Zhou Y. Diagnostic accuracy of liver stiffness measurement in chronic hepatitis B patients with normal or mildly elevated alanine transaminase levels. Sci Rep. 2018;8:5224.
Li Q, Li W, Huang Y, Chen L. The gamma-glutamyl transpeptidase-to-platelet ratio predicts liver fibrosis and cirrhosis in HBeAg-positive chronic HBV infection patients with high HBV DNA and normal or mildly elevated alanine transaminase levels in China. J Viral Hepat. 2016;23:912–9.
Li Q, Song J, Huang Y, et al. The gamma-glutamyl-transpeptidase to platelet ratio does not show advantages than APRI and Fib-4 in diagnosing significant fibrosis and cirrhosis in patients with chronic hepatitis B: a retrospective cohort study in China. Medicine (Baltimore). 2016;95:e3372.
Kim MN, Kim SU, Park JY, et al. Risk assessment of liver-related events using transient elastography in patients with chronic hepatitis B receiving entecavir. J Clin Gastroenterol. 2014;48:272–8.
European Association for the Study of the Liver, Clinical Practice Guideline Panel, Chair, EASL Governing Board representative, Panel members. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J Hepatol 2021;75:659–89
Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–93.
Kim MN, Kim SU, Kim BK, et al. Long-term changes of liver stiffness values assessed using transient elastography in patients with chronic hepatitis B receiving entecavir. Liver Int. 2014;34:1216–23.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Kim SU, Park JY, Kim DY, et al. Non-invasive assessment of changes in liver fibrosis via liver stiffness measurement in patients with chronic hepatitis B: impact of antiviral treatment on fibrosis regression. Hepatol Int. 2010;4:673–80.
Merriman RB, Ferrell LD, Patti MG, et al. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–80.
Kweon YO, Goodman ZD, Dienstag JL, et al. Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol. 2001;35:749–55.
Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–47.
Wang JH, Changchien CS, Hung CH, et al. Liver stiffness decrease after effective antiviral therapy in patients with chronic hepatitis C: longitudinal study using FibroScan. J Gastroenterol Hepatol. 2010;25:964–9.
Vergniol J, Foucher J, Castera L, et al. Changes of non-invasive markers and FibroScan values during HCV treatment. J Viral Hepat. 2009;16:132–40.
Enomoto M, Mori M, Ogawa T, et al. Usefulness of transient elastography for assessment of liver fibrosis in chronic hepatitis B: regression of liver stiffness during entecavir therapy. Hepatol Res. 2010;40:853–61.
Lu M, Li J, Zhang T, et al. Serum biomarkers indicate long-term reduction in liver fibrosis in patients with sustained virological response to treatment for HCV infection. Clin Gastroenterol Hepatol. 2016;14:1044–1055.e3.
Al-Mohri H, Murphy T, Lu Y, Lalonde RG, Klein MB. Evaluating liver fibrosis progression and the impact of antiretroviral therapy in HIV and hepatitis C coinfection using a noninvasive marker. J Acquir Immune Defic Syndr. 2007;44:463–9.
Wang XD, Pan CW, Zhou GY, et al. Effect of liver steatosis on liver stiffness measurement in chronic hepatitis B patients with normal serum alanine aminotransferase levels: a multicentre cohort study. J Viral Hepat. 2022;29:196–204.
Kim WR, Berg T, Asselah T, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64:773–80.
Li Q, Huang C, Xu W, Hu Q, Chen L. Accuracy of FibroScan in analysis of liver fibrosis in patients with concomitant chronic hepatitis B and nonalcoholic fatty liver disease. Medicine (Baltimore). 2020;99:e20616.
Acknowledgements
We thank the participants of the study.
Funding
This study was supported by Grant 21S11905600 from the Shanghai Association for Science and Technology, and Grant SHDC12020109 from the Shanghai Shenkang Hospital Development Center. The rapid service fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Study concept and design: Qiang Li. Data collection: Wei Xu, Qiankun Hu, Chong Chen, and WeiXia Li. Analysis and interpretation of data: Wei Xu, Qiankun Hu, Chong Chen, WeiXia Li, and Qiang Li. Drafting of the manuscript: Qiang Li. Critical revision of the manuscript: Liang Chen.
Disclosures
All named authors—Wei Xu, Qiankun Hu, Chong Chen, Weixia Li, Qiang Li and Liang Chen—have nothing to declare.
Compliance with Ethics Guidelines
The study was approved by the Clinical Research Ethics Committee of Shanghai Public Health Clinical Center (No. 2022-Y051-01). Although this is a retrospective study, all patients provided verbal consent that their clinical data might be used for further medical study. The procedures were in accordance with the ethical standards of the Helsinki Declaration (1964, amended most recently in 2008) of the World Medical Association.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Xu, W., Hu, Q., Chen, C. et al. Non-invasive Assessment of Liver Fibrosis Regression in Patients with Chronic Hepatitis B: A Retrospective Cohort Study. Infect Dis Ther 12, 487–498 (2023). https://doi.org/10.1007/s40121-022-00738-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00738-1